Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Others
Intraoperative electron radiation therapy (IOeRT) was developed to improve precision in local cancer treatment by combining real-time surgical exploration and resection with high-energy electron irradiation.
- IORT
- IOeRT
- electrons
- FLASH
- radiotherapy
1. Introduction
Cancer accounts for nearly 20% of deaths worldwide, representing nearly 10 million of the 55.4 million deaths worldwide [1]. The ratio between mortality and incidence varies globally a lot among countries based on tumor type distribution and performance of the healthcare system based on patient- and tumor related characteristics and cancer management innovation to tailor diagnostic and treatment into more precise medical treatment. Moreover, close multidisciplinary collaboration facilitates the search for an optimal blend of different treatments to obtain higher rates of tumor control while sparing organs and improving quality of life. IOeRT (Intraoperative electron Radiation Therapy) is a component of precise irradiation evidence based in multidisciplinary oncology.
Preclinical developments made a breakthrough in 2014, when the first publication about very promising preclinical results obtained with ultra-high dose rate electron beams, called “FLASH-RT”, attracted a huge amount of attention worldwide [2] due to an unexpected reduction of toxicities in normal tissue compared to conventional radiotherapy, while still achieving local tumor control. Confirmed by different groups and on many preclinical models [3,4], the FLASH effect is defined as the combination of a relative absence of normal tissue toxicities compared to isodose of conventional dose rate RT combined with maintained anti-tumor efficacy. It has been observed after exposure of biological tissues to high doses in extremely short treatment times and with specific beam parameters including mean and instantaneous dose rates [5] mainly, using electrons [6], but also X-rays [7] and protons [8]. As transferring X-ray- and proton-FLASH into the clinics encounters numerous and important technological challenges, FLASH-RT using electrons is the logical first choice to being investigated for the transition from preclinical research to the first clinical applications. For this, both superficial tumors as well as deeper-seated tumors in the context of IOeRT are possible targets. The technical and practical ability to deliver electron FLASH-RT intraoperatively offers a new and fascinating challenge to further explore improvements of therapeutic interventions.
Integrated management of human cancer in the 21st century requires a complete and broad consensus within the oncology community on a coordinated interdisciplinary approach, as well as established guidelines [9]. The most advanced surgical, medical, and radiation oncology requires sustained MTB (Multidisciplinary Tumor Board) enrichment [10].
Breast cancer is a disease model with continuous innovation in local treatment and associated improvement [11,12]. Similar clinical and biological relationships have been demonstrated or expected for many cancer subtypes, based both on locoregional progression and systemic progression of the cancer. Presently, surgically guided RT is the best option for guided real-time irradiation. IOeRT allows delivering the required dose to post-resected high-risk target volumes. Displacement from the electron beam of dose-sensitive normal tissues uninvolved by cancer is major protective maneuver during surgical procedure. Since obtaining local control in cancer therapy is an essential requirement of achieving long-term survivorship with maintained functional normal tissues, real-time radio-surgical collaboration, a stunning new feature of precise radiation oncology technology exemplified by IOeRT, is highly beneficial to achieving long-term survivorship [13].
In an effort to improve patient safety in practice [14], technological innovation has led to the implementation of simulation and treatment planning systems [15] and in vivo dosimetry has been explored assessing real-time dose-delivering in clinical scenarios [16,17] or based in interactive Monte Carlo algorithms estimated in phantoms [18]. Failure mode and effect analysis (FMEA) tested in the IOeRT clinical process has the potential to reduce risk and improve quality [19].
Progress in surgery is adaptable to progress in IOeRT. This model of laparoscopic surgery is fully compatible with IOeRT (with locally advanced rectal cancer as a comparison) [20]. The feasibility of combining robotic surgery technology with IOeRT procedures with miniaturized-mobile electron linear accelerators has been tested and subsequently clinically successfully applied (Figure 1) [21].
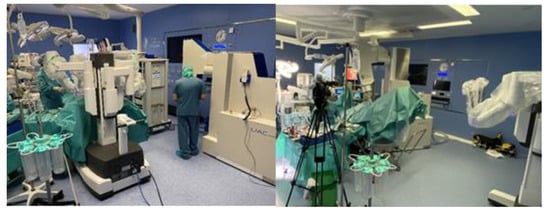
Figure 1. A modern operating room with a miniaturized-mobile electron linear accelerator and a robotic Da Vinci system used together to treat a prostate cancer patient with IOeRT (post-resection of oligonodal relapse) Liac HWL, Sordina IORT Technologies.
Surgical navigation is feasible during open surgical procedures [22] (Figure 2a). Imaging advances will make it easier to guide radio-surgical manoeuvres during intra-planning, and to register and document technical parameters in real time using ultrasound (Figure 2b).
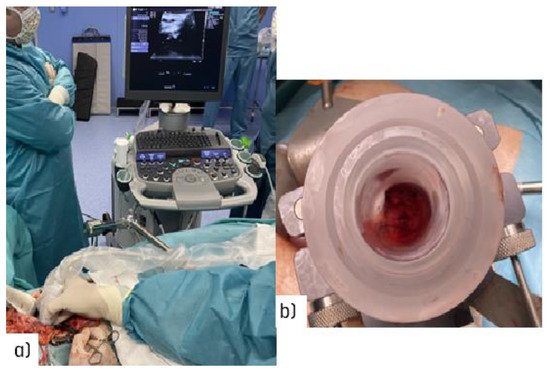
Figure 2. Illustration of the target definition imaging procedure during an open abdominal procedure: (a) real-time ultrasound assessment of the coeliac trunk with an unresectable nodal recurrence; (b) view through the electron beam applicator during the procedure in a patient with recurrent gastric cancer (uninvolved normal sensitive tissues in the upper abdomen are displaced out of the IOeRT target volume).
In academic expert institutions, a special interest is generated for the development of normalized clinical practice based on prospective data recording and e-learning resources [23].
2. Evidence-Based Data from IOeRT in 6 ESTRO/ACROP Guidelines 2020
The performance and quality of intraoperative radiation therapy (IORT) publications identified in medical databases during a recent period in terms of bibliographic metrics has been reported [24]. An updated extensive bibliometric search revealed a total of 19,148 patients registered in 116 publications evaluated analyzing publications up to 2020 (period 1982–2020) (Figure 3).
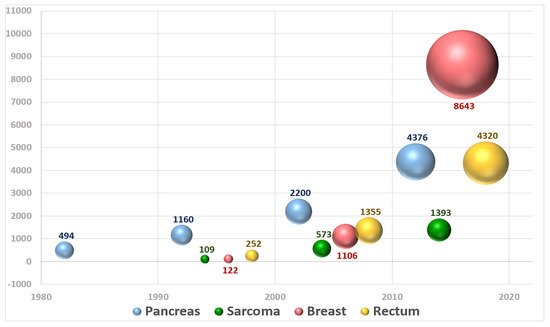
Figure 3. Graphical representation of the number of patients included in publications about IOeRT per year over the period 1982–2020.
An expert-based task force under the umbrella of the European Society for Radiotherapy and Oncology (ESTRO) condensed the available data in combination with expertise/expert opinion into a total of 6 guidelines concerning soft tissue sarcomas, unresected and borderline-resected pancreatic cancer, locally recurrent and locally advanced rectal cancer, and breast cancer. The ESTRO/ACROP (Advisory Committee for Radiation Oncology Practice) recommendations of all guidelines included the background and requirements for every aspect of clinical IOeRT practice, including patient selection, diagnostic and therapeutic procedures, quality assurance, and reporting [25,26,27,28,29,30].
The breast cancer IOeRT model is the most numerous in terms of registered patients (4229 in 12 studies on IOeRT as a partial breast irradiation technique with a single fraction and 4414 patients reported in 9 publications using IOeRT as an anticipated boost, both after breast conserving surgery). The chronology of breast cancer data is peculiar: it is the last actor in published recording (no relevant publications are available before the year 2000), but in the last two decades, the growth of IOeRT-related publications has been exponential [25].
On the other side, pancreatic cancer is the clinical model that is better documented in the early publications (1980s and 1990s) with a sustained bibliometric representation along 40 years (total of 2307 patients described in 36 publications with unresected localized disease and 2087 post-resected patients in 33 papers) [26,27].
The case of rectal cancer is particularly relevant due to the fact that it contains a significant proportion of patients treated with IOeRT for the rescue of oligo-recurrent disease (total of 1730, 10 publications). A remarkable finding from these data is the existence of a quite large and constant group of patients that remain alive and controlled, over long time periods, something that was not reported previously with other surgical or RT techniques [28]. The integration of an IOeRT component of treatment in the combined management of locally advanced primary rectal cancer (2590 patients, 18 studies) is a successful extension of the adaptation of IOeRT boost to further increase local control in a cancer site that has witnessed the introduction of multiple multimodality treatment approaches through the course of the four decades that we analyzed [29].
The last guideline to be mentioned refers to the use of IOeRT in the management of soft tissue sarcomas which has two well-defined populations: extremity (922 patients in 14 reports) and retroperitoneal (871, 24). Sarcoma publications appeared only in the 21st century and the increment in patients reported is superior in the extremity involvement model using IOeRT as an anticipated boost [30].
This entry is adapted from the peer-reviewed paper 10.3390/cancers14153693
This entry is offline, you can click here to edit this entry!
