Iron-based nanoparticles have been extensively studied to enhance the AD performance. nZVI and Fe
3O
4 NPs have been used as additives to enhance the conversion efficiency and methane generation of AD. However, their effects on the performance of microorganisms are different owing to their physical and chemical properties. The addition of nZVI has showed an increase in the methane and hydrogen production in AD of municipal wastewater and industrial wastewater from brewery and sewage plants [
47]. It was reported that the addition of Fe
3O
4 NPs at a 7 nm size and 100 ppm concentration increased methane production by 234% due to the presence of non-toxic Fe
3+ and Fe
2+ ions [
41]. The main effect of iron NPs in an AD system is to change the interspecies electron transfer in the syntrophic process of AD in which butyrate or hydrogen are used to produce methane. The nZVI can serve as a suitable low release electron donor for methanogenesis in an AD process, resulting in the increase of biogas yield. The magnetite Fe
3O
4 NPs in an AD system can act as the electron conduit when the particles are attached to the membrane surface of different cells to accelerate electron transfer among different microorganisms, leading to the improvement of methane generation [
48]. Another positive effect is the Fe
2+/Fe
3+ which can promote the growth of microorganisms. Other properties including magnetism, absorptivity, and biocompatibility of Fe
3O
4 NPs which can strengthen the digestion efficiency of pollutants in AD. Fe
3O
4 NPs have been applied in the AD treatment of industrial, municipal, and agricultural wastewater, as well as the solid waste produced from agricultural and municipal activities. Aulenta et al. discovered that the kinetics during the AD of trichloroethene (TCE) dichlorination was increased by the addition of a small amount of Fe
3O
4 NPs at 10 mg Fe/L because of the promoted electron transfer in the dechlorinating culture and the enrichment of
Desulforomonas species in microcosms [
49]. The positive effects of Fe
3O
4 NPs on AD were also demonstrated by the increase in the H
2 production and biogas yield [
50]. Similar to nZVI, Fe
3O
4 NPs could also remove heavy metal pollutants such as Cr(VI) in the wastewater due to their adsorptive capacity [
51,
52]. However, the effects of nZVI and Fe
3O
4 NPs on methane production depend on their concentration. The inhibitory impact of nZVI and Fe
3O
4 NPs at high concentrations on methanogenesis can be attributed to the deactivation of bacteria and the damage of bacterial cell membrane [
51].
3. Nanoparticles for the Enhancement of Microalgae Cultivation
The first generations of biofuels derived from edible oil seeds, food crops, and animal fats, and the second generation of biofuels derived from low-value feedstocks such as non-edible oilseeds, used cooking oil, and lignocellulosic biomass have several disadvantages as alternative fuels including the destruction of vital soil resources, deforestation, and the use of large amounts of fresh water and arable land for the supply of those feedstocks. The third and fourth generations of biofuels by taking the advantage of algae and algae/microbes, respectively, offer several advantages over the previous generations of biofuels including high productivity and growth rate, short harvesting cycle (i.e., one to ten days), higher carbon sequestration capacity (10 to 50 times higher than terrestrial plants), less water and land requirements, high oil yield per acre, having the capacity to grow in the waste stream and extreme weather conditions, and no competition with food chain [
62,
63,
64]. In spite of the mentioned advantages, more studies are needed to improve the microalgae cultivation for the large-scale economic production of biofuels by increasing their productivity, lipid content, and usage efficiencies of CO
2 and light [
63]. Among various methods, there is an increasing attention to add functional nanomaterials to algal culture to improve CO
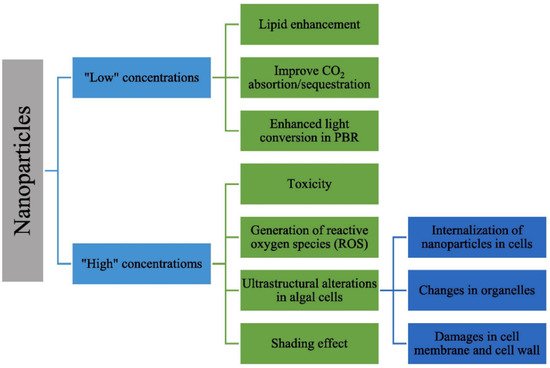
2 adsorption and light conversion efficiency for enhancing photosynthesis and algae growth. On the other hand, some destructive impacts of nanomaterials on algae growth were reported which depended on their concentrations and characteristic properties (e.g., size, crystal structure, and oxidation state), culture medium, and algae species. The effects of nanoparticles at low and high concentrations on microalgae were summarized in the literature as shown in
Figure 1 [
63].
Figure 1. Effects of nanoparticles on microalgae (reprinted with permission of Elsevier) [
63].
3.1. Metallic Nanoparticles as Micronutrients for Algal Cultivation
Trace metals play a key role as micronutrients in microalgae growth. Their effectiveness depends on their concentrations in the culture media and their synergy or antagonistic effect with other environmental factors [
62]. However, the enhancement of algal growth and lipid production strongly depends on the type and concentration of nanomaterials. Among various nanomaterials, iron has received considerable attentions due to its low toxicity, biocompatibility and high effectiveness. Microalgae require iron as an essential micronutrient in their fundamental cellular functions of photosynthesis and respiration [
65]. As shown by Pádrová et al., adding a trace amount of nZVI (1.7 to 5.1 mg L
−1) could increase the growth of green algae (
Desmodesmus subspicatus, Dunaliella salina, Parachlorella kessleri, and
Raphidocelis subcapitata) and eustigmatophycean algae (
Nannochloropsis limnetica and
Trachydiscus minutus) [
62]. Iron is a vital regulatory element in the gene expression and metabolism of algae. The presence of iron in the culture media can prolong the exponential growth phase and enhance the final cell density [
62]. Iron plays a critical role in fundamental cellular functions by acting as a cofactor of key enzymes in photosynthesis and respiration. It can promote chlorophyll biosynthesis and biomass growth by activating the
Crd1 enzyme which plays a key role in the Calvin Benson cycle [
63].
The increase of the iron content of algal culture leads to the simultaneous enhancement of the growth rate and lipid content in some microalgae species [
62]. Pádrová et al. found that the addition of 1.7–5.1 mg L
−1 of nZVI in the culture media for green algae
subcapitata and
eustigmatophycean algae enhanced the growth of those algae, and 5.1 mg L
−1 nZVI could dramatically increase lipid accumulation [
66]. Kadar et al. showed the lipid enhancement in
Tetraselims suecica and
Pavlova lutheri by the rate of 41.9% and 46.34%, respectively, after exposing to uncoated nZVI and coated nZVI powder [
67]. Pádrová et al. in another study reported the addition of 5.1 mg/L nZVI to the
Trachydiscus minutus and
Desmodesmus subspicatus cultures increased the lipid content by about 9% and 38%, respectively. They claimed that nZVI could provide a suitable source of iron to enhance the cell growth and lipid contents and induce changes in lipids’ metabolic pathways resulting in the alteration of the lipid composition [
66]. Therefore, iron, especially nZVI, may be a suitable source for the increase of microalgae growth and algal lipid production, and alternation of algal lipid profile by increasing polyunsaturated fatty acids contents [
66]. Another study further showed that the growth of the algae was even favored by the iron nanoparticles in comparison with their bulk analogues [
67].
Although increasing iron concentration below a given threshold can increase the growth rate and lipid content, iron at concentrations above the threshold negatively impacts the algal biomass and lipid production [
62]. Abd El Baky et al. found the concentration of FeCl
3 below 20 mg/L increased the total lipid accumulation, total lipid productivity, and biomass production of
Scenedesmus obliquus by 28.13%, 95.35 mg per day, and 1.25 mg per liter, respectively, in the period of 18 days [
68]. Cao et al. found that the optimum concentration of FeCl
3·6H
2O for the highest lipid content and growth rate of
Chlorella minutissima was between 0.05 to 0.1 mM [
69]. Most of the studies suggest that the increase of iron concentrations can increase the algal growth rate and lipid content but the concentrations above 0.002 g L
−1 and 0.001 g L
−1 have negative impacts on biomass and lipid production, respectively, due to inhibitory effects [
62]. Owing to the high activity, iron NPs can produce various reactive oxygen species (ROS) via Fenton-type reactions that cause oxidative injury to cells via lipid peroxidation and oxidation of thiol groups of proteins and DNA [
70].
Algal growth and lipid production are also affected by the environmental stress. The typical response of algae to environmental stress, especially nutrient shortage, is to accumulate a tremendous amount of carbon in the forms of carbohydrates and lipids for self-protection against damage. In the case of lipid accumulation under an environmental stress, the contents of most saturated and monounsaturated fatty acids increase and the content of polyunsaturated fatty acids associated with polar membrane decreases, which leads to the decrease in cellular growth [
63]. Several studies showed the additives of nanomaterials could improve algal lipid production via induced stress [
66,
68,
71]. The addition of SiC NPs under xenon illumination can improve lipid biosynthesis through inducing oxidative stress and enhancing the activity of acetyl-CoA carboxylase which is a key enzyme for catalyzing the lipid biosynthesis. Although the SiC NPs at an optimal concentration of 150 mg/L could increase lipid content by 40.26%, the TiO
2 and TiC NPs showed the inhibitory effect on the same algae [
71].
3.2. Nanoparticles for CO2 Supply
Microalgae can fix and convert atmospheric CO
2 to oxygen and biomass through photosynthesis. By considering the role of CO
2 as a carbon source in green algae cultivation, the improvement of CO
2 biofixation can increase the algal productivity and atmospheric CO
2 mitigation [
72].
pH is an important factor that affects the CO
2 fixation. Alkaline pH can activate rubisco enzyme which is responsible for CO
2 fixation through the Calvin cycle. Therefore, alkaline pH can enhance algal biomass yield and subsequent photosynthesis efficiency. In fact, photosynthesis and microalgae growth lead to alkalize the culture medium. The generated OH
− ions in reaction with CO
2 can form bicarbonate (
CO2+ OH−↔HCO−3) which can further be used as carbon sources for microalgae growth. However, an excessive amount of CO
2 entering the culture would acidify the culture medium through the formation of carbonic acid (
CO2+ H2O ↔H2CO3). Acidic pH decreases the activity of rubisco enzymes which will decrease the CO
2 biofixation efficiency and result in CO
2 loss. Nanostructured adsorbents can be used to adsorb CO
2 at an acidic pH value to retain the CO
2 gas in the culture and consequently enhance algal growth by controlling the availability of CO
2 in the culture medium through an adsorption/desorption cycle [
72].
CO
2 adsorption can be achieved through physical and chemical processes. The physical adsorption of CO
2 on nanostructured adsorbents is affected by the surface area, and pore size and volume. The pore size of adsorbents is responsible for the selectivity of an adsorption process. By considering the size of CO
2 molecules (~0.33 nm), the smaller pore size enhances the CO
2 adsorption rather than oxygen (~0.36 nm) and nitrogen (~0.35 nm) adsorption. Moreover, the CO
2 adsorption capacity and selectivity of adsorbents can be enhanced by adding some heteroatoms such as nitrogen, oxygen, and sulfur to the nanostructured surface to offer basic sites [
73]. The presence of ammonium groups and OH ions on the aerogel consisting of quaternized chitosan and polyvinyl alcohol increases its CO
2 sorption capacity up to 0.18 mmol/g. It was also reported that the selectivity of the aerogel towards CO
2 was better than the commercial membranes [
74]. Another study showed the impregnation of amine on zeolite improved CO
2 adsorption up to 4.44 mmol/g [
75]. Therefore, besides the surface area and porosity of nanostructured adsorbents, their surface chemistry and the existence of specific functional groups can significantly influence their adsorption capacity for CO
2 [
72,
76].
Recent studies showed that nanostructured adsorbents had higher CO
2 capturing capacity and reusability over several adsorption/desorption cycles than other popular adsorbents. This might be attributed to their high specific surface area and functionality that can provide more accessible adsorption sites for CO
2 [
72,
76]. Carbonization of pine cone shells at 650 °C and subsequent activation by KOH enhanced the CO
2 adsorption capacity up to 7.63 mmol g
−1 and 2.35 mmol g
−1 at 0 °C under 1 and 0.15 bar pressure, respectively, due to increasing specific surface area and porosity [
77]. Several studies reported the promising performance of polyacrylonitrile (PAN) nanofiber in adsorbing and supplying CO
2 for microalgae cultivation [
76,
78,
79]. The addition of PAN nanofiber at 0.1·g·L
−1 to the
Chlorella fusca LEB 111 culture was found to improve biofixation and carbohydrate production by 45% and 2.3%, respectively, compared to the control without the nanofiber [
78]. Metal nanoparticles can be added to the nanofiber to further enhance CO
2 biofixation. The addition of iron oxide NPs at 4% w/v to PAN nanofiber significantly enhanced the CO
2 fixation in
Chlorella fusca LEB 111 to 216.2 mg·L
−1·d
−1 due to the high CO
2 adsorption capacity of Fe
2O
3 NPs (164.2·mg·g
−1), increasing the contact time between gas and microorganisms by enhancing the porosity and providing higher surface area [
79] and creating the microorganism–nanoparticle hybrid [
80].
4. Nanomaterials for Microbial Fuel Cells
4.1. Nanostructured Bioelectrodes
An MFC requires anode and cathode electrodes. Biofilms on the anode electrode oxidize biodegradable organic matters in wastes to liberate electrons and protons. Protons and electrons from an anode will be combined with oxygen at the cathode electrode to produce water. The electrodes should have high surface area and excellent abilities in extracellular electron transport and electrical conductivity to achieve high power density.
Colonization of microorganisms requires anodes with a high porosity, proper pore size, biocompatibility, low cytotoxicity, and resistance to decomposition. Metal components have high conductivity, but their bacterial adhesion is poor. Furthermore, they may be susceptible to corrosion and liberate toxic heavy metals. Carbon-based materials such as graphene and carbon nanotubes provide great surface area facilitating the colonization of microbes, biocompatibility and high conductivity [
91]. Nanostructured biocatalyst electrode architectures can be designed and optimized as an excellent MFC anode [
93].
One economic and effective way to increase power output of an MFC is to depose of metal or metal oxide (e.g., gold and ruthenium oxide) nanoparticles on the surface of electrodes [
94,
95]. It was found that carbon cloth-based anode electrodes deposited with Au with a thickness of 50 nm and 100 nm on each side achieved power density 1.22–1.88 times higher than that obtained with a plain carbon cloth electrode [
95]. Another study showed that the dual chamber MFC with a RuO
2-coated carbon felt anode increased the power density by 17 times as compared to that obtained with the MFC using a bare carbon felt anode [
94].
Polyaniline that has high electrical conductivity can facilitate the electron transfer from bacteria to external circuit on an MFC anode. Study showed that an anode formed by coating polyaniline nanofiber and electrochemically reducing graphene oxide on the surface of carbon cloth yielded a maximum power density of 1390 mW/m
2, which was three times larger than that of the MFC with a carbon cloth anode [
96]. It was reported that an anode formed by a hydrogel composite with bacterial cellulose as a continuous phase and polyaniline as a dispersed phase could achieve a maximum power density of 117.6 mW/m
2 in a current density of 617 mA/m
2, compared to 1 mW/m
2 and 10 mA/m
2 using a graphite plate anode at the same condition [
97]. Hydrogel can achieve excellent nutrient transfer from the culture medium to attached microbial biofilm, provide favorable conditions for bacteria colonization, and prevent it from spoilage. Polyaniline with a high electrical conductivity can facilitate the electron transfer from bacteria to external circuit [
97].
Various metal and non-mental nano-composites such as carbon nanotubes and graphene have been studied as cathode catalysts [
98]. Owing to its availability and the high electrochemical oxygen potential, an air-breathing cathode MFC is considered as the most promising configuration. An air-breathing cathode usually consists of an electrode substrate, oxygen reduction reaction (ORR) catalyst layer, and air-diffusion layer [
99]. The air-cathode catalyst is pivotal in the performance of MFCs because of its role in improving the intrinsic overpotential and poor kinetics of ORR. Although exhibiting the best ORR performance, Pt group metals-based electrocatalysts have high cost, low abundance, and easy deactivation in the presence of MFC metabolites, hindering them from broad industrial application. So far, numerous non-noble metal inexpensive electrocatalysts with an excellent catalytic ability such as Fe-N/C catalysts have been studied. Various Fe-N/C catalysts were synthesized to introduce Fe and N as dual-dopants on the carbon so that the catalytic sites, i.e., Fe-Nx and N-Cx, can be generated [
100]. The typical synthesis method is to pyrolyze the widely available precursors comprising of iron salts, nitrogen-rich molecules, and carbon precursors under a high temperature in either N
2 or NH
3 environment. The carbon component in the catalysts serves as the conductive support and the host of active moieties.
In general, an ideal electrocatalyst should possess good electrical conductivity, hierarchical pore structure, large specific surface area, and competent active sites. Therefore, the iron-containing precursors are normally introduced in various carbonaceous materials including active carbon, graphene, carbon nanotube (CNT), conducting polymers, and porous carbons. The heteroatoms (Fe and N) can be introduced into the carbon materials by pyrolyzing FePc-coated activated carbon [
101]; by co-doping hierarchical porous iron and nitrogen in carbons via coupling polypyrrole with iron cation [
102], and by using metal-organic-framework (MOF) of dual metal- and nitrogen-doped carbon as a precursor [
103].