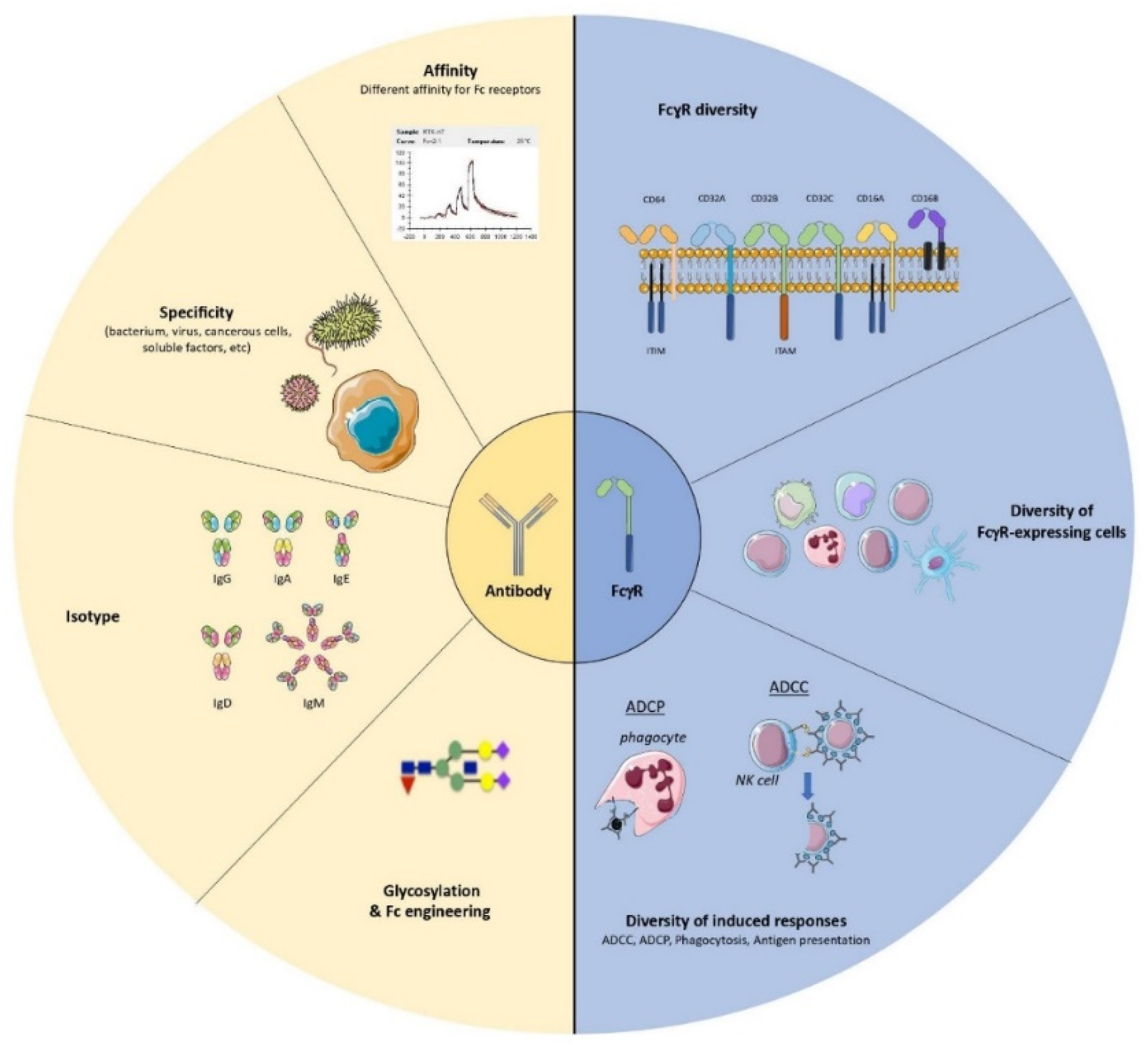
The multiple mechanisms of action of antiviral monoclonal antibodies (mAbs) have made these molecules a potential therapeutic alternative for treating severe viral infections. In addition to their direct effect on viral propagation, several studies have shown that mAbs are able to enhance the host’s adaptive immune response and generate long-lasting protective immunity. Such immunomodulatory effects occur in an Fc-dependent manner and rely on Fc-FcγR interactions. It is noteworthy that several FcγR-expressing cells have been shown to play a key role in enhancing humoral and cellular immune responses (so-called “vaccinal effects”) in different experimental settings.
- antiviral immunity
- antiviral monoclonal antibodies
- immunotherapy
- FcγR
- vaccinal effect
- immunomodulation
1. Introduction
2. Multiple FcγR-Expressing Immune Cells Are Involved in the Induction of Vaccinal Effects: Lessons Learned from a Murine Model of Retroviral Infection
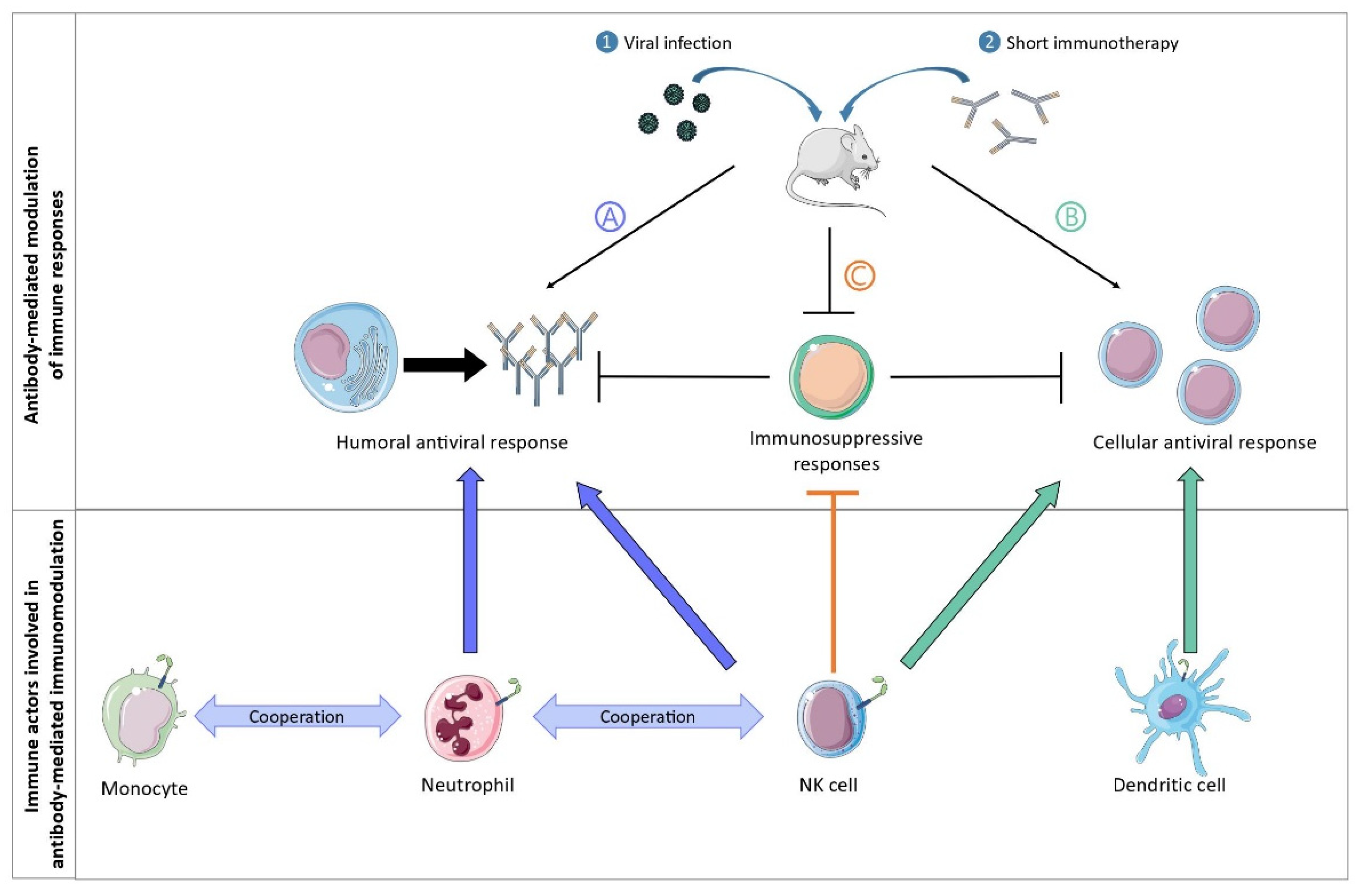
- (i).
-
The vaccinal effects of mAbs strictly depend on Fc-FcγR interactions. In particular, the formation of ICs composed of the administered mAb and infected cells (rather than with virions) enhances the cytotoxic cellular response via the interaction with FcγRs expressed on dendritic cells (DC). These observations also highlighted that the nature of ICs matters to generate protective immunity, as infected cells display immunodominant peptides that are poorly incorporated into virions [12][13],
- (ii).
-
MAb treatment prevents the development of the regulatory T (Treg) response in an Fc-dependent manner, with specific antibody isotypes involved in such Treg inhibition [14]. Thus, whereas the administration of anti-FrCasE mAbs of the IgG2a isotype prevented the development of Treg responses in infected mice, neither anti-FrCasE mAbs of the IgM isotype nor F(ab’)2 antibody fragment administration had the same effect. However, the mechanisms involved in this Fc-dependent inhibition of the Treg response by the therapeutic mAbs were not elucidated.
- (iii).
-
Neutrophils have a crucial role in the induction of a protective humoral immune response during immunotherapy with neutralizing mAbs [15]. The immunomodulatory potential of neutrophils was evaluated by performing neutrophil depletion experiments. These experiments showed that the absence of neutrophils in infected, mAb-treated mice resulted in a decrease in serum levels of specific anti-FrCasE IgGs as well as a decrease in the frequency of marginal zone B cells and plasma cells in the spleen and bone marrow, respectively. Importantly, neutrophils acquired B cell helper functions upon FcγR-triggering (i.e., secretion of B cell activating factor; BAFF) leading to the induction of a sustained and protective humoral response that was key for the survival of the mice [15].
- (iv).
-
Neutrophils and monocytes cooperate in the induction of a protective immune response [16]. Notably, upon antibody therapy, neutrophils and inflammatory monocytes display distinct functional activation states and sequentially modulate the antiviral immune response by secreting Th1-type polarizing cytokines and chemokines, which occur in an FcγRIV-dependent manner. Notably, mAb-treatment of infected mice led to a strong upregulation of FcγRIV in neutrophils and inflammatory monocytes, as well as an enhanced functional activation of both cell types (i.e., upregulation of several activation markers and enhanced secretion of cytokines/chemokines). Interestingly, neutrophils showed a higher and a wider induction of chemokines and cytokines release than monocytes at day 8 p.i, while monocytes secreted strong quantities of Th1-polarizing cytokines and chemokines at day 14 p.i., suggesting a potential role for neutrophils as early drivers of the induction of vaccinal effects by mAbs. In addition, FcγRIV-blocking in mAb-treated mice led to decreased secretion of cytokines and chemokines by both myeloid cell-types, as well as reduced mAb-mediated protection.
- (v).
-
NK cells, in addition to their role in the elimination of infected cells, also have a key immunomodulatory role in the induction of a protective immune response after mAb treatment. This was demonstrated using an NK depletion approach that led to the abrogation of the vaccinal effects induced by mAb therapy (i.e., decreased virus-specific antibody titers and CD8+ T cell responses) [17]. The immunomodulatory effects of NK cells are two-fold. Firstly, control of viral propagation by NK cells prevents immune cell exhaustion and the establishment of immunosuppressive mechanisms (i.e., upregulation of molecules involved in immunosuppressive pathways, such as PD-1, PD-L1, and CD39 on dendritic cells and T cells). Secondly, IFNγ-producing NK cells play a role in the enhancement of the B cell responses through the potentiation of the B cell helper properties of neutrophils [17].
3. Fc-Mediated Immunomodulatory Properties of mAbs Directed against Human Viruses: Evidence from Mouse, NHP Preclinical Models and Clinical Studies
Table 1 summarizes vaccinal effects reported in preclinical and clinical studies of human viral infections.
Table 1. Vaccinal effects reported in preclinical and clinical studies of human viral infections.
|
Type of Study |
Infection |
Ab |
Animal Model/Patients |
Immune Outcome (Observed Vaccinal Effect) |
Mechanism |
Reference |
|---|---|---|---|---|---|---|
|
Preclinical |
Henipaviruses |
m102.4 |
African green monkeys |
Humoral responses |
||
|
Preclinical |
Influenza virus |
3C05 (GAALIE variant) |
Transgenic FcγRs humanized mice |
CD8+ T cell responses |
Dendritic cell activation |
[21] |
|
Preclinical |
SARS-CoV-2 |
COV2-2050 |
Mice and hamsters |
Increased numbers and more activated CD8+ T cells. Decreased inflammation |
Potential monocyte involvement in decreasing inflammation |
[22] |
|
Preclinical |
SHIV-SF162P3 |
PGT121/3BNC117/b12 mAb cocktail |
Rhesus macaques (Macaca mulatta) |
Increased frequencies and decreased exhaustion of Gag-specific CD8+ and CD4+ T cells |
[23] |
|
|
Preclinical |
SHIVAD8-EO |
3BNC117 and 10–1074 |
Rhesus macaques (Macaca mulatta) |
Polyfunctional CD8+ T cells |
[24] |
|
|
Clinical |
HIV |
3BNC117 |
Viremic and aviremic subjects on antiretroviral therapy (ART) |
Humoral response |
[25] |
|
|
Clinical |
HIV |
3BNC117 and 10–1074 |
HIV-1-infected individuals and ART interruption |
Virus-specific T cell immunity |
[26] |
4. Induction of Vaccinal Effects by mAbs in HIV-Infected Patients
This entry is adapted from the peer-reviewed paper 10.3390/antib11030050
References
- Mullard, A. FDA Approves 100th Monoclonal Antibody Product. Nat. Rev. Drug Discov. 2021, 20, 491–495.
- Dibo, M.; Battocchio, E.C.; dos Santos Souza, L.M.; da Silva, M.D.V.; Banin-Hirata, B.K.; Sapla, M.M.M.; Marinello, P.; Rocha, S.P.D.; Faccin-Galhardi, L.C. Antibody Therapy for the Control of Viral Diseases: An Update. Curr. Pharm. Biotechnol. 2019, 20, 1108–1121.
- Hooft van Huijsduijnen, R.; Kojima, S.; Carter, D.; Okabe, H.; Sato, A.; Akahata, W.; Wells, T.N.C.; Katsuno, K. Reassessing Therapeutic Antibodies for Neglected and Tropical Diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007860.
- Salazar, G.; Zhang, N.; Fu, T.-M.; An, Z. Antibody Therapies for the Prevention and Treatment of Viral Infections. NPJ Vaccines 2017, 2, 19.
- Corti, D.; Purcell, L.A.; Snell, G.; Veesler, D. Tackling COVID-19 with Neutralizing Monoclonal Antibodies. Cell 2021, 184, 3086–3108.
- Crowe, J.E. Human Antibodies for Viral Infections. Annu. Rev. Immunol. 2022, 40, 349–386.
- Cameroni, E.; Bowen, J.E.; Rosen, L.E.; Saliba, C.; Zepeda, S.K.; Culap, K.; Pinto, D.; VanBlargan, L.A.; De Marco, A.; di Iulio, J.; et al. Broadly Neutralizing Antibodies Overcome SARS-CoV-2 Omicron Antigenic Shift. Nature 2022, 602, 664–670.
- Forthal, D.N.; Landucci, G.; Daar, E.S. Antibody from Patients with Acute Human Immunodeficiency Virus (HIV) Infection Inhibits Primary Strains of HIV Type 1 in the Presence of Natural-Killer Effector Cells. J. Virol. 2001, 75, 6953–6961.
- Pelegrin, M.; Naranjo-Gomez, M.; Piechaczyk, M. Antiviral Monoclonal Antibodies: Can They Be More Than Simple Neutralizing Agents? Trends Microbiol. 2015, 23, 653–665.
- Lambour, J.; Naranjo-Gomez, M.; Piechaczyk, M.; Pelegrin, M. Converting Monoclonal Antibody-Based Immunotherapies from Passive to Active: Bringing Immune Complexes into Play. Emerg. Microbes Infect. 2016, 5, e92.
- Portis, J.L.; Czub, S.; Garon, C.F.; McAtee, F.J. Neurodegenerative Disease Induced by the Wild Mouse Ecotropic Retrovirus Is Markedly Accelerated by Long Terminal Repeat and Gag-Pol Sequences from Nondefective Friend Murine Leukemia Virus. J. Virol. 1990, 64, 1648–1656.
- Michaud, H.A.; Gomard, T.; Gros, L.; Thiolon, K.; Nasser, R.; Jacquet, C.; Hernandez, J.; Piechaczyk, M.; Pelegrin, M. A Crucial Role for Infected-Cell/Antibody Immune Complexes in the Enhancement of Endogenous Antiviral Immunity by Short Passive Immunotherapy. PLoS Pathog. 2010, 6, e1000948.
- Nasser, R.; Pelegrin, M.; Michaud, H.A.; Plays, M.; Piechaczyk, M.; Gros, L. Long-Lasting Protective Antiviral Immunity Induced by Passive Immunotherapies Requires Both Neutralizing and Effector Functions of the Administered Monoclonal Antibody. J. Virol. 2010, 84, 10169–10181.
- Nasser, R.; Pelegrin, M.; Plays, M.; Gros, L.; Piechaczyk, M. Control of Regulatory T Cells Is Necessary for Vaccine-like Effects of Antiviral Immunotherapy by Monoclonal Antibodies. Blood 2013, 121, 1102–1111.
- Naranjo-Gomez, M.; Lambour, J.; Piechaczyk, M.; Pelegrin, M. Neutrophils Are Essential for Induction of Vaccine-like Effects by Antiviral Monoclonal Antibody Immunotherapies. JCI Insight 2018, 3, e97339.
- Lambour, J.; Naranjo-Gomez, M.; Boyer-Clavel, M.; Pelegrin, M. Differential and Sequential Immunomodulatory Role of Neutrophils and Ly6Chi Inflammatory Monocytes during Antiviral Antibody Therapy. Emerg. Microbes Infect. 2021, 10, 964–981.
- Naranjo-Gomez, M.; Cahen, M.; Lambour, J.; Boyer-Clavel, M.; Pelegrin, M. Immunomodulatory Role of NK Cells during Antiviral Antibody Therapy. Vaccines 2021, 9, 137.
- Wen, Y.-M.; Mu, L.; Shi, Y. Immunoregulatory Functions of Immune Complexes in Vaccine and Therapy. EMBO Mol. Med. 2016, 8, 1120–1133.
- Bossart, K.N.; Geisbert, T.W.; Feldmann, H.; Zhu, Z.; Feldmann, F.; Geisbert, J.B.; Yan, L.; Feng, Y.R.; Brining, D.; Scott, D.; et al. A Neutralizing Human Monoclonal Antibody Protects African Green Monkeys from Hendra Virus Challenge. Sci. Transl. Med. 2011, 3, 105ra103.
- Geisbert, T.W.; Mire, C.E.; Geisbert, J.B.; Chan, Y.P.; Agans, K.N.; Feldmann, F.; Fenton, K.A.; Zhu, Z.; Dimitrov, D.S.; Scott, D.P.; et al. Therapeutic Treatment of Nipah Virus Infection in Nonhuman Primates with a Neutralizing Human Monoclonal Antibody. Sci. Transl. Med. 2014, 6, 242ra82.
- Bournazos, S.; Corti, D.; Virgin, H.W.; Ravetch, J.V. Fc-Optimized Antibodies Elicit CD8 Immunity to Viral Respiratory Infection. Nature 2020, 588, 485–490.
- Winkler, E.S.; Gilchuk, P.; Yu, J.; Bailey, A.L.; Chen, R.E.; Chong, Z.; Zost, S.J.; Jang, H.; Huang, Y.; Allen, J.D.; et al. Human Neutralizing Antibodies against SARS-CoV-2 Require Intact Fc Effector Functions for Optimal Therapeutic Protection. Cell 2021, 184, 1804–1820.e16.
- Barouch, D.H.; Whitney, J.B.; Moldt, B.; Klein, F.; Oliveira, T.Y.; Liu, J.; Stephenson, K.E.; Chang, H.W.; Shekhar, K.; Gupta, S.; et al. Therapeutic Efficacy of Potent Neutralizing HIV-1-Specific Monoclonal Antibodies in SHIV-Infected Rhesus Monkeys. Nature 2013, 503, 224–228.
- Nishimura, Y.; Gautam, R.; Chun, T.-W.; Sadjadpour, R.; Foulds, K.E.; Shingai, M.; Klein, F.; Gazumyan, A.; Golijanin, J.; Donaldson, M.; et al. Early Antibody Therapy Can Induce Long-Lasting Immunity to SHIV. Nature 2017, 543, 559–563.
- Schoofs, T.; Klein, F.; Braunschweig, M.; Kreider, E.F.; Feldmann, A.; Nogueira, L.; Oliveira, T.; Lorenzi, L.C.C.; Parrish, E.H.; Learn, G.H.; et al. HIV-1 Therapy with Monoclonal Antibody 3BNC117 Elicits Hoost Immune Responses against HIV-1. Science 2016, 352, 997–1001.
- Niessl, J.; Baxter, A.E.; Mendoza, P.; Jankovic, M.; Cohen, Y.Z.; Butler, A.L.; Lu, C.-L.; Dubé, M.; Shimeliovich, I.; Gruell, H.; et al. Combination Anti-HIV-1 Antibody Therapy Is Associated with Increased Virus-Specific T Cell Immunity. Nat. Med. 2020, 26, 222–227.
