Quercetin is a flavonoid, present in various natural sources, which has demonstrated in vitro and in vivo antidiabetic properties. It improves oral glucose tolerance, as well as pancreatic β-cell function to secrete insulin. It inhibits the α-glucosidase and DPP-IV enzymes, which prolong the half-life of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Quercetin also suppresses the release of pro-inflammatory markers such as IL-1β, IL-4, IL-6, and TNF-α.
- quercetin
- diabetes
- inflammatory markers
- medicinal plants
- insulin
1. Introduction
Diabetes mellitus (DM) is a chronic disease that is one of the leading causes of illness and mortality across the globe. DM is diagnosed as a result of an elevated blood glucose level (hyperglycaemia) caused by inadequate insulin secretion, defective insulin action, or both. The improper control of insulin has also been linked to abnormalities in the metabolism of lipids and proteins. If proper treatment is not received on time, or if left untreated, DM can lead to hyperglycaemic coma, and severe damage to the eyes, kidneys, blood vessels, and nervous and cardiovascular system. It can even lead to death due to ketoacidosis and nonketotic hyperosmolar syndrome [1][2]. These metabolic disruptions result from low insulin levels or insulin resistance in skeletal muscles, adipose tissue, and other target tissues. The development, pathogenesis, and complications of DM have been strongly correlated with high levels of oxidative stress, free radicals, and other metabolic stressors [3][4]. According to reports from 2021, 465 million people suffer from DM worldwide [5]. This number is anticipated to rise to 700 million by 2045. The majority of DM sufferers are from middle and low-income countries [5].
The American Diabetes Association has categorized diabetes as Type 1, Type 2, and gestational DM [6]. Type 1 diabetes, also known as juvenile diabetes, causes a decrease in glucose sensitivity to clonal pancreatic β-cells [7]. It has no cure but can be controlled by lifestyle changes, blood sugar monitoring, and the administration of insulin. This type of diabetes occurs in approximately 80–90% of children and adolescents [8]. Type 2 diabetes mellitus (T2DM) is the most prevalent and occurs due to the insufficient production of insulin by the body, insulin resistance, and obesity [9]. It can be controlled by lifestyle/dietary changes and oral antidiabetic drugs but requires insulin in severe cases [10]. Whilst the majority of T2DM sufferers are adults (more than 90% of the patient population), it affects people of all ages. Individuals over 40 years of age and with obesity issues and a family history of the disease are at a higher risk of developing T2DM [11].
A range of antidiabetic drugs such as metformin, sulfonylureas, meglitinides, thiazolidinediones, GLP-1 mimetics, DPP-IV, and SGLT2 inhibitors are currently used to treat T2DM. However, many of these are costly and present notable adverse side effects (Table 1) [12].
Table 1. Pharmacological actions and side effects of antidiabetic drugs.
|
Type 2 Antidiabetic Agents |
Pharmacological Actions |
Side Effects |
References |
|---|---|---|---|
|
α-glucosidase inhibitors (Acarbose, miglitol) |
Inhibit the intestinal absorption of carbohydrates |
Flatulence, bloating, diarrhoea |
|
|
Biguanides (Metformin) |
Inhibit hepatic gluconeogenesis, Reduce the liver and intestinal absorption of sugar Increase insulin sensitivity and glucose uptake |
Kidney complications, upset stomach, tiredness, and dizziness |
|
|
Dopamine agonists (Bromocriptine, cabergoline, apomorphine) |
Regulate plasma glucose, free fatty acids, and triglyceride levels in insulin-resistant patients |
Visual hallucinations and confusion, edema |
|
|
Dipeptidyl peptidase-4 (DPP-4) inhibitors (Sitagliptin, saxagliptin, linagliptin) |
Increase the half-life of GLP-1 and GIP |
Gastrointestinal problems, flu-like symptoms (headache, runny nose, sore throat) |
|
|
GLP-1 agonists (Dulaglutide, exenatide, albiglutide) |
Enhance insulin release Reduce glucagon release |
Gastrointestinal problems and nausea |
|
|
Meglitinides (Nateglinide, repaglinide) |
Stimulate the release of insulin |
Weight gain, hypoglycaemia, excessive sweating |
|
|
Sodium-glucose Co-transporter-2 (SGLT-2) inhibitors (Dapagliflozin, canagliflozin, empagliflozin) |
Inhibit glucose reabsorption in the renal tubule |
Urinary tract infection and increased urination, upper respiratory tract infections, joint pain, nausea, and thirst |
|
|
Sulfonylureas (Tolbutamide, tolazamide, chlorpropamide) |
Inhibit ATP-sensitive potassium (KATP) channel in pancreatic β-cells |
Hypoglycaemia, upset stomach, skin rash, and itching |
[27] |
|
Thiazolidinediones (Rosiglitazone, pioglitazone) |
Bind with the peroxisome proliferator-activated receptor (PPAR)-γ receptor resulting in the activation of several genes that regulate glucose metabolism in the liver |
Anaemia risk, weight gain, edema, heart failure |
2. Chemistry of Quercetin
The term quercetin is derived from the Latin word “Quercetum” which means oak forest. The main dietary sources of quercetin are fruits, vegetables, and various medicinal plants. Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is a compound yellow in color, fully soluble in lipids and alcohol, insoluble in cold water, and sparingly soluble in hot water, that was isolated as a flavonoid glycoside for the first time in 1854. Its chemical structure was elucidated in 1899 [30]. Quercetin belongs to the flavonol subclass of flavonoids, with two aromatic rings (A and B) interlinked by a three-carbon linked γ-pyrone ring (C), and five hydroxyl (OH) groups that can be variously substituted (Figure 1).
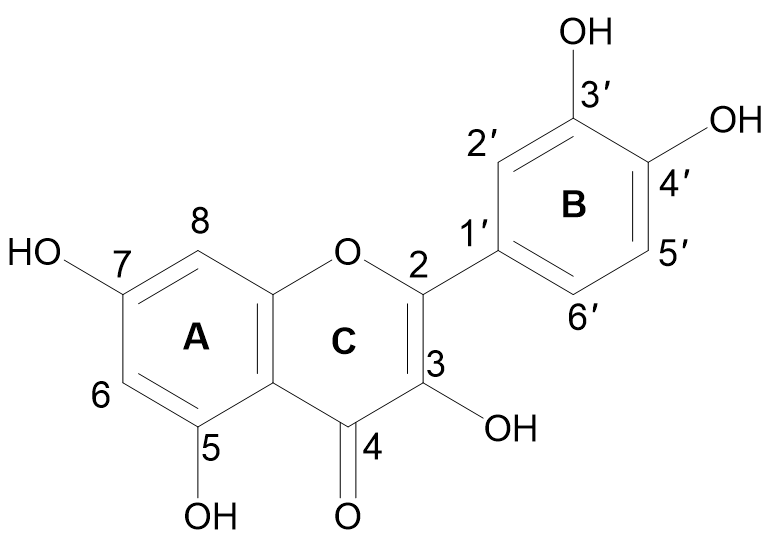
Figure 1. Chemical structure of quercetin with (A, B) and (C) representing the aromatic and γ-pyrone rings, respectively.
The majority of quercetin derivatives are found in a glycoside form in which one or more hydroxyl group is substituted by different types of sugars [31]. Its polyphenolic structure, catechol moiety in the B ring, OH groups at positions 3 and 5 in the A ring, and 2,3-double bond conjugated with a 4-oxo function in the C ring have been identified as important features responsible for the well-known antioxidant effect of quercetin [32][33].
3. Pharmacological Actions of Quercetin in Diabetes and Associated Metabolic Disorders
Quercetin possesses various pharmacological properties and has been reported as one of the most widely used flavonoids to treat metabolic and inflammatory disorders [34]. In vitro studies on human retinal endothelial cells demonstrated that quercetin could inhibit the proliferation of high-glucose-induced cells by lowering the production of vascular endothelial growth factor (VEGF) (Figure 2) [35]. Quercetin also inhibited carbohydrate digesting-enzymes (intestinal α-glucosidase and pancreatic α-amylase), reduced starch hydrolysis, decreased the rate of glucose absorption, as well as slowed down the progression of postprandial hyperglycaemia in in vitro settings (Figure 2) [36][37].
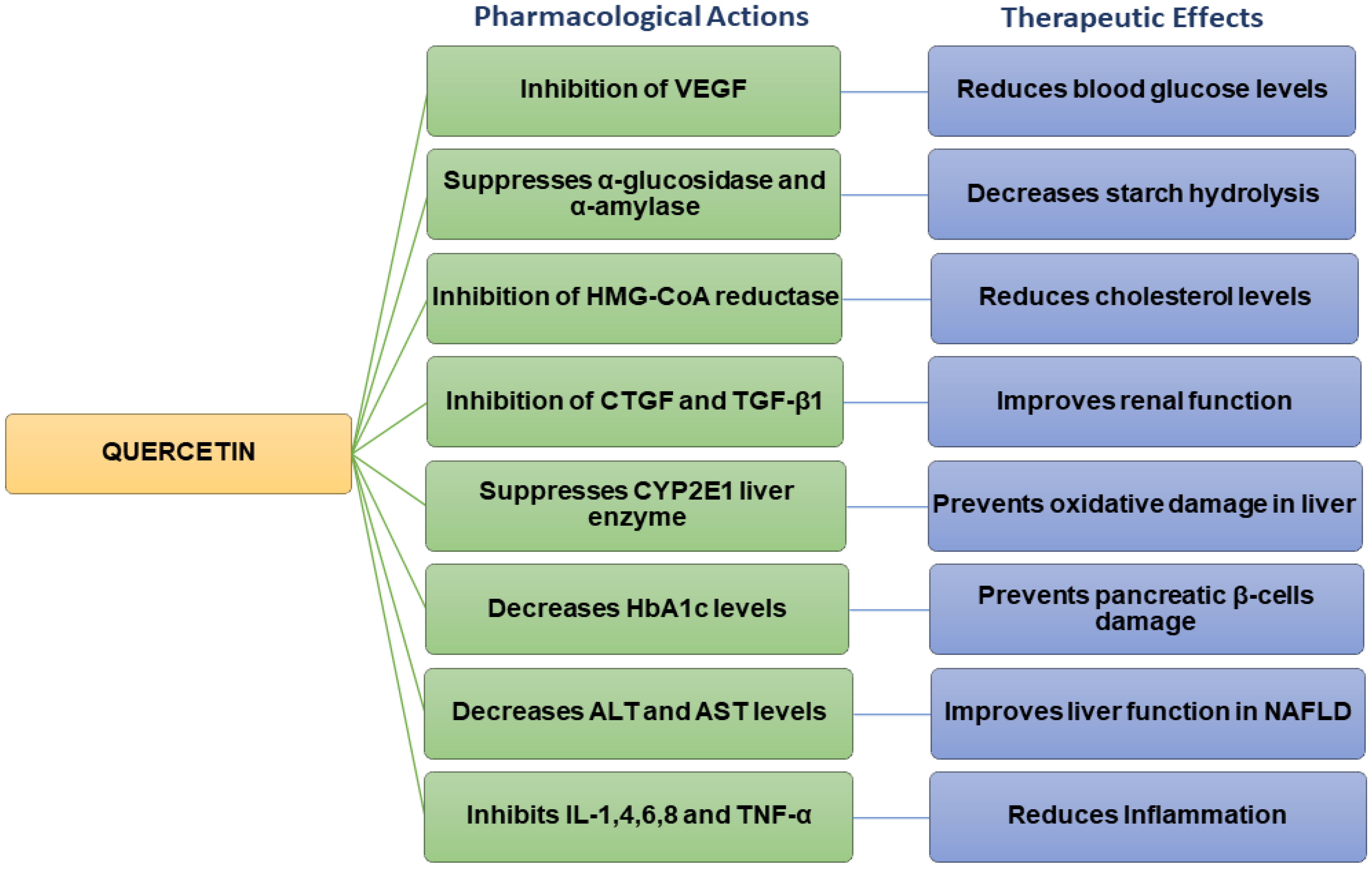
Figure 2. Flow chart summarizing the pharmacological actions and therapeutic effects of quercetin.
Studies carried out on streptozotocin (STZ)-induced diabetic rats have revealed that quercetin could reduce blood glucose levels and improve glucose tolerance [38]. Quercetin decreased plasma glucose levels in Type 2 diabetic rats [39]. In hyperlipidaemic animals, quercetin lowered the levels of triglycerides (TG), total cholesterol (TC), LDL, and VLDL cholesterol, inhibited 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase, and increased adiponectin and HDL cholesterol levels [39][40]. Previous findings also indicated that quercetin could improve the high-fat diet (HFD)-induced dyslipidaemia in Swiss albino mice [41]. Other studies have shown that quercetin inhibited the overexpression of connective tissue growth factor (CTGF) and transforming growth factor beta-1 (TGF-β1) and contributed to improving renal function in diabetic nephropathic rats (Figure 2) [42].
4. Mechanisms of Action of Quercetin
Quercetin maintains glucose homeostasis by interacting with molecular targets in the small intestine, pancreas, skeletal muscle, adipose tissue, and liver. Studies carried out on STZ-induced diabetic rats have revealed that quercetin could restore the impaired protein expression of insulin signaling molecules, such as phosphatidylinositol 3 kinases (PI3K) and insulin receptor substrate-1 (IRS-1), resulting in increased insulin-mediated glucose uptake [54]. Quercetin has also been shown to activate adenosine monophosphate-activated protein kinase (AMPK) in the livers of rats, which reduces glucose synthesis primarily via downregulating glycogenic isoenzymes, such as phosphoenolpyruvate carboxylase (PEPCK) and glucose-6-phosphatase (G6Pase) [46][55]. In mouse skeletal muscle cells, it has been reported to enhance glucose uptake by promoting the translocation of GLUT4 to the cell membrane [56]. These findings indicate that quercetin regulates the metabolism of glucose, increasing glycolysis while decreasing gluconeogenesis [57]. In healthy individuals, around 80% of the absorbed glucose is stored in the form of glycogen in skeletal muscles upon the action of insulin. A reduction in this uptake has been shown to contribute to the etiology of T2DM as irregularities in the expression of the GLUT4 transporter lower the rate of glucose entering the cells, leading to a rise in blood glucose levels [58]. In skeletal muscles, quercetin activates AMPK, which in turn stimulates GLUT4 receptors and Akt (protein kinase B) in the cell membrane [59]. This allows glucose to enter the cells via the GLUT4 transporter, thereby regulating glycaemia [54]. Similarly, exercise is a potent activator of GLUT4 expression, which increases insulin activity and muscle glycogen storage. Defective activation of AMPK leads to insulin resistance, which causes T2DM [60]. Quercetin-induced AMPK activation in hepatocytes inhibits glucose-6 phosphatases [55]. Treatment with quercetin decreases GLUT2 expression and the intestinal sodium-dependent glucose uptake, in turn reducing glucose absorption in the gastrointestinal tract and controlling glycaemia (Figure 3) [56].

Figure 3. Pharmacological action of quercetin via different mechanistic pathways: Quercetin enhances pancreatic β-cell function and increases insulin release by inhibiting apoptosis, NF-κB, and JNK pathways; decreases glucose absorption in the kidney by inhibiting DPP-IV and COX-2 activity; decreases gluconeogenesis through inhibition of TNF-α and IL-4 in the liver; suppresses glucose reabsorption in the gastrointestinal tract by decreasing α-glucosidase activity; reduces blood glucose levels and oxidative stress by inhibiting IL-6 activity in the heart and blood vessels.
In addition, quercetin has been reported to enhance the AMP/ATP ratio and scavenge reactive oxygen species (ROS) in clonal pancreatic β-cells, reducing oxidative stress (Figure 3) [61]. The increased AMP/ATP ratio activates the mitochondrial target of rapamycin (mTOR), which induces mitogenesis (via transduction signaling pathways activation) and stimulates insulin secretion [62]. mTOR plays a significant role in the regulation of transcription, protein synthesis, and cell nutrition [63]. Under hyperglycaemic conditions, proteins, lipids, and nucleic acids undergo non-enzymatic glycation to form Advanced Glycation End (AGE)-products. The latter cause diabetic complications such as cardiovascular diseases, nephropathy, retinopathy, and neuropathy. Quercetin has been found to inhibit protein glycation more potently than the synthetic drug aminoguanidine [59][60]. It can reduce the formation of AGE-products by trapping methylglyoxal and glyoxal [64] and improve diabetic complications due to its antioxidant, anti-inflammatory, and antihyperglycemic properties [41].
The release of pro-inflammatory mediators such as IL-1, IL-6, IL-8, IL-4, TNF-α, and histamine in brown adipose tissue has been linked with increased insulin resistance and high blood glucose levels (Figure 3) [70]. Quercetin inhibits these mediators and prevents oxidative stress [71]. In the kidneys, quercetin reduces DPP-IV and cyclooxygenase-2 (COX-2) activity, leading to a decrease in blood glucose reabsorption (Figure 3) [72]. Quercetin also activates leukocytes and targets various enzymes such as kinases, membrane proteins, and phosphatases to control inflammation and the immune response [66]. It suppresses lipoxygenase and cyclooxygenase enzymes, which suppresses the release of pro-inflammatory mediators including leukotrienes and prostaglandins [73]. Quercetin also inhibits TNF-α, a cytokine that plays a vital role in leukocyte formation, proliferation, and differentiation, specifically in the liver and gastrointestinal tract [62][72][74]. This causes a reduction in gluconeogenesis, glucose reabsorption, and α-glucosidase activity (Figure 3) [72]. Pancreatic β-cell apoptosis may occur due to hyperglycaemia-induced oxidative stress, and this can lead to diabetes mellitus. Glutathione peroxidase 4 (GPX4), an enzyme that protects cells against lipid peroxidation, suppresses the ferroptosis or apoptosis of pancreatic β-cells [75]. It has been demonstrated that quercetin can increase GPX4 activity in the pancreas, reducing oxidative stress, increased β-cell production, and insulin secretion [76]. Quercetin has also been reported to reduce the intestinal absorption of cholesterol by reducing the expression of the epithelial cholesterol transporter Niemann-Pick C1-Like 1 (NPC1L1) [77] and it has been suggested that the consumption of quercetin in the diet could lower systolic, diastolic, and mean arterial pressure in hypertensive individuals [78]. Quercetin can also lower blood pressure by reducing oxidative stress, enhancing the renin-angiotensin-aldosterone system (RAAS), and increasing vascular activity [70].
This entry is adapted from the peer-reviewed paper 10.3390/life12081146
References
- Kharroubi, A.T.; Darwish, H.M. Diabetes Mellitus: The Epidemic of the Century. World J. Diabetes 2015, 6, 850.
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013, 37, S81–S90.
- Lamb, M.M.; Yin, X.; Zerbe, G.O.; Klingensmith, G.J.; Dabelea, D.; Fingerlin, T.E.; Rewers, M.; Norris, J.M. Height Growth Velocity, Islet Autoimmunity and Type 1 Diabetes Development: The Diabetes Autoimmunity Study in the Young. Diabetologia 2009, 52, 2064–2071.
- Robertson, R.P.; Harmon, J.; Tran, P.O.; Tanaka, Y.; Takahashi, H. Glucose Toxicity in β-Cells: Type 2 Diabetes, Good Radicals Gone Bad, and the Glutathione Connection. Diabetes 2003, 52, 581–587.
- Akhtar, S.; Nasir, J.A.; Sarwar, A.; Nasr, N.; Javed, A.; Majeed, R.; Salam, M.A.; Billah, B. Prevalence of Diabetes and Pre-Diabetes in Bangladesh: A Systematic Review and Meta-Analysis. BMJ Open 2020, 10, e036086.
- Craig, M.E.; Hattersley, A.; Donaghue, K.C. Definition, Epidemiology and Classification of Diabetes in Children and Adolescents. Pediatr. Diabetes 2009, 10, 3–12.
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 Diabetes Mellitus. Nat. Rev. Dis. Primers 2017, 3, 17016.
- Kilic, G.; Alvarez-Mercado, A.I.; Zarrouki, B.; Opland, D.; Liew, C.W.; Alonso, L.C.; Myers, M.G.; Jonas, J.-C.; Poitout, V.; Kulkarni, R.N.; et al. The Islet Estrogen Receptor-α Is Induced by Hyperglycaemia and Protects against Oxidative Stress-Induced Insulin-Deficient Diabetes. PLoS ONE 2014, 9, e87941.
- Devendra, D.; Liu, E.; Eisenbarth, G.S. Type 1 Diabetes: Recent Developments. BMJ 2004, 328, 750–754.
- Dabelea, D.; Mayer-Davis, E.J.; Saydah, S.; Imperatore, G.; Linder, B.; Divers, J.; Bell, R.; Badaru, A.; Talton, J.W.; Crume, T.; et al. Prevalence of Type 1 and Type 2 Diabetes among Children and Adolescents from 2001 to 2009. JAMA 2014, 311, 1778–1786.
- Chiang, J.L.; Kirkman, M.S.; Laffel, L.M.B.; Peters, A.L. Type 1 Diabetes through the Life Span: A Position Statement of the American Diabetes Association. Diabetes Care 2014, 37, 2034–2054.
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Insulin Secretory and Antidiabetic Actions of Heritiera Fomes Bark Together with Isolation of Active Phytomolecules. PLoS ONE 2022, 17, e0264632.
- Halban, P.A.; Polonsky, K.S.; Bowden, D.W.; Hawkins, M.A.; Ling, C.; Mather, K.J.; Powers, A.C.; Rhodes, C.J.; Sussel, L.; Weir, G.C. β-Cell Failure in Type 2 Diabetes: Postulated Mechanisms and Prospects for Prevention and Treatment. J. Clin. Endocrinol. Metab. 2014, 99, 1983–1992.
- Usman, B.; Sharma, N.; Satija, S.; Mehta, M.; Vyas, M.; Khatik, G.L.; Khurana, N.; Hansbro, P.M.; Williams, K.; Dua, K. Recent Developments in Alpha-Glucosidase Inhibitors for Management of Type-2 Diabetes: An Update. Curr. Pharm. Des. 2019, 25, 2510–2525.
- Kumar, S.; Kumar, V.; Prakash, O. Enzymes Inhibition and Antidiabetic Effect of Isolated Constituents from Dillenia Indica. Biomed Res. Int. 2013, 2013, e382063.
- Wang, G.S.; Hoyte, C. Review of Biguanide (Metformin) Toxicity. J. Intensive Care Med. 2018, 34, 863–876.
- Rena, G.; Hardie, D.G.; Pearson, E.R. The Mechanisms of Action of Metformin. Diabetologia 2017, 60, 1577–1585.
- Lamos, E.M.; Levitt, D.L.; Munir, K.M. A Review of Dopamine Agonist Therapy in Type 2 Diabetes and Effects on Cardio-Metabolic Parameters. Prim. Care Diabetes 2016, 10, 60–65.
- Andersen, I.B.; Andreassen, M.; Krogh, J. The Effect of Dopamine Agonists on Metabolic Variables in Adults with Type 2 Diabetes: A Systematic Review with Meta-Analysis and Trial Sequential Analysis of Randomized Clinical Trials. Diabetes Obes. Metab. 2020, 23, 58–67.
- Pathak, R.; Bridgeman, M.B. Dipeptidyl Peptidase-4 (DPP-4) Inhibitors in the Management of Diabetes. Pharmacol. Ther. 2010, 35, 509–513.
- Capuano, A.; Sportiello, L.; Maiorino, M.I.; Rossi, F.; Giugliano, D.; Esposito, K. Dipeptidyl Peptidase-4 Inhibitors in Type 2 Diabetes therapy–Focus on Alogliptin. Drug Des. Devel. Ther. 2013, 7, 989–1001.
- Gastaldelli, A.; Marchesini, G. Time for Glucagon like Peptide-1 Receptor Agonists Treatment for Patients with NAFLD? J. Hepatol. 2016, 64, 262–264.
- Unger, J.R.; Parkin, C.G. Glucagon-like Peptide-1 (GLP-1) Receptor Agonists: Differentiating the New Medications. Diabetes Ther. 2011, 2, 29–39.
- Black, C.; Donnelly, P.; McIntyre, L.; Royle, P.; Shepherd, J.J.; Thomas, S. Meglitinide Analogues for Type 2 Diabetes Mellitus. Cochrane Database Syst. Rev. 2007, 2010, CD004654.
- Ekanayake, P.; Hupfeld, C.; Mudaliar, S. Sodium-Glucose Cotransporter Type 2 (SGLT-2) Inhibitors and Ketogenesis: The Good and the Bad. Curr. Diab. Rep. 2020, 20, 74.
- Scholtes, R.A.; Baar, M.J.B.; Lytvyn, Y.; Bjornstad, P.; Nieuwdorp, M.; Cherney, D.Z.I.; Raalte, D.H. Sodium Glucose Cotransporter (SGLT)-2 Inhibitors: Do We Need Them for Glucose-Lowering, for Cardiorenal Protection or Both? Diabetes Obes. Metab. 2019, 21, 24–33.
- Sola, D.; Rossi, L.; Schianca, G.P.C.; Maffioli, P.; Bigliocca, M.; Mella, R.; Corlianò, F.; Fra, G.P.; Bartoli, E.; Derosa, G. State of the Art Paper Sulfonylureas and Their Use in Clinical Practice. Arch. Med. Sci. 2015, 4, 840–848.
- Kalra, S.; Aamir, A.H.; Raza, A.; Das, A.K.; Khan, A.A.; Shrestha, D.; Qureshi, M.F.; Fariduddin, M.; Pathan, M.F.; Jawad, F.; et al. Place of Sulfonylureas in the Management of Type 2 Diabetes Mellitus in South Asia: A Consensus Statement. Indian J. Endocrinol. Metab. 2015, 19, 577.
- Rizos, C.V.; Kei, A.; Elisaf, M.S. The Current Role of Thiazolidinediones in Diabetes Management. Arch. Toxicol. 2016, 90, 1861–1881.
- Shebeko, S.K.; Zupanets, I.A.; Popov, O.S.; Tarasenko, O.O.; Shalamay, A.S. Effects of Quercetin and Its Combinations on Health. In Polyphenols: Mechanisms of Action in Human Health and Disease; Academic Press: Cambridge, MA, USA, 2018; pp. 373–394.
- Williams, C.A.; Grayer, R.J. Anthocyanins and Other Flavonoids. Nat. Prod. Rep. 2004, 21, 539.
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and Prooxidant Behavior of Flavonoids: Structure-Activity Relationships. Free Radic. Biol. Med. 1997, 22, 749–760.
- Metodiewa, D.; Jaiswal, A.K.; Cenas, N.; Dickancaité, E.; Segura-Aguilar, J. Quercetin May Act as a Cytotoxic Prooxidant after Its Metabolic Activation to Semiquinone and Quinoidal Product. Free Radic. Biol. Med. 1999, 26, 107–116.
- Parasuraman, S.; Anand David, A.; Arulmoli, R. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84.
- Oboh, G.; Ademosun, A.; Ayeni, P.O.; Omojokun, O.S.; Bello, F.O. Comparative Effect of Quercetin and Rutin on α-Amylase, α-Glucosidase, and Some Pro-Oxidant-Induced Lipid Peroxidation in Rat Pancreas. Comp. Clin. Path. 2014, 24, 1103–1110.
- Spínola, V.; Llorent-Martínez, E.J.; Castilho, P.C. Inhibition of α-Amylase, α-Glucosidase and Pancreatic Lipase by Phenolic Compounds of Rumex Maderensis (Madeira Sorrel). Influence of Simulated Gastrointestinal Digestion on Hyperglycaemia-Related Damage Linked with Aldose Reductase Activity and Protein Glycation. LWT 2020, 118, 108727.
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-Amylase and α-Glucosidase: Potential Linkage for Whole Cereal Foods on Prevention of Hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337.
- Yang, D.K.; Kang, H.-S. Anti-Diabetic Effect of Cotreatment with Quercetin and Resveratrol in Streptozotocin-Induced Diabetic Rats. Biomol. Ther. 2018, 26, 130–138.
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes. Mediators Inflamm. 2016, 2016, 9340637.
- Adewole, S.; Caxton-Martins, E.; Ojewole, J. Protective Effect of Quercetin on the Morphology of Pancreatic β-Cells of Streptozotocin-Treated Diabetic Rats. Afr. J. Tradit. Complement Altern. Med. 2007, 4, 64–74.
- Dhanya, R.; Arya, A.D.; Nisha, P.; Jayamurthy, P. Quercetin, a Lead Compound against Type 2 Diabetes Ameliorates Glucose Uptake via AMPK Pathway in Skeletal Muscle Cell Line. Front. Pharmacol. 2017, 8, 336.
- Chen, P.; Chen, J.; Zheng, Q.; Chen, W.; Wang, Y.; Xu, X. Pioglitazone, Extract of Compound Danshen Dripping Pill, and Quercetin Ameliorate Diabetic Nephropathy in Diabetic Rats. J. Endocrinol. Investig. 2013, 36, 422–427.
- Thomas, A.A.; Feng, B.; Chakrabarti, S. ANRIL: A Regulator of VEGF in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 470.
- Vessal, M.; Hemmati, M.; Vasei, M. Antidiabetic Effects of Quercetin in Streptozocin-Induced Diabetic Rats. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 135, 357–364.
- Shi, G.-J.; Li, Y.; Cao, Q.-H.; Wu, H.-X.; Tang, X.-Y.; Gao, X.-H.; Yu, J.-Q.; Chen, Z.; Yang, Y. In Vitro and in Vivo Evidence That Quercetin Protects against Diabetes and Its Complications: A Systematic Review of the Literature. Biomed. Pharmacother. 2019, 109, 1085–1099.
- Zhou, M.; Wang, S.; Zhao, A.; Wang, K.; Fan, Z.; Yang, H.; Liao, W.; Bao, S.; Zhao, L.; Zhang, Y.; et al. Transcriptomic and Metabonomic Profiling Reveal Synergistic Effects of Quercetin and Resveratrol Supplementation in High Fat Diet Fed Mice. J. Proteome Res. 2012, 11, 4961–4971.
- Saisho, Y.; Kou, K.; Tanaka, K.; Abe, T.; Kurosawa, H.; Shimada, A.; Meguro, S.; Kawai, T.; Itoh, H. Postprandial Serum C-Peptide to Plasma Glucose Ratio as a Predictor of Subsequent Insulin Treatment in Patients with Type 2 Diabetes. Endocr. J. 2011, 58, 315–322.
- Kulkarni, C.R.; Joglekar, M.M.; Patil, S.B.; Arvindekar, A.U. Antihyperglycemic and Antihyperlipidemic Effect OfSantalum Albumin Streptozotocin Induced Diabetic Rats. Pharm. Biol. 2011, 50, 360–365.
- Yim, S.; Malhotra, A.; Veves, A. Antioxidants and CVD in Diabetes: Where Do We Stand Now? Curr. Diab. Rep. 2007, 7, 8–13.
- Spencer, J.P.E.; Vauzour, D.; Rendeiro, C. Flavonoids and Cognition: The Molecular Mechanisms Underlying Their Behavioural Effects. Arch. Biochem. Biophys. 2009, 492, 1–9.
- Sikder, K.; Das, N.; Kesh, S.B.; Dey, S. Quercetin and Beta-Sitosterol Prevent High Fat Diet Induced Dyslipidemia and Hepatotoxicity in Swiss Albino Mice. Indian J. Exp. Biol. 2014, 52, 60–66.
- Mazloom, Z.; Abdollahzadeh, S.M.; Dabbaghmanesh, M.H.; Rezaianzadeh, A. The Effect of Quercetin Supplementation on Oxidative Stress, Glycemic Control, Lipid Profile, and Insulin Resistance in Type 2 Diabetes: A Randomized Clinical Trial. J. Health Sci. Surveill. Sys. 2014, 2, 8–14.
- Youl, E.; Bardy, G.; Magous, R.; Cros, G.; Sejalon, F.; Virsolvy, A.; Richard, S.; Quignard, J.; Gross, R.; Petit, P.; et al. Quercetin Potentiates Insulin Secretion and Protects INS-1 Pancreatic β-Cells against Oxidative Damage via the ERK1/2 Pathway. Br. J. Pharmacol. 2010, 161, 799–814.
- Fontana Pereira, D.; Cazarolli, L.H.; Lavado, C.; Mengatto, V.; Figueiredo, M.S.R.B.; Guedes, A.; Pizzolatti, M.G.; Silva, F.R.M.B. Effects of Flavonoids on α-Glucosidase Activity: Potential Targets for Glucose Homeostasis. Nutrition 2011, 27, 1161–1167.
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; et al. Quercetin and Cancer: New Insights into Its Therapeutic Effects on Ovarian Cancer Cells. Cell Biosci. 2020, 10, 32.
- Borghi, S.M.; Mizokami, S.S.; Pinho-Ribeiro, F.A.; Fattori, V.; Crespigio, J.; Clemente-Napimoga, J.T.; Napimoga, M.H.; Pitol, D.L.; Issa, J.P.M.; Fukada, S.Y.; et al. The Flavonoid Quercetin Inhibits Titanium Dioxide (TiO2)-Induced Chronic Arthritis in Mice. J. Nutr. Biochem. 2018, 53, 81–95.
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic Effect of Quercetin as an Antibiotic Alternative in Vivo and Its Antibacterial Mechanism in Vitro. J. Food Prot. 2018, 81, 68–78.
- Alam, F.; Islam, A.; Khalil, I.; Hua Gan, S. Metabolic Control of Type 2 Diabetes by Targeting the GLUT4 Glucose Transporter: Intervention Approaches. Curr. Pharm. Des. 2016, 22, 3034–3049.
- Dhanya, R.; Arun, K.B.; Syama, H.P.; Nisha, P.; Sundaresan, A.; Santhosh Kumar, T.R.; Jayamurthy, P. Rutin and Quercetin Enhance Glucose Uptake in L6 Myotubes under Oxidative Stress Induced by Tertiary Butyl Hydrogen Peroxide. Food Chem. 2014, 158, 546–554.
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and Skeletal Muscle Glucose Uptake. Physiol. Rev. 2013, 93, 993–1017.
- Ashraf, J.M.; Shahab, U.; Tabrez, S.; Lee, E.J.; Choi, I.; Ahmad, S. Quercetin as a Finer Substitute to Aminoguanidine in the Inhibition of Glycation Products. Int. J. Biol. Macromol. 2015, 77, 188–192.
- Dhanya, R.; Kartha, C.C. Quercetin Improves Oxidative Stress-Induced Pancreatic Beta Cell Alterations via MTOR-Signaling. Mol. Cell. Biochem. 2021, 476, 3879–3887.
- Saxton, R.A.; Sabatini, D.M. MTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 169, 361–371.
- Li, X.; Zheng, T.; Sang, S.; Lv, L. Quercetin Inhibits Advanced Glycation End Product Formation by Trapping Methylglyoxal and Glyoxal. J. Agric. Food Chem. 2014, 62, 12152–12158.
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin Reduces Blood Pressure in Hypertensive Subjects. J. Nutr. 2007, 137, 2405–2411.
- Kalupahana, N.S.; Claycombe, K.J.; Moustaid-Moussa, N. (N-3) Fatty Acids Alleviate Adipose Tissue Inflammation and Insulin Resistance: Mechanistic Insights. Adv. Nutr. 2011, 2, 304–316.
- Ferreira, P.E.B. Diabetic Neuropathy: An Evaluation of the Use of Quercetin in the Cecum of Rats. World J. Gastroenterol. 2013, 19, 6416.
- Shoelson, S.E. Inflammation and Insulin Resistance. J. Clin. Investig. 2006, 116, 1793–1801.
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. Rev. 2019, 14, 50.
- Tziomalos, K.; Athyros, V.G. Diabetic Nephropathy: New Risk Factors and Improvements in Diagnosis. Rev. Diabet. Stud. 2015, 12, 110–118.
- Cermak, R.; Landgraf, S.; Wolffram, S. Quercetin Glucosides Inhibit Glucose Uptake into Brush-Border-Membrane Vesicles of Porcine Jejunum. Br. J. Nutr. 2004, 91, 849–855.
- Cao, Z.; Zeng, Z.; Wang, B.; Liu, C.; Liu, C.; Wang, Z.; Li, S. Identification of Potential Bioactive Compounds and Mechanisms of GegenQinlian Decoction on Improving Insulin Resistance in Adipose, Liver, and Muscle Tissue by Integrating System Pharmacology and Bioinformatics Analysis. J. Ethnopharmacol. 2021, 264, 113289.
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.; Kruhlak, M.; Levine, M. Inhibition of the Intestinal Glucose Transporter GLUT2 by Flavonoids. FASEB J. 2006, 21, 366–377.
- D’Andrea, G. Quercetin: A Flavonol with Multifaceted Therapeutic Applications? Fitoterapia 2015, 106, 256–271.
- Sha, W.; Hu, F.; Xi, Y.; Chu, Y.; Bu, S. Mechanism of Ferroptosis and Its Role in Type 2 Diabetes Mellitus. J. Diabetes Res. 2021, 2021, 9999612.
- Li, D.; Jiang, C.; Mei, G.; Zhao, Y.; Chen, L.; Liu, J.; Tang, Y.; Gao, C.; Yao, P. Quercetin Alleviates Ferroptosis of Pancreatic β Cells in Type 2 Diabetes. Nutrients 2020, 12, 2954.
- Huseini, H.F.; Hasani-Rnjbar, S.; Nayebi, N.; Heshmat, R.; Sigaroodi, F.K.; Ahvazi, M.; Alaei, B.A.; Kianbakht, S. Capparis spinosa L. (Caper) Fruit Extract in Treatment of Type 2 Diabetic Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Complement. Ther. Med. 2013, 21, 447–452.
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, Inflammation, and Insulin Resistance. Gastroenterology 2007, 132, 2169–2180.
