Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Biomaterials
Hydrogels are polymeric materials with a characteristic hydrophilic structure that enables the storage of large amounts of water and biological fluids in their 3D network. For hydrogel synthesis, the incorporation of a cross-linking agent is important to achieve a better structuring due to its ability to form new polymeric chains within the structure through a large variety of reactions between different polymeric molecules or fibrous proteins
- hydrogel
- synthetic polymers
- biopolymers
- cross-linking
1. Synthesis of Hydrogels
A hydrogel is generally obtained by synthesis through the hydrolysis and condensation of the chosen precursors, causing the creation of a solid nanostructured network [199]. In this sense, for the synthesis of the hydrogel, most of the studies focus primarily on physical and chemical cross-linking methods (Figure 3), and the current challenges by means of synthesis procedures are oriented to obtain structures with higher cross-linking density for specific applications such as limbal stem cells [200] or hard-tissue engineering [201] and the use of green alternative procedures, solvents and cross-linking agents to assess efficient hydrogel synthesis without impairing biological properties such as biocompatibility and cytotoxicity [202,203,204].
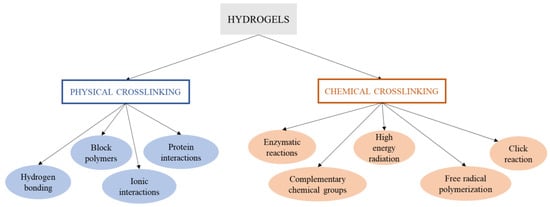
Figure 3. Schematic overview of the different physical and chemical cross-linking methods of synthesis of hydrogels.
2. Physical Cross-Linked Hydrogels
When the liquid phase changes to a gel due to an environmental change (pH, temperature, mixing of two components or ionic concentration) the hydrogels formed are known as physical hydrogels [205]. Their main interest lies in the absence of cross-linking agents in the synthesis. Figure 4 shows schematically the formation of physically cross-linked networks, specifying the interaction that constitutes the mentioned structures for each of the four types of cross-links that generate physical hydrogels, as depicted in Figure 3 [206].
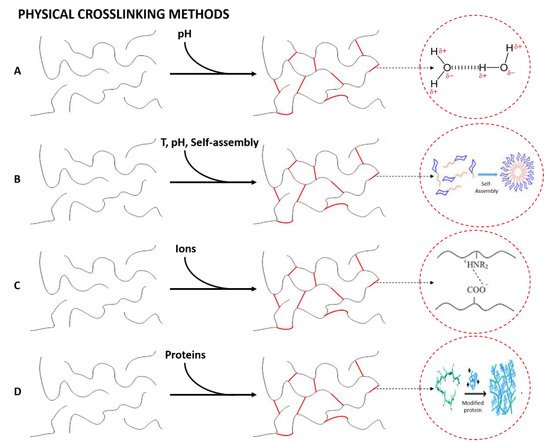
Figure 4. Formation of physically cross-linked hydrogels by (A) hydrogen bonding, (B) amphiphilic grafts and block polymers, (C) ionic interactions and (D) protein interactions.
Hydrogen bonding. This cross-linking method is based on the formation of hydrogen bonds between the polymeric chains to form a nanostructured network [207]. However, Jing et al. (2022) showed that this type of bond has a strong dependence on the pH of the gel. In their study, they obtained pH-responsive alginate/chitosan hydrogels whose properties may be tuned depending on the pH of the solution [208].
Amphiphilic grafts and block polymers. This group is formed by molecules with the ability to self-assemble in aqueous solutions to form hydrogels and polymeric micelles, in which the hydrophobic part of the polymer is concentrated. Interestingly, block polymer-based hydrogels can also be formed via crystallization, as is shown by Castillo and Müller (2009) in their study about the use of crystallization to produce a block copolymer material with good hydrophilicity and suitable mechanical and physical properties to be used as potential biomaterials [209].
Ionic interactions. Hydrogel formation is favored by the presence of ions to form the internal network [210]. This method is normally carried out at room temperature and physiological pH. The resulting hydrogels are nontoxic, do not cause skin irritation, are easily extensible and have adequate adhesion strength to be applied as a polymeric film on the skin [211].
Protein interactions. This cross-linking method is based on the use of genetically modified proteins or through antigen−antibody interactions. The former is produced by modifying the peptide sequence, enabling the control of the hydrogels’ physicochemical properties. On the other hand, the addition of a free antigen as a cross-linking agent generates a slight swelling of the hydrogel due to the replacement of the antigen bound to the polymer, generating the release of antibodies together with a decrease in the cross-linking density [212].
3. Chemical Cross-Linked Hydrogels
Chemical cross-linking is an irreversible process, where covalent bonds can be induced by the methods described below [15]. This type of hydrogel is of special interest thanks to their good mechanical resistance after cross-linking. For these hydrogels, there are mainly five ways to promote chemical cross-linking (Figure 3), whose specific interactions and conditions for the formation of the cross-linked structures are represented in Figure 5 [206].
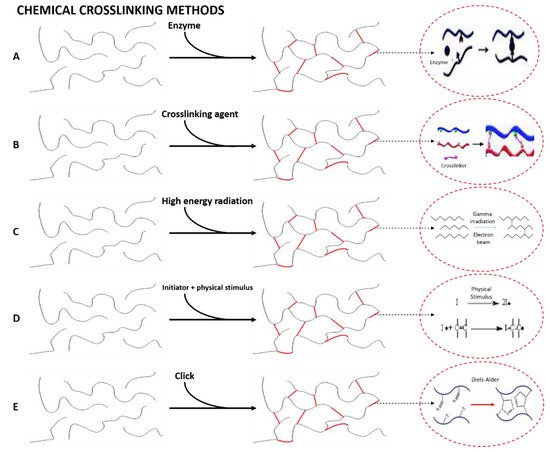
Figure 5. Formation of chemically cross-linked hydrogels by (A) enzymatic reactions, (B) chemical reaction promoted by a cross-linking agent, (C) high-energy radiation, (D) free-radical polymerization and (E) click reactions.
Enzymatic reactions. Enzymatic cross-linking requires the use of enzymes as reactants in order to reduce the potential toxicity of the chemical reagents traditionally used [213]. For this reason, there is a controversy, since some authors have considered enzymatic cross-linking as a third cross-linking method, whereas other researchers include it as a specific type of chemical cross-linking. This type of cross-linking is generally carried out with biopolymers. Therefore, the formation of hydrogels can be favored by the presence of enzymes that act as additives to generate new bonds between the polymer chains [214]. In this line, transglutaminase is the most used enzyme to carry out this type of cross-linking [215].
Reaction of complementary chemical groups. These hydrogels are formed by the use of agents that generate secondary reactions to promote cross-linking of the hydrogel. This group includes aldehydes and those additives that promote condensation reactions [216].
High energy radiation. This cross-linking method is promoted by the use of gamma radiation or an electron beam. Recent studies have combined this cross-linking method with enzymes to produce a chemical gelling of hydrogels obtained with macromolecules [217].
Free-radical polymerization. The manufacture of hydrogels following this route requires the use of synthetic, semi-synthetic or natural hydrophilic polymers [218]. It is also necessary to use enzymes as catalysts to favor the reaction [219] or, most commonly, free radical initiators, which are compounds that can generate free radicals by different stimuli. Temperature, UV irradiation, oxidation, microwave irradiation and gamma radiation have been used to induce radical generation [220].
Click reactions. This term describes a type of rapid, spontaneous, versatile and extremely selective reactions that do not lead to the formation of secondary products and result in high yields of heteroatom-linked molecular systems with high efficiency in a wide variety of mild reaction conditions [221]. The “click chemistry” approach allows a wide variety of synthetic strategies to accomplish the cross-linking and chemical functionalization of hydrogels with tailored properties. Several well-known reactions, which can be classified into three groups, comply with the “click chemistry” approach for hydrogel manufacture. These three groups include: (1) copper-free click reactions, such as Diels–Alder (DA), strain-promoted azide-alkyne cycloaddition (SPAAC), oxime-forming reactions and radical mediated thiol-ene; (2) copper-catalyzed azide-alkyne cycloaddition (Cu-AAC), and (3) pseudo click reactions, which include aldehyde-hydrazide reactions (Schiff-base reactions) and thiol-Michael addition [176].
In this section, different synthesis procedures have been discussed for hydrogel manufacturing, leading to mainly two types of cross-linked structures, namely physical and chemical cross-linking. The main difference between these two cross-linking approaches is the formation or not of covalent bonds, respectively, in the resulting structure [222]. Thus, physically cross-linked hydrogels have some advantages, such as remarkable versatility and the absence of chemical compounds to attain the desired cross-linked structure, which commonly harms fundamental properties such as biocompatibility or causes toxicity problems once implanted within the body [205]. Nevertheless, as described previously, the main drawback of these methods is the reversible demeanor that they present and the strong dependence on environmental parameters such as pH or temperature, which leads to unstable properties. This is the main challenge for the physically cross-linked hydrogel, though they still represent a very interesting strategy to reinforce hydrogels’ structure [21].
On the other hand, chemical cross-linking methods stand out for the better stability and mechanical properties of the synthesized hydrogels, rather than physically cross-linked ones, due to the formation of covalent bonds. However, most of the compounds used to induce secondary reactions or to form free radicals, as well as common reagents for some click reactions are presumably toxic and negatively affect biological properties and induce cytotoxicity issues [223]. Therefore, the main challenge of these procedures would be to move towards the implementation of the abovementioned green alternative compounds to induce complementary group reactions or free radical initiators that do not harm the biological properties of the resulting systems [224,225]. In addition, further research to obtain specific enzymes would be another interesting approach to attain chemically cross-linked structures avoiding the use of toxic compounds [226,227].
The combination of cross-linking procedures and materials is apparently the best solution to obtain adequate hydrogel systems for biomedical applications [228]. Not only combining polymers, but also including other materials and further cross-linking stages could be beneficial in order to obtain suitable hydrogels for more specific and demanding biomedical applications. This statement will be discussed in the following section.
This entry is adapted from the peer-reviewed paper 10.3390/polym14153023
This entry is offline, you can click here to edit this entry!
