Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Kidney function highly depends on mitochondria, organelles that regulate different metabolic pathways. Mitochondria-altered function and structure are present during acute kidney injury (AKI) and chronic kidney disease (CKD).
- mitochondrial alterations
- kidney diseases
- mitochondria targeting therapy
1. Introduction
Kidneys are among the most energy-demanding organs due to their filtration and reabsorption functions. In particular, the proximal tubular segment of the nephron consumes large amounts of energy in the form of adenosine triphosphate (ATP) provided by oxidative phosphorylation, a metabolic process performed in the mitochondria [1].
In addition to their energy-producing function, mitochondria also exert other metabolic processes such as glutaminolysis, the catabolism of branched-chain amino acids, fatty acid beta-oxidation, nucleotide biosynthesis, heme metabolism, redox balance, the management of metabolic by-products, cellular death regulation, calcium homeostasis, etc. [2][3].
Acute kidney injury (AKI) is characterized by an abrupt reduction in kidney function due to pre-renal, renal, and post-renal causes such as the reduction of blood supply, nephrotoxins, and obstruction, respectively [4]. On the other hand, chronic kidney disease (CKD) is characterized by the progressive and irreversible loss of kidney function and structure for more than three months and may be a consequence of other conditions such as diabetes, hypertension, or aging [5].
AKI and CKD are related to each other since the presence of one could predispose the development of the other [6][7][8]. In addition to their complicated pathophysiology, several mitochondrial alterations have been reported in both pathologies, contributing to their progression.
In different experimental AKI models, mitochondrial morphological alterations are prevalent in tubular segments, showing fragmentation, swelling, and the loss of cristae; moreover, functionality is also compromised, with reduced electron transport chain (ETC) activity, a loss of membrane potential, and increased reactive oxygen species (ROS) production as a consequence [9][10][11][12][13][14][15]. Interestingly, these alterations also persist during AKI to CKD progression [16][17][18]. Similarly, in established CKD, mitochondrial alterations are present, showing low membrane potential and consequently reduced ETC activity and overproduction of ROS [19][20][21]; on the other hand, morphological alterations such as mitochondrial fragmentation have been noticed, especially in podocytes [21][22][23][24].
ROS overproduction is present in AKI and CKD, representing a therapeutic target since their abrogation reduces tissue damage and improves kidney function [25][26][27][28][29][30][31][32][33][34]. ROS are well-known inducers of the inflammatory response through the activation of the transcription factor nuclear factor kappa B (NF-kB) [35]; moreover, mitochondria-derived ROS are activators of the NLR family pyrin domain containing 3 (NLRP3) inflammasome/interleukin (IL)-1β axis [36][37], which has been reported to promote kidney injury [38]. The specific blocking of mitochondria-derived ROS also reduces kidney damage and improves kidney function [15][23].
Hence, specific mitochondrial targeting in order to block excessive ROS production and restore some mitochondrial functions could be a suitable complementary therapeutic strategy for kidney diseases.
Mitochondria targeting strategies include the use of small molecules, peptides, nanocarriers, and mitochondrial transplantation (Figure 1).
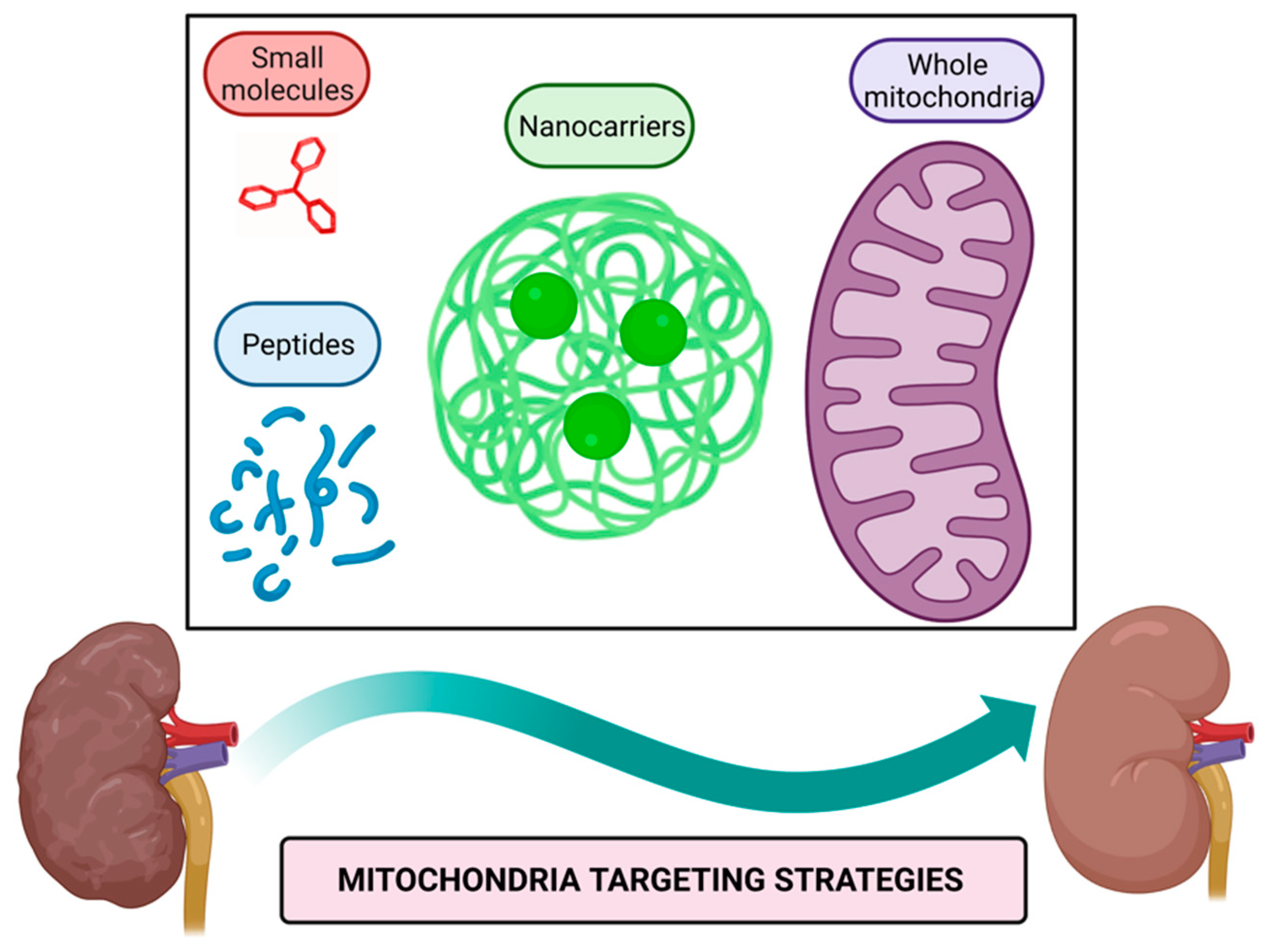
Figure 1. Mitochondria targeting strategies. Small molecules, peptides, nanocarriers, and whole mitochondria transplantation represent therapeutic strategies targeted to mitochondria to alleviate their dysfunction in kidney diseases. Figure created with BioRender.com.
Mitochondria targeting compounds include lipophilic cationic small molecules and peptides that can be used alone or conjugated with other bioactive molecules [39][40]; additionally, nanocarriers of drugs harboring signals that direct them to mitochondria or even whole mitochondria transferred to target tissue could be used to alleviate mitochondrial dysfunction [41][42].
2. Mitochondria Targeting Peptides
Peptides as therapeutics have emerged recently and show several advantages over other molecules, such as their chemical synthesis, selectivity, and minimal side effects. These could be used alone or conjugated with another bioactive compound [43][44].
Nowadays, the peptide peginesatide, an antagonist of the erythropoietin receptor, is used to treat CKD-associated anemia in humans [45]. Hence, other experimental approaches focused on mitochondria have been explored; for example, using a peptide to block the interaction of nucleophosmin with Bcl-2-associated X protein (Bax) inhibits apoptotic cell death; thus, resulting in decreased renal damage caused by ischemia [46] and suggesting that mitochondria targeting peptides could also be a potential therapy for kidney diseases.
Therapeutic peptides are classified as cell-targeting peptides (CTP) if they are directed specifically to a receptor or as cell-penetrating peptides (CPP) if they pass the plasma membrane to reach the cytoplasm [43][44]. Mitochondria targeting peptides require CPP characteristics to enter cells, and to reach mitochondria requires CTP characteristics harboring a mitochondria targeting sequence (MTS) or possessing cationic charges.
2.1. MTS-Containing Peptides
Mitochondrial proteome mainly is constituted by nuclear-encoded proteins that once synthesized possess an MTS to reach mitochondria through the recognition by the mitochondrial TOM complex eliciting the integration to mitochondrial membranes. The conserved pattern residues on MTS are φχχφφ, where φ represents an aromatic or hydrophobic residue, whereas χ represents any kind of residue, for example, the pattern LSRLL; additionally, MTS acquires an alpha-helix conformation that facilitates the insertion to the mitochondrial outer membrane. Once inside, MTS is degraded by mitochondrial processing proteinases (MPP) [3][47][48]. Considering those mentioned above, synthetic MTS-containing peptides have been developed and used as carriers of other compounds to facilitate their delivery into mitochondria to exert biological functions. The construct of a CPP with an MTS improves cellular and mitochondrial uptake [49], as has been demonstrated in vitro with peptides conjugated with DNase, human metallothionein 1A (hMT1A), and manganese-porphyrin [50][51][52]. Moreover, the cell-penetrating artificial mitochondria targeting peptide (CAMP)-hMT1A conjugate has been tested in a Parkinson’s disease model in rats and demonstrated to restore tyrosine hydroxylase levels in striatum and substantia nigra resulting in improved motor coordination when it is administrated intracerebrally [51]; similarly, using a recombinant MTS-containing mitochondrial transcription factor A (TFAM) IV injected in mice also improves motor coordination, although it could be by the increase in complex I of the ETC [53]. MTS-containing TFAM has also been proven in a septic shock model, increasing animal survival and, in healthy mice, increasing the brain and muscle complex I level of the ETC [53][54].
Although for kidney diseases, there are no reports of the use of MTS-containing peptides, the conjugation of these with antioxidant molecules such as the mentioned metallothionein and manganese–porphyrin could have promising results, since both molecules have been reported to reduce renal damage in aristocholic acid-induced CKD and I/R-induced AKI, respectively [55][56]. Moreover, recombinant MTS-containing TFAM could also help maintain mitochondrial DNA and increase complex I levels in kidney tubular epithelial cells.
One advantage of MTS-containing peptides over other molecules that target mitochondria is that cationic charges are expendable to enter mitochondria; hence, their insertion mechanism is independent of mitochondrial membrane potential.
2.2. Cationic Mitochondrial Penetrating Peptides
Positive charges and alpha-helix structures are basal characteristics of these peptides, and they differ from each other due to other structural features.
CAPH peptides possess the ability to enter the cell through endocytosis and reach mitochondria due to enriched proline residues in their structures, such as P11LRR and P14LRR peptides [57][58]; moreover, the addition of a dimethyl tyrosine (Dmt) residue to P11LRR structure exert antioxidant functions demonstrated in vitro [57][58].
As mentioned above, oxidative stress is a hallmark of kidney diseases, in which mitochondria are the primary sources of ROS [59]. Ergo, the use CAPH-Dmt has excellent potential to explore in AKI and CKD models.
The plant derivate roseltide rT1 is a cationic cysteine-rich peptide recognized by the TOM complex in an MTS-independent way; interestingly, roseltide rT1 by itself can bind ATP synthase and enhance ATP production in different cell lines [60]. During AKI and CKD, ATP production is compromised, as demonstrated in experimental models [11][16][17][18][26][31][61][62], and for this reason, roseltide rT1 by itself without conjugation with another bioactive compound could be helpful in the treatment of kidney disease.
Hexapeptides with delocalized lipophilic cations contain the modified residue cyclohexyl alanine in their structure to bring hydrophobicity and facilitate cellular uptake; positive charge residues such as lysine and arginine also are incorporated. In addition to these characteristics, cationic moieties of pyridyl salts in alanine residues bring mitochondrial selectivity [63]. Although these peptides are not proven in any disease model, it seems to have great potential as a drug delivery system to mitochondria. As described above, non-peptidic cationic molecules are a comprehensive system to target mitochondria due to the charge affinity.
Peptoids resemble backbone peptide structures but are resistant to proteolysis due to structural modifications, in which side chains are attached to the nitrogen atom instead of the alpha carbon [64]. These peptoids also require the lipophilic, cationic, and alpha-helix structure characteristics to enter the cell and mitochondria [65]. Although peptoids conjugated with any kind of drugs are not assessed in any disease models in animals, they represent a vast field to explore in kidney diseases, in which the conjugation with molecules that require more stability, such as transcription factors, bioactive lipids, or proteins involved in mitochondrial dynamics.
SS peptides are aromatic and cationic tetrapeptides able to enter mitochondria and, if they possess a tyrosine or Dmt residue in their structure, also function as antioxidants themselves. SS-01 and SS-20 that lack tyrosine or Dmt residues can enter mitochondria but lack antioxidant activity, whereas SS-02 and SS-31, which possess any of those two residues, enter mitochondria and are potent antioxidants.
Among SS peptides, SS-31 (also known as MTP-131, Bendavia, and elamipretide) has gained great attention for the potent antioxidant activity and safety demonstrated in experimental models; in fact, SS-31 has been proved in clinical trials for human mitochondrial myopathies, Barth syndrome, cardiovascular diseases, and renal arterial stenosis [66][67][68][69][70].
In AKI, SS-31 IP administration reduces structural and functional damage induced by cisplatin in mice; moreover, it decreases oxidative damage and NLPR3-derived IL-1β synthesis [71]. Similarly, in I/R-induced AKI in rats, SS-31 subcutaneous administration reaches a high concentration in kidneys and reduces epithelial and endothelial damage; at the subcellular level, it avoids mitochondrial swelling, maintains cristae structure by its binding with cardiolipin, and recover ATP levels [72][73][74]. SS-31 peptide has been modified with CPP characteristics or encapsulated in nanopolyplexes to increase its cellular uptake and mitochondrial accumulation, thus resulting in enhanced antioxidant capacity demonstrated in vitro [75][76]. Moreover, the efficiency of SS-31 encapsulated in nanopolyplexes has been demonstrated in lipopolysaccharide (LPS)-induced AKI model in mice, showing better results than SS-31 alone [76]. Although cisplatin, I/R, and LPS-induced AKI SS-31 have demonstrated promising results, for other models such as aristocholic acid (AA) and adriamycin-induced AKI, there are controversial results [77] that could be explained by the specific physiopathology induced by these compounds or even by their chemical interaction with SS-31. It is known that AKI predisposes to CKD development; as has been reported in CKD development induced I/R, in which the treatment with SS-31 for six weeks and started four weeks after ischemic injury reduces the structural damage of kidneys, fibrotic damage, and mitochondrial swelling; surprisingly, this protective effect persists even nine months after I/R induction [78].
On the other hand, during diabetic nephropathy, the IP or subcutaneous administration of SS-31for at least four weeks of SS-31 does not affect glycemic levels; however, it improves kidney function, preserves podocyte structure, diminishes inflammatory and fibrotic markers, and reduces oxidative stress [79][80][81][82][83][84].
SS-02 and SS-20 peptides in conjugation with deferoxamine have also been demonstrated to possess mitochondrial antioxidant properties in vitro [85], suggesting their potential use.
A summary of proven and not proven mitochondria targeting peptides in kidney disease models is shown in Figure 2.
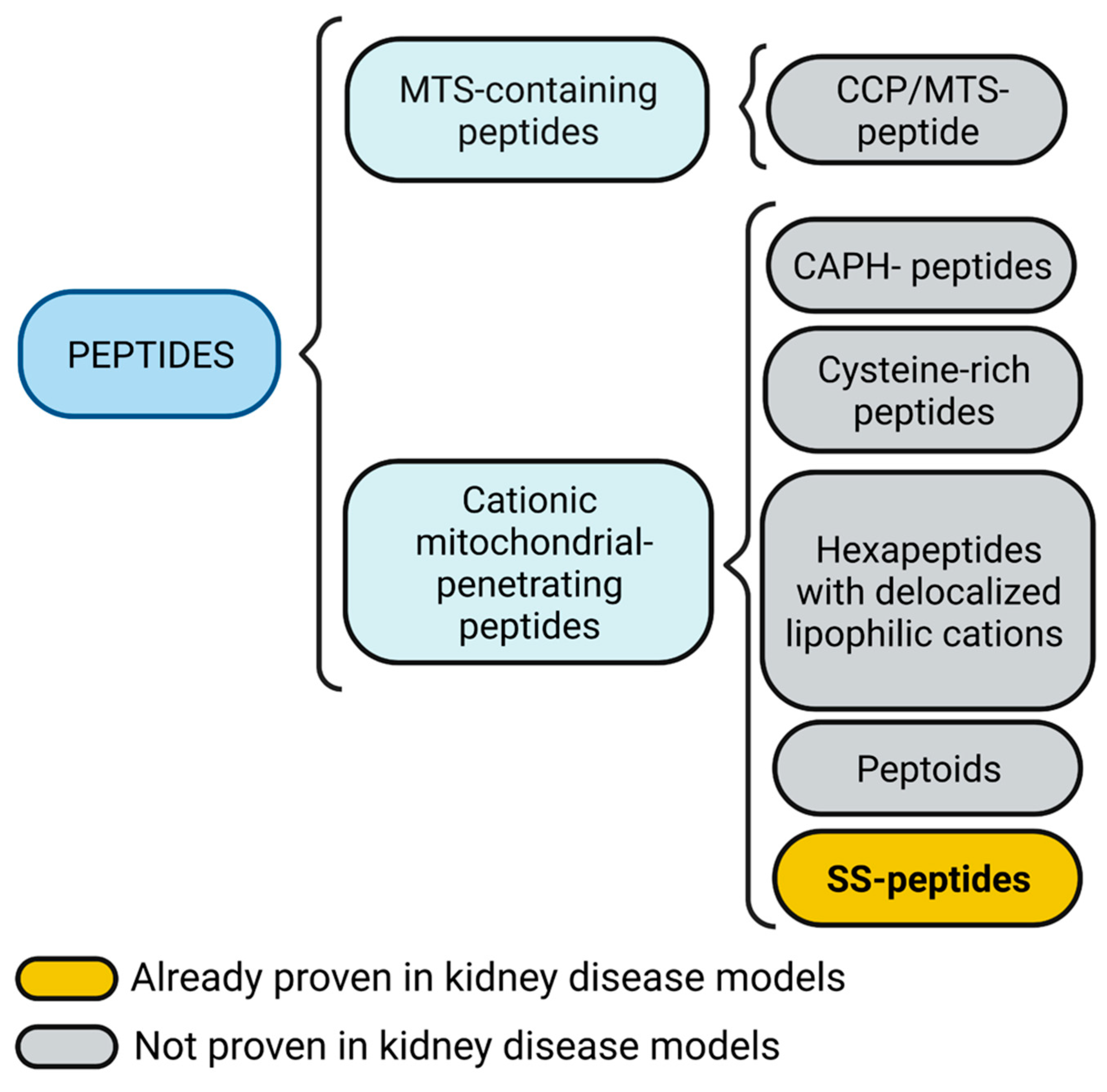
Figure 2. Mitochondria targeting peptides. Mitochondria targeting sequence (MTS)-containing peptides also could include a cell-penetrating peptide (CPP) feature. Cationic mitochondrial penetrating peptides could be subdivided into cationic amphiphilic polyproline helix (CAPH) peptides, cysteine-rich peptides, and hexapeptides with delocalized lipophilic cations, peptoids, and Szeto-Schiller (SS) peptides. Figure created with BioRender.com.
3. Mitochondrial Replacement
Mitochondrial replacement, also known as mitochondrial transplantation, is a novel experimental therapeutic strategy to transfer healthy mitochondria to the target tissue to recover mitochondrial function (Figure 3). This strategy has already been used in pediatric patients after cardiogenic shock, in which mitochondria isolated from their muscles are directly injected into the myocardium, demonstrating that patients with mitochondrial transplantation do not suffer short adverse effects and show fewer cardiovascular events several months after the intervention [86][87].
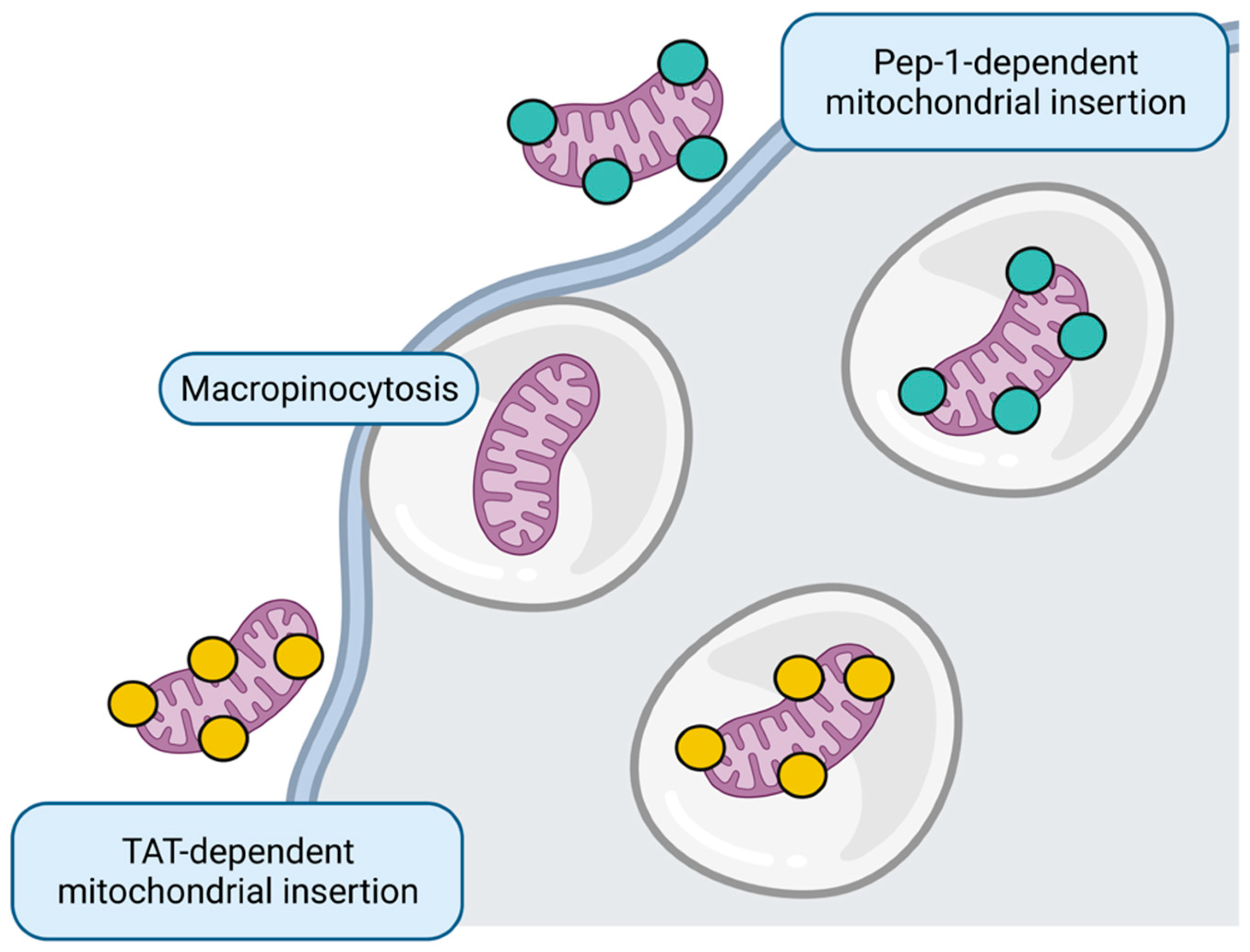
Figure 3. Mitochondrial replacement. Whole healthy mitochondria insertion to target cell could occur through micropinocytosis or directed through Pep-1 and transactivator of transcription (TAT) peptides. Figure created with BioRender.com.
Only AKI models have explored the effect of mitochondrial replacement. In the doxorubicin-induced AKI model, the transplantation of mesenchymal stem cell (MSC)-derived mitochondria to the renal subcapsular region results in improved kidney function and increased antioxidant enzyme levels; however, although tubular regeneration was increased, tubular dilation persists [88]. Similarly, in I/R-induced AKI in rats and pigs, the intra-arterial administration of muscle-derived mitochondria improves renal function in the first 24 to 48 h [89][90] and even promotes proliferation of renal cells [89] and reduces inflammation [90]. However, for CKD, mitochondrial replacement remains unexplored.
This entry is adapted from the peer-reviewed paper 10.3390/futurepharmacol2030017
References
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646.
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754.
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Calcium and mitochondria in the regulation of cell death. Biochem. Biophys. Res. Commun. 2015, 460, 72–81.
- Rahman, M.; Shad, F.; Smith, M.C. Acute kidney injury: A guide to diagnosis and management. Am. Fam. Physician 2012, 86, 631–639.
- Charles, C.; Ferris, A.H. Chronic Kidney Disease. Prim. Care Clin. Off. Pract. 2020, 47, 585–595.
- Sato, Y.; Takahashi, M.; Yanagita, M. Pathophysiology of AKI to CKD progression. Semin. Nephrol. 2020, 40, 206–215.
- Singh, P.; Rifkin, D.E.; Blantz, R.C. Chronic kidney disease: An inherent risk factor for acute kidney injury? Clin. J. Am. Soc. Nephrol. 2010, 5, 1690–1695.
- Fiorentino, M.; Grandaliano, G.; Gesualdo, L.; Castellano, G. Acute Kidney Injury to Chronic Kidney Disease Transition. Contrib. Nephrol. 2018, 193, 45–54.
- Li, M.; Li, C.M.; Ye, Z.C.; Huang, J.; Li, Y.; Lai, W.; Peng, H.; Lou, T.Q. Sirt3 modulates fatty acid oxidation and attenuates cisplatin-induced AKI in mice. J. Cell. Mol. Med. 2020, 24, 5109–5121.
- Liang, N.N.; Zhao, Y.; Guo, Y.Y.; Zhang, Z.H.; Gao, L.; Yu, D.X.; Xu, D.X.; Xu, S. Mitochondria-derived reactive oxygen species are involved in renal cell ferroptosis during lipopolysaccharide-induced acute kidney injury. Int. Immunopharmacol. 2022, 107, 108687.
- Aparicio-Trejo, O.E.; Reyes-Fermin, L.M.; Briones-Herrera, A.; Tapia, E.; Leon-Contreras, J.C.; Hernandez-Pando, R.; Sanchez-Lozada, L.G.; Pedraza-Chaverri, J. Protective effects of N-acetyl-cysteine in mitochondria bioenergetics, oxidative stress, dynamics and S-glutathionylation alterations in acute kidney damage induced by folic acid. Free Radic. Biol. Med. 2019, 130, 379–396.
- Funk, J.A.; Schnellmann, R.G. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol.-Ren. Physiol. 2012, 302, F853–F864.
- Adil, M.; Kandhare, A.D.; Dalvi, G.; Ghosh, P.; Venkata, S.; Raygude, K.S.; Bodhankar, S.L. Ameliorative effect of berberine against gentamicin-induced nephrotoxicity in rats via attenuation of oxidative stress, inflammation, apoptosis and mitochondrial dysfunction. Ren. Fail. 2016, 38, 996–1006.
- Hall, A.M.; Rhodes, G.J.; Sandoval, R.M.; Corridon, P.R.; Molitoris, B.A. In vivo multiphoton imaging of mitochondrial structure and function during acute kidney injury. Kidney Int. 2013, 83, 72–83.
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863.
- Jimenez-Uribe, A.P.; Bellido, B.; Aparicio-Trejo, O.E.; Tapia, E.; Sanchez-Lozada, L.G.; Hernandez-Santos, J.A.; Fernandez-Valverde, F.; Hernandez-Cruz, E.Y.; Orozco-Ibarra, M.; Pedraza-Chaverri, J. Temporal characterization of mitochondrial impairment in the unilateral ureteral obstruction model in rats. Free Radic. Biol. Med. 2021, 172, 358–371.
- Aparicio-Trejo, O.E.; Avila-Rojas, S.H.; Tapia, E.; Rojas-Morales, P.; Leon-Contreras, J.C.; Martinez-Klimova, E.; Hernandez-Pando, R.; Sanchez-Lozada, L.G.; Pedraza-Chaverri, J. Chronic impairment of mitochondrial bioenergetics and beta-oxidation promotes experimental AKI-to-CKD transition induced by folic acid. Free Radic. Biol. Med. 2020, 154, 18–32.
- Aparicio-Trejo, O.E.; Rojas-Morales, P.; Avila-Rojas, S.H.; Leon-Contreras, J.C.; Hernandez-Pando, R.; Jimenez-Uribe, A.P.; Prieto-Carrasco, R.; Sanchez-Lozada, L.G.; Pedraza-Chaverri, J.; Tapia, E. Temporal Alterations in Mitochondrial beta-Oxidation and Oxidative Stress Aggravate Chronic Kidney Disease Development in 5/6 Nephrectomy Induced Renal Damage. Int. J. Mol. Sci. 2020, 21, 6512.
- Thome, T.; Coleman, M.D.; Ryan, T.E. Mitochondrial Bioenergetic and Proteomic Phenotyping Reveals Organ-Specific Consequences of Chronic Kidney Disease in Mice. Cells 2021, 10, 3282.
- Liu, X.; Huang, S.; Wang, F.; Zheng, L.; Lu, J.; Chen, J.; Li, S. Huangqi-Danshen Decoction Ameliorates Adenine-Induced Chronic Kidney Disease by Modulating Mitochondrial Dynamics. Evid.-Based Complement. Altern. Med. 2019, 2019, 9574045.
- Qin, X.; Jiang, M.; Zhao, Y.; Gong, J.; Su, H.; Yuan, F.; Fang, K.; Yuan, X.; Yu, X.; Dong, H.; et al. Berberine protects against diabetic kidney disease via promoting PGC-1alpha-regulated mitochondrial energy homeostasis. Br. J. Pharmacol. 2020, 177, 3646–3661.
- Fan, Y.; Yang, Q.; Yang, Y.; Gao, Z.; Ma, Y.; Zhang, L.; Liang, W.; Ding, G. Sirt6 Suppresses High Glucose-Induced Mitochondrial Dysfunction and Apoptosis in Podocytes through AMPK Activation. Int. J. Biol. Sci. 2019, 15, 701–713.
- Zhu, Z.; Liang, W.; Chen, Z.; Hu, J.; Feng, J.; Cao, Y.; Ma, Y.; Ding, G. Mitoquinone Protects Podocytes from Angiotensin II-Induced Mitochondrial Dysfunction and Injury via the Keap1-Nrf2 Signaling Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 1394486.
- Ma, Y.; Chen, Z.; Tao, Y.; Zhu, J.; Yang, H.; Liang, W.; Ding, G. Increased mitochondrial fission of glomerular podocytes in diabetic nephropathy. Endocr. Connect. 2019, 8, 1206–1212.
- Ortega-Dominguez, B.; Aparicio-Trejo, O.E.; Garcia-Arroyo, F.E.; Leon-Contreras, J.C.; Tapia, E.; Molina-Jijon, E.; Hernandez-Pando, R.; Sanchez-Lozada, L.G.; Barrera-Oviedo, D.; Pedraza-Chaverri, J. Curcumin prevents cisplatin-induced renal alterations in mitochondrial bioenergetics and dynamic. Food Chem. Toxicol. 2017, 107, 373–385.
- Avila-Rojas, S.H.; Aparicio-Trejo, O.E.; Briones-Herrera, A.; Medina-Campos, O.N.; Reyes-Fermin, L.M.; Martinez-Klimova, E.; Leon-Contreras, J.C.; Hernandez-Pando, R.; Tapia, E.; Pedraza-Chaverri, J. Alterations in mitochondrial homeostasis in a potassium dichromate model of acute kidney injury and their mitigation by curcumin. Food Chem. Toxicol. 2020, 145, 111774.
- Molina-Jijon, E.; Tapia, E.; Zazueta, C.; El Hafidi, M.; Zatarain-Barron, Z.L.; Hernandez-Pando, R.; Medina-Campos, O.N.; Zarco-Marquez, G.; Torres, I.; Pedraza-Chaverri, J. Curcumin prevents Cr(VI)-induced renal oxidant damage by a mitochondrial pathway. Free Radic. Biol. Med. 2011, 51, 1543–1557.
- Molina-Jijon, E.; Aparicio-Trejo, O.E.; Rodriguez-Munoz, R.; Leon-Contreras, J.C.; Del Carmen Cardenas-Aguayo, M.; Medina-Campos, O.N.; Tapia, E.; Sanchez-Lozada, L.G.; Hernandez-Pando, R.; Reyes, J.L.; et al. The nephroprotection exerted by curcumin in maleate-induced renal damage is associated with decreased mitochondrial fission and autophagy. Biofactors 2016, 42, 686–702.
- Negrette-Guzman, M.; Garcia-Nino, W.R.; Tapia, E.; Zazueta, C.; Huerta-Yepez, S.; Leon-Contreras, J.C.; Hernandez-Pando, R.; Aparicio-Trejo, O.E.; Madero, M.; Pedraza-Chaverri, J. Curcumin Attenuates Gentamicin-Induced Kidney Mitochondrial Alterations: Possible Role of a Mitochondrial Biogenesis Mechanism. Evid.-Based Complement. Altern. Med. 2015, 2015, 917435.
- Tapia, E.; Sanchez-Lozada, L.G.; Garcia-Nino, W.R.; Garcia, E.; Cerecedo, A.; Garcia-Arroyo, F.E.; Osorio, H.; Arellano, A.; Cristobal-Garcia, M.; Loredo, M.L.; et al. Curcumin prevents maleate-induced nephrotoxicity: Relation to hemodynamic alterations, oxidative stress, mitochondrial oxygen consumption and activity of respiratory complex I. Free Radic. Res. 2014, 48, 1342–1354.
- Aparicio-Trejo, O.E.; Tapia, E.; Molina-Jijon, E.; Medina-Campos, O.N.; Macias-Ruvalcaba, N.A.; Leon-Contreras, J.C.; Hernandez-Pando, R.; Garcia-Arroyo, F.E.; Cristobal, M.; Sanchez-Lozada, L.G.; et al. Curcumin prevents mitochondrial dynamics disturbances in early 5/6 nephrectomy: Relation to oxidative stress and mitochondrial bioenergetics. Biofactors 2017, 43, 293–310.
- Wang, D.; Yang, Y.; Zou, X.; Zheng, Z.; Zhang, J. Curcumin ameliorates CKD-induced mitochondrial dysfunction and oxidative stress through inhibiting GSK-3beta activity. J. Nutr. Biochem. 2020, 83, 108404.
- Chen, S.; Liu, G.; Long, M.; Zou, H.; Cui, H. Alpha lipoic acid attenuates cadmium-induced nephrotoxicity via the mitochondrial apoptotic pathways in rat. J. Inorg. Biochem. 2018, 184, 19–26.
- Wang, L.; Wu, C.G.; Fang, C.Q.; Gao, J.; Liu, Y.Z.; Chen, Y.; Chen, Y.N.; Xu, Z.G. The protective effect of alpha-Lipoic acid on mitochondria in the kidney of diabetic rats. Int. J. Clin. Exp. Med. 2013, 6, 90–97.
- Lingappan, K. NF-kappaB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86.
- Hou, Y.; Wang, Q.; Han, B.; Chen, Y.; Qiao, X.; Wang, L. CD36 promotes NLRP3 inflammasome activation via the mtROS pathway in renal tubular epithelial cells of diabetic kidneys. Cell Death Dis. 2021, 12, 523.
- Han, Y.; Xu, X.; Tang, C.; Gao, P.; Chen, X.; Xiong, X.; Yang, M.; Yang, S.; Zhu, X.; Yuan, S.; et al. Reactive oxygen species promote tubular injury in diabetic nephropathy: The role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 2018, 16, 32–46.
- Zhang, Y.; Liu, Y.; Bi, X.; Hu, C.; Ding, W. NLRP3 Deletion Attenuated Angiotensin II-Induced Renal Fibrosis by Improving Mitochondrial Dysfunction and Endoplasmic Reticulum Stress. Nephron 2021, 145, 518–527.
- Battogtokh, G.; Cho, Y.Y.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Mitochondrial-Targeting Anticancer Agent Conjugates and Nanocarrier Systems for Cancer Treatment. Front. Pharm. 2018, 9, 922.
- Zinovkin, R.A.; Zamyatnin, A.A. Mitochondria-Targeted Drugs. Curr. Mol. Pharm. 2019, 12, 202–214.
- Pathak, R.K.; Kolishetti, N.; Dhar, S. Targeted nanoparticles in mitochondrial medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 315–329.
- Yamada, Y.; Ito, M.; Arai, M.; Hibino, M.; Tsujioka, T.; Harashima, H. Challenges in Promoting Mitochondrial Transplantation Therapy. Int. J. Mol. Sci. 2020, 21, 6365.
- Erak, M.; Bellmann-Sickert, K.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide chemistry toolbox—Transforming natural peptides into peptide therapeutics. Bioorg. Med. Chem. 2018, 26, 2759–2765.
- Ma, L.; Wang, C.; He, Z.; Cheng, B.; Zheng, L.; Huang, K. Peptide-Drug Conjugate: A Novel Drug Design Approach. Curr. Med. Chem. 2017, 24, 3373–3396.
- Fishbane, S.; Schiller, B.; Locatelli, F.; Covic, A.C.; Provenzano, R.; Wiecek, A.; Levin, N.W.; Kaplan, M.; Macdougall, I.C.; Francisco, C.; et al. Peginesatide in patients with anemia undergoing hemodialysis. N. Engl. J. Med. 2013, 368, 307–319.
- Wang, Z.; Gall, J.M.; Bonegio, R.; Havasi, A.; Illanes, K.; Schwartz, J.H.; Borkan, S.C. Nucleophosmin, a critical Bax cofactor in ischemia-induced cell death. Mol. Cell Biol. 2013, 33, 1916–1924.
- Saitoh, T.; Igura, M.; Obita, T.; Ose, T.; Kojima, R.; Maenaka, K.; Endo, T.; Kohda, D. Tom20 recognizes mitochondrial presequences through dynamic equilibrium among multiple bound states. EMBO J. 2007, 26, 4777–4787.
- Wiedemann, N.; Pfanner, N. Mitochondrial Machineries for Protein Import and Assembly. Annu. Rev. Biochem. 2017, 86, 685–714.
- Lin, R.; Zhang, P.; Cheetham, A.G.; Walston, J.; Abadir, P.; Cui, H. Dual peptide conjugation strategy for improved cellular uptake and mitochondria targeting. Bioconjug. Chem. 2015, 26, 71–77.
- Jain, A.; Chugh, A. Mitochondrial transit peptide exhibits cell penetration ability and efficiently delivers macromolecules to mitochondria. FEBS Lett. 2016, 590, 2896–2905.
- Kang, Y.C.; Son, M.; Kang, S.; Im, S.; Piao, Y.; Lim, K.S.; Song, M.Y.; Park, K.S.; Kim, Y.H.; Pak, Y.K. Cell-penetrating artificial mitochondria-targeting peptide-conjugated metallothionein 1A alleviates mitochondrial damage in Parkinson’s disease models. Exp. Mol. Med. 2018, 50, 1–13.
- Asayama, S.; Kawamura, E.; Nagaoka, S.; Kawakami, H. Design of manganese porphyrin modified with mitochondrial signal peptide for a new antioxidant. Mol. Pharm. 2006, 3, 468–470.
- Thomas, R.R.; Khan, S.M.; Portell, F.R.; Smigrodzki, R.M.; Bennett, J.P., Jr. Recombinant human mitochondrial transcription factor A stimulates mitochondrial biogenesis and ATP synthesis, improves motor function after MPTP, reduces oxidative stress and increases survival after endotoxin. Mitochondrion 2011, 11, 108–118.
- Iyer, S.; Thomas, R.R.; Portell, F.R.; Dunham, L.D.; Quigley, C.K.; Bennett, J.P., Jr. Recombinant mitochondrial transcription factor A with N-terminal mitochondrial transduction domain increases respiration and mitochondrial gene expression. Mitochondrion 2009, 9, 196–203.
- Lu, Y.J.; Wu, Y.J.; Chen, L.J.; Ko, B.S.; Chang, T.C.; Wu, Y.J.; Liang, S.M.; Jan, Y.J.; Liou, J.Y. Reduced Expression of Metallothionein-I/II in Renal Proximal Tubules Is Associated with Advanced Chronic Kidney Disease. Toxins 2021, 13, 568.
- Saba, H.; Batinic-Haberle, I.; Munusamy, S.; Mitchell, T.; Lichti, C.; Megyesi, J.; MacMillan-Crow, L.A. Manganese porphyrin reduces renal injury and mitochondrial damage during ischemia/reperfusion. Free Radic. Biol. Med. 2007, 42, 1571–1578.
- Li, L.; Geisler, I.; Chmielewski, J.; Cheng, J.X. Cationic amphiphilic polyproline helix P11LRR targets intracellular mitochondria. J. Control. Release 2010, 142, 259–266.
- Kalafut, D.; Anderson, T.N.; Chmielewski, J. Mitochondrial targeting of a cationic amphiphilic polyproline helix. Bioorg. Med. Chem. Lett. 2012, 22, 561–563.
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Aparicio-Trejo, O.E.; Pedraza-Chaverri, J. Mitochondrial Redox Signaling and Oxidative Stress in Kidney Diseases. Biomolecules 2021, 11, 1144.
- Kam, A.; Loo, S.; Dutta, B.; Sze, S.K.; Tam, J.P. Plant-derived mitochondria-targeting cysteine-rich peptide modulates cellular bioenergetics. J. Biol. Chem. 2019, 294, 4000–4011.
- Rojas-Morales, P.; Leon-Contreras, J.C.; Aparicio-Trejo, O.E.; Reyes-Ocampo, J.G.; Medina-Campos, O.N.; Jimenez-Osorio, A.S.; Gonzalez-Reyes, S.; Marquina-Castillo, B.; Hernandez-Pando, R.; Barrera-Oviedo, D.; et al. Fasting reduces oxidative stress, mitochondrial dysfunction and fibrosis induced by renal ischemia-reperfusion injury. Free Radic. Biol. Med. 2019, 135, 60–67.
- Briones-Herrera, A.; Avila-Rojas, S.H.; Aparicio-Trejo, O.E.; Cristobal, M.; Leon-Contreras, J.C.; Hernandez-Pando, R.; Pinzon, E.; Pedraza-Chaverri, J.; Sanchez-Lozada, L.G.; Tapia, E. Sulforaphane prevents maleic acid-induced nephropathy by modulating renal hemodynamics, mitochondrial bioenergetics and oxidative stress. Food Chem. Toxicol. 2018, 115, 185–197.
- Kelley, S.O.; Stewart, K.M.; Mourtada, R. Development of novel peptides for mitochondrial drug delivery: Amino acids featuring delocalized lipophilic cations. Pharm. Res. 2011, 28, 2808–2819.
- Yoo, B.; Kirshenbaum, K. Peptoid architectures: Elaboration, actuation, and application. Curr. Opin. Chem. Biol. 2008, 12, 714–721.
- Nam, H.Y.; Hong, J.A.; Choi, J.; Shin, S.; Cho, S.K.; Seo, J.; Lee, J. Mitochondria-Targeting Peptoids. Bioconjug. Chem. 2018, 29, 1669–1676.
- Karaa, A.; Haas, R.; Goldstein, A.; Vockley, J.; Weaver, W.D.; Cohen, B.H. Randomized dose-escalation trial of elamipretide in adults with primary mitochondrial myopathy. Neurology 2018, 90, e1212–e1221.
- Reid Thompson, W.; Hornby, B.; Manuel, R.; Bradley, E.; Laux, J.; Carr, J.; Vernon, H.J. A phase 2/3 randomized clinical trial followed by an open-label extension to evaluate the effectiveness of elamipretide in Barth syndrome, a genetic disorder of mitochondrial cardiolipin metabolism. Genet. Med. 2021, 23, 471–478.
- Chakrabarti, A.K.; Feeney, K.; Abueg, C.; Brown, D.A.; Czyz, E.; Tendera, M.; Janosi, A.; Giugliano, R.P.; Kloner, R.A.; Weaver, W.D.; et al. Rationale and design of the EMBRACE STEMI study: A phase 2a, randomized, double-blind, placebo-controlled trial to evaluate the safety, tolerability and efficacy of intravenous Bendavia on reperfusion injury in patients treated with standard therapy including primary percutaneous coronary intervention and stenting for ST-segment elevation myocardial infarction. Am. Heart. J. 2013, 165, 509–514 e507.
- Butler, J.; Khan, M.S.; Anker, S.D.; Fonarow, G.C.; Kim, R.J.; Nodari, S.; O’Connor, C.M.; Pieske, B.; Pieske-Kraigher, E.; Sabbah, H.N.; et al. Effects of Elamipretide on Left Ventricular Function in Patients With Heart Failure With Reduced Ejection Fraction: The PROGRESS-HF Phase 2 Trial. J. Card. Fail. 2020, 26, 429–437.
- Saad, A.; Herrmann, S.M.S.; Eirin, A.; Ferguson, C.M.; Glockner, J.F.; Bjarnason, H.; McKusick, M.A.; Misra, S.; Lerman, L.O.; Textor, S.C. Phase 2a Clinical Trial of Mitochondrial Protection (Elamipretide) During Stent Revascularization in Patients With Atherosclerotic Renal Artery Stenosis. Circ. Cardiovasc. Interv. 2017, 10, e005487.
- Yang, S.K.; Han, Y.C.; He, J.R.; Yang, M.; Zhang, W.; Zhan, M.; Li, A.M.; Li, L.; Na, S.; Liu, Y.T.; et al. Mitochondria targeted peptide SS-31 prevent on cisplatin-induced acute kidney injury via regulating mitochondrial ROS-NLRP3 pathway. Biomed. Pharm. 2020, 130, 110521.
- Szeto, H.H.; Liu, S.; Soong, Y.; Wu, D.; Darrah, S.F.; Cheng, F.Y.; Zhao, Z.; Ganger, M.; Tow, C.Y.; Seshan, S.V. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J. Am. Soc. Nephrol. 2011, 22, 1041–1052.
- Liu, S.; Soong, Y.; Seshan, S.V.; Szeto, H.H. Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis. Am. J. Physiol. Ren. Physiol. 2014, 306, F970–F980.
- Birk, A.V.; Liu, S.; Soong, Y.; Mills, W.; Singh, P.; Warren, J.D.; Seshan, S.V.; Pardee, J.D.; Szeto, H.H. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J. Am. Soc. Nephrol. 2013, 24, 1250–1261.
- Cerrato, C.P.; Pirisinu, M.; Vlachos, E.N.; Langel, U. Novel cell-penetrating peptide targeting mitochondria. FASEB J. 2015, 29, 4589–4599.
- Liu, D.; Jin, F.; Shu, G.; Xu, X.; Qi, J.; Kang, X.; Yu, H.; Lu, K.; Jiang, S.; Han, F.; et al. Enhanced efficiency of mitochondria-targeted peptide SS-31 for acute kidney injury by pH-responsive and AKI-kidney targeted nanopolyplexes. Biomaterials 2019, 211, 57–67.
- Wyss, J.C.; Kumar, R.; Mikulic, J.; Schneider, M.; Mary, J.L.; Aebi, J.D.; Juillerat-Jeanneret, L.; Golshayan, D. Differential Effects of the Mitochondria-Active Tetrapeptide SS-31 (D-Arg-dimethylTyr-Lys-Phe-NH2) and Its Peptidase-Targeted Prodrugs in Experimental Acute Kidney Injury. Front. Pharm. 2019, 10, 1209.
- Szeto, H.H.; Liu, S.; Soong, Y.; Seshan, S.V.; Cohen-Gould, L.; Manichev, V.; Feldman, L.C.; Gustafsson, T. Mitochondria Protection after Acute Ischemia Prevents Prolonged Upregulation of IL-1beta and IL-18 and Arrests CKD. J. Am. Soc. Nephrol. 2017, 28, 1437–1449.
- Hou, Y.; Shi, Y.; Han, B.; Liu, X.; Qiao, X.; Qi, Y.; Wang, L. The antioxidant peptide SS31 prevents oxidative stress, downregulates CD36 and improves renal function in diabetic nephropathy. Nephrol. Dial. Transpl. 2018, 33, 1908–1918.
- Yang, S.K.; Li, A.M.; Han, Y.C.; Peng, C.H.; Song, N.; Yang, M.; Zhan, M.; Zeng, L.F.; Song, P.A.; Zhang, W.; et al. Mitochondria-Targeted Peptide SS31 Attenuates Renal Tubulointerstitial Injury via Inhibiting Mitochondrial Fission in Diabetic Mice. Oxidative Med. Cell. Longev. 2019, 2019, 2346580.
- Yang, Q.; Xie, W.; Wang, X.; Luo, J.; Zhou, Y.; Cao, H.; Sun, Q.; Jiang, L.; Yang, J. SS31 Ameliorates Podocyte Injury via Inhibiting OMA1-Mediated Hydrolysis of OPA1 in Diabetic Kidney Disease. Front. Pharmacol. 2022, 12, 707006.
- Hou, Y.; Li, S.; Wu, M.; Wei, J.; Ren, Y.; Du, C.; Wu, H.; Han, C.; Duan, H.; Shi, Y. Mitochondria-targeted peptide SS-31 attenuates renal injury via an antioxidant effect in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2016, 310, F547–F559.
- Miyamoto, S.; Zhang, G.; Hall, D.; Oates, P.J.; Maity, S.; Madesh, M.; Han, X.; Sharma, K. Restoring mitochondrial superoxide levels with elamipretide (MTP-131) protects db/db mice against progression of diabetic kidney diease. J. Biol. Chem. 2020, 295, 7249–7260.
- Szeto, H.H.; Liu, S.; Soong, Y.; Alam, N.; Prusky, G.T.; Seshan, S.V. Protection of mitochondria prevents high-fat diet-induced glomerulopathy and proximal tubular injury. Kidney Int. 2016, 90, 997–1011.
- Alta, R.Y.; Vitorino, H.A.; Goswami, D.; Liria, C.W.; Wisnovsky, S.P.; Kelley, S.O.; Machini, M.T.; Esposito, B.P. Mitochondria-penetrating peptides conjugated to desferrioxamine as chelators for mitochondrial labile iron. PLoS ONE 2017, 12, e0171729.
- Guariento, A.; Piekarski, B.L.; Doulamis, I.P.; Blitzer, D.; Ferraro, A.M.; Harrild, D.M.; Zurakowski, D.; Del Nido, P.J.; McCully, J.D.; Emani, S.M. Autologous mitochondrial transplantation for cardiogenic shock in pediatric patients following ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 2021, 162, 992–1001.
- Emani, S.M.; Piekarski, B.L.; Harrild, D.; Del Nido, P.J.; McCully, J.D. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 2017, 154, 286–289.
- Kubat, G.B.; Ozler, M.; Ulger, O.; Ekinci, O.; Atalay, O.; Celik, E.; Safali, M.; Budak, M.T. The effects of mesenchymal stem cell mitochondrial transplantation on doxorubicin-mediated nephrotoxicity in rats. J. Biochem. Mol. Toxicol. 2021, 35, e22612.
- Jabbari, H.; Roushandeh, A.M.; Rostami, M.K.; Razavi-Toosi, M.T.; Shokrgozar, M.A.; Jahanian-Najafabadi, A.; Kuwahara, Y.; Roudkenar, M.H. Mitochondrial transplantation ameliorates ischemia/reperfusion-induced kidney injury in rat. Biochim. Biophys. Acta Mol. Basis. Dis. 2020, 1866, 165809.
- Doulamis, I.P.; Guariento, A.; Duignan, T.; Kido, T.; Orfany, A.; Saeed, M.Y.; Weixler, V.H.; Blitzer, D.; Shin, B.; Snay, E.R.; et al. Mitochondrial transplantation by intra-arterial injection for acute kidney injury. Am. J. Physiol. Ren. Physiol. 2020, 319, F403–F413.
- Kitani, T.; Kami, D.; Matoba, S.; Gojo, S. Internalization of isolated functional mitochondria: Involvement of macropinocytosis. J. Cell Mol. Med. 2014, 18, 1694–1703.
- Chang, J.C.; Hoel, F.; Liu, K.H.; Wei, Y.H.; Cheng, F.C.; Kuo, S.J.; Tronstad, K.J.; Liu, C.S. Peptide-mediated delivery of donor mitochondria improves mitochondrial function and cell viability in human cybrid cells with the MELAS A3243G mutation. Sci. Rep. 2017, 7, 10710.
- Chang, J.C.; Liu, K.H.; Li, Y.C.; Kou, S.J.; Wei, Y.H.; Chuang, C.S.; Hsieh, M.; Liu, C.S. Functional recovery of human cells harbouring the mitochondrial DNA mutation MERRF A8344G via peptide-mediated mitochondrial delivery. Neurosignals 2013, 21, 160–173.
- Chang, J.C.; Chang, H.S.; Wu, Y.C.; Cheng, W.L.; Lin, T.T.; Chang, H.J.; Kuo, S.J.; Chen, S.T.; Liu, C.S. Mitochondrial transplantation regulates antitumour activity, chemoresistance and mitochondrial dynamics in breast cancer. J. Exp. Clin. Cancer. Res. 2019, 38, 30.
- Maeda, H.; Kami, D.; Maeda, R.; Murata, Y.; Jo, J.I.; Kitani, T.; Tabata, Y.; Matoba, S.; Gojo, S. TAT-dextran-mediated mitochondrial transfer enhances recovery from models of reperfusion injury in cultured cardiomyocytes. J. Cell Mol. Med. 2020, 24, 5007–5020.
This entry is offline, you can click here to edit this entry!
