Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Immunology
Human Natural Killer (NK) cells are all round players in immunity thanks to their powerful and immediate response against transformed cells and the ability to modulate the subsequent adaptive immune response.
- NK cells
- monoclonal antibodies
- bispecific antibodies
- trispecific engagers
1. Introduction
Colorectal cancer (CRC) is the third-most-common cancer worldwide and claims almost 1 million deaths per year, despite effective cancer prevention screening plans and relatively good prognosis compared to other gastrointestinal malignancies (WHO and UEG) [1]. The five-year survival rate is 90 percent for CRC diagnosed at an early stage compared with 13 percent for those diagnosed later [2]. In this context, immunotherapy could represent an additional strategy to complement surgery, radiotherapy, and chemotherapy to increase the survival of CRC patients, especially when the disease is diagnosed at later stages. Indeed, several immunotherapeutic approaches have been developed for different cancer types, including CRC, with promising clinical results [3]. Immunotherapy for CRC patients includes monoclonal antibodies (mAbs) targeting tumor-associated antigens (TAAs), immune checkpoint inhibitors (ICIs), adoptive cell therapy, anti-cancer vaccines, and oncolytic viruses treatment [3,4,5,6]. However, given the high heterogeneity of CRCs, the therapeutic efficacy of these approaches is variable [7]. In particular, ICI therapy is only effective in a small group of CRC patients characterized by microsatellite instability (MSI) and mismatch-repair deficiency (dMMR), which accounts for less than 20% of patients [7]. Conversely to the other most diffuse types of CRC, marked by mismatch-repair proficiency (pMMR) and microsatellite stability (MSS), MSI and dMMR cancers are usually characterized by enriched immune cell infiltration. The presence of tumor-infiltrating lymphocytes (TILs) has been correlated with the containment of metastases [8], a good clinical outcome [9,10] and a positive response to ICI immunotherapies [11,12,13]. In particular, high numbers of CD8 and CD4 T cells with Th1 profile and NK cells have been correlated with better prognosis in CRC patients [14,15,16]. Based on these clinical data correlating NK cell infiltration with better survival in CRC patients, it is conceivable that novel immune-mediated therapies aimed at increasing the number and/or function of NK cells in tumor lesions could be a useful strategy in CRC containment. In this regard, NK cells, which are innate lymphocytes able to carry out a powerful and immediate response against cancerous cells, may importantly contribute to immune-mediated therapies. NK cells are cytotoxic members of the large and plastic family of Innate Lymphoid Cells (ILCs) [17], whose role in the development of CRC is controversial [18]. NK cells circulate among blood, lymphoid, and non-lymphoid tissues patrolling almost the entire human body, while ILCs are mainly located at mucosal surfaces and are usually not cytolytic [17]. However, NK cells can also be resident in tissues displaying highly variable expression patterns. Thus a clear distinction between tissue-resident NK cells and ILCs, especially ILC1, can be challenging [19,20].
Thanks to their multitude of effector capabilities, including killing activity and modulation of the adaptive immune response, NK cells are ever more considered strategic effectors of the immunotherapeutic approach. In the present review, we discuss the different immunotherapeutic strategies focused on enhancing NK cell function in the fight against CRC.
2. Human NK Cells
NK cell capabilities to recognize transformed cells without prior antigen exposure, kill target cells, and release immune-regulating cytokines/chemokines are mainly due to the large repertoire of germline-encoded activating receptors that provide the “on” signal following the interaction with putative ligands (Figure 1). Among all, the non-HLA-specific natural cytotoxicity receptors NKp46, NKp30, and NKp44 (collectively named NCRs), NKG2D, and DNAM-1 are the main activating NK receptors [21,22,23,24,25,26,27].
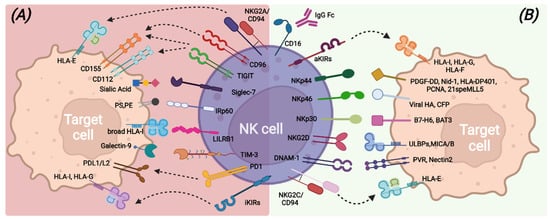
Figure 1. Main inhibitory (A) and activating (B) NK-cell surface receptors and their cognate ligands on target cells.
NCRs belong to the Ig superfamily and possess, in the transmembrane domain, positively charged amino acids that allow association with ITAM-bearing molecules, such as CD3-ζ and/or FcεRI-γ for NKp30 and NKp46 and KARAP/DAP12 for NKp44, and, therefore, the transduction of an activation signal upon ligand recognition [28]. Different membrane-bound, intracellular, and soluble extracellular molecules have been identified as NCRs ligands, including some molecules of viral origin [26,29,30,31,32,33,34,35,36].
NKG2D is a type II transmembrane protein with a C-type lectin-like extracellular domain and transduces an activation signal through association with the adapter ITAM-bearing molecule DAP-10 upon recognition of stress-inducible molecules, namely ULBPs and MICA/B. NKG2D ligands can also be shed from tumor-transformed cells and contribute to tumor escape mechanisms [25,37,38].
DNAM-1 is a transmembrane glycoprotein characterized by two extracellular Ig-like domains and by a cytoplasmic portion containing tyrosine residues involved in lymphocyte adhesion and signaling [39]. DNAM-1 recognizes PVR and Nectin-2 [24,40] that are highly expressed on antigen-presenting cells (APCs), tumors, and virus-infected cells. In addition, DNAM-1 shares the same ligands of the TIGIT and CD96 inhibitory receptors but exhibits opposite functions with respect to them, suggesting a complementary role in the regulation of tumor immunity and inflammatory response [40,41].
Another relevant activating receptor is CD16 (FcγRIIIa), a low-affinity receptor for the immunoglobulin G (IgG) Fc fragment, whose binding to opsonized target cells triggers efficient NK cell-mediated killing through antibody-dependent cell-mediated cytotoxicity (ADCC) [42,43].
In healthy conditions, all these activating receptors are under the control of inhibitory signals transduced by HLA class I-specific receptors (such as inhibitory KIRs, CD94/NKG2A, LILRB-1) upon recognition of self-HLA molecules [44,45,46] (Figure 1). Mature and functional NK cells usually express at least one inhibitory receptor, specific for self-HLA class I molecules. Indeed, during the cell differentiation process, only NK cells expressing inhibitory receptors recognizing self-HLA class I molecules undergo a process called “education”, consisting of the acquisition of functional competencies in terms of cytotoxicity ability and cytokine secretion [47,48]. This process ensures, on the one hand, self-tolerance towards healthy cells and on the other hand, an efficient response against transformed cells, which usually lack or down-regulate HLA class I expression and acquire or up-regulate the expression of ligands for the non-HLA specific activating NK receptors [49]. NK cells can also express other inhibitory receptors, the non-HLA class I specific receptors, which can regulate the NK cell function by acting as immune checkpoints (ICs), similarly to KIR, NKG2A, and LILRB1. These additional ICs include PD-1, TIM-3, TIGIT, CD96, LAG3, CD161, Siglec-7, and IRp60 and can be up-regulated or de novo expressed by NK cells in pathologic conditions [50,51,52], thus contributing to avoid exacerbated immune responses and also favoring tumor escape (Figure 1).
In this regard, the immunosuppressive CRC tumor microenvironment (TME) due to the activity of tumor cells and the related presence of other immune cells with immune-modulatory properties, such as myeloid-derived suppressor cells (MDSCs) and Th17 cells, can create a disadvantageous milieu affecting the killing properties of NK cells [53]. Several studies have demonstrated that NK cells have reduced functionality in CRC patients [10]. Decreased expression of NKp46 and NKp30 has been demonstrated in CRC-infiltrating NK cells, and the low expression of NKp46 in peripheral blood (PB) NK cells of CRC patients has been correlated with lower relapse-free survival (RFS). Moreover, NKG2D and DNAM-1 expression has been demonstrated to be down-modulated in both PB-NK cells and tissue-infiltrating NK cells of CRC patients [54,55].
3. Monoclonal Antibodies-Based Treatments to Enhance NK Cell Cytotoxicity
The potential of immunotherapies based on the use of mAbs that target distinct cancer-specific cell markers and may trigger T cell anti-tumor function and NK cell-mediated ADCC has been initially revealed in the hematological setting. Indeed, the successful use of Rituximab, a chimeric anti-CD20 mAb targeting CD20+ lymphoma cells [56,57], has prompted the design of different immune tools to also be applied against solid tumors, including CRC. In this context, several mAbs targeting CRC tumor antigens (e.g., EGFR, CEA, Her2) or the TME (e.g., VEGF) that may simultaneously trigger NK cell cytotoxicity have been developed. More recently, the use of mAbs targeting different ICs, represented by inhibitory receptors (e.g., PD-1) that control the function of effector cells, such as T and NK cells, by disrupting inhibitory interactions and restoring anti-tumor capabilities by cytotoxic lymphocytes, has offered unexpected possibilities to cure solid tumors [58,59,60].
3.1. Anti-Tumor Associated Antigen (TAA) mAbs, BiKe, and Engagers Enhancing CRC Killing via ADCC
The use of mAbs directed against surface antigens expressed by tumor cells has shown clinical efficacy in different tumors. Treatment with anti-TAA mAbs can induce tumor cell death by several mechanisms, such as directly inducing tumor apoptosis or via ADCC, that is, mediated by CD16 engagement with IgG-opsonized tumor cells. Indeed, the efficacy of an anti-TAA mAb largely employed in the treatment of metastatic CRC, i.e., anti-EGFR (known by the commercial names Cetuximab or Panitumumab or Necitumumab [61]) also relies on enhanced NK-mediated killing via ADCC (Figure 2a). The epidermal growth factor receptor (EGFR; ErbB-1; HER1 in humans), a transmembrane tyrosine kinase receptor, affects cell adhesion, survival and proliferation and is overexpressed in most CRC (75%) and is associated with poor prognosis. Anti-EGFR mAbs can act by blocking ligand binding and thus prevent proliferation in response to EGF. However, in a fraction of CRC patients (36–55% [62]), mutations in KRAS, a signaling molecule downstream of EGFR, render the receptor constitutively active and the anti-EGFR treatment ineffective [63,64,65]. On the other hand, in KRAS-mutated patients, anti-EGFR mAbs could still efficaciously induce ADCC by NK cells as demonstrated in vitro [66] and also in vivo in a recent clinical study (NCT01450319) [67]. Interestingly, this work also reported that anti-EGFR-treated, KRAS-mutant patients carrying homozygous KIR genotypes (AA or BB) have a worse outcome than KIR heterozygotes (AB). The mechanisms underlying this observation are unknown but could be related to altered HLA class-I expression on tumor cells in response to factors, such as IFN-γ, possibly released by CD16-engaged NK cells.
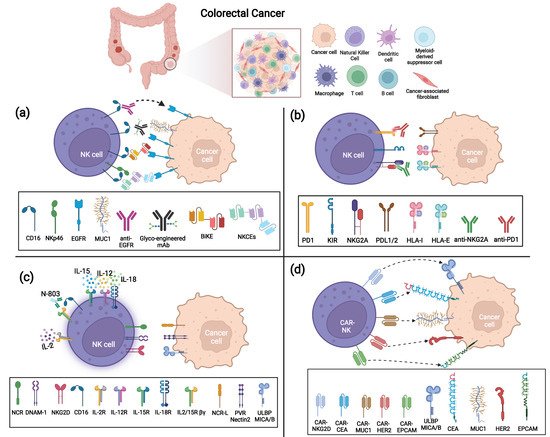
Figure 2. Strategies to enhance anti-tumor NK cell function against Colorectal Cancer (CRC). (a) ADCC triggering strategies via anti-TAA mAbs, BiKe, or NKCEs; (b) IC blockade via anti-NKG2A and anti-PD-1 mAbs; (c) Cytokine activation of NK cells by IL-2, IL-15/IL-12/IL-18 combination, or N-803; (d) CAR-modified NK cells targeting several TAAs (CEA, MUC-1, EpCAM, HER-2, and NKG2D ligands MICA/B and ULBPs).
However, anti-EGFR clinical benefits are confined to a fraction of patients [68], and many efforts to optimize this treatment are in progress. One possibility that has been explored is the manipulation of the Fc fragment to augment its affinity for CD16. For example, a glycoengineered anti-EGFR mAb (GA201, Imgatuzumab, RO5083945) has been demonstrated to induce superior ADCC than the non-manipulated mAb (Cetuximab), allowing both the NK cell impairment often observed in CRC patients [69] and the lower Fc affinity of given CD16 variants that display an amino-acid substitution at position 158 (V158F polymorphism) to be overcome [64,70]. GA201 is not currently employed in clinical trials or therapies, but a similar glycoengineered antibody derived from Cetuximab, Tomuzotuximab, has been tested in combination with a glycoengineered humanized anti-Mucin-1 (MUC-1) antibody (Gatipotuzumab) that targets a tumor-specific epitope of MUC-1, showing promising results in CRC patients [71]. Indeed, Fc-optimized mAbs can elicit potent NK-mediated ADCC and possibly replace the standard antibody in several tumor settings. In this context, anti-EGFR efficacy can be limited not only by KRAS mutations or CD16 variants but also by low Ab penetrance due to TME factors. To overcome these hurdles, novel immune tools such as BiKe and engagers have been designed and have proved their efficacy in vitro and in preclinical models [72,73]. Similar to BiTe (i.e., bispecific single-chain T-cell engager, such as Blinatumomab that couples anti-CD3 to anti-CD20), BiKe are composed of a single-chain variable fragment (scFv) of an antibody specific for a given TAA connected through a short peptide linker to an anti-CD16 scFv, which triggers stronger cytolytic signals in NK cells, favoring the formation of the NK-tumor immunological synapse [35]. Interestingly, a bispecific single domain antibody that targets CD16 on NK cells and recognizes EGFR on tumor cells induces a strong NK cell effector response against both EGFR-expressing CRC cell lines and ex vivo CRC-derived tumor cells, regardless of tumor KRAS mutation status [73]. Moreover, this bispecific antibody induces a consistent release of CXCL10 when ex vivo metastatic CRC tumor cells are co-cultured with autologous PBMC, possibly favoring the in vivo recruitment of effector T and NK cells at the tumor site. Similarly, a tetravalent bispecific (i.e., displaying two binding domains instead of a single chain) fusion antibody targeting CD16 and EGFR on tumor cells also results in highly effective toward a variety of EGFR-expressing tumor cells, regardless of the EGFR expression level [74], thus suggesting its potential efficacy despite CRC heterogeneity.
MAbs and BiKes engaging CD16 to induce ADCC can fail to efficiently trigger NK-mediated cytotoxicity when CD16 is shed from the NK cell surface by matrix metalloproteases such as ADAM17 [75] that can be up-regulated in the TME [76]. Indeed, low CD16 expression levels have been described in tumor-infiltrating NK cells from CRC biopsies [54]. Besides the use of metalloprotease inhibitors to prevent CD16 shedding [77], other immune tools that hold promise to circumvent this issue are trifunctional NK cell engagers (NKCEs). This innovative tool simultaneously targeting NKp46 (or NKp30) and CD16 on NK cells and EGFR on cancer cells has proved to induce superior anti-tumor activity than the standard therapeutic mAbs (e.g., Cetuximab) in preclinical models [72]. Indeed, NKCEs represent plastic tools that could be easily modified with different anti-tumor antigens or anti-NK receptor moieties to better fit both cancer features and NK cell heterogeneity in distinct patients.
Of note, bispecific antibodies simultaneously binding two different tumor antigens that could potently induce NK-mediated ADCC are currently in clinical trials for advanced CRC or in preclinical models, such as anti-EGFR and anti-c-MET (Mesenchymal Epithelial Transition receptor, highly expressed or amplified in subsets of CRC, NCT04930432) or anti-EGFR and anti-LGR5 (cancer stem cell marker) [78].
Beyond EGFR, several other TAAs associated with CRC, such as MUC-1, CEA, gpa33, HER2, PD-L1, and CD73, are currently being explored as targets of mAb-mediated immunotherapy, possibly benefitting NK cell ADCC [53,79]
3.2. Immune Checkpoint Inhibitors (ICI) to Unleash NK Cell Killing against CRC
In the TME, NK cell function can be dampened mainly by the down-modulation of activating receptors in response to soluble factors released by tumor or tumor-associated cells (e.g., TGF-β, PGE2, soluble B7-H6, IDO1-derived catabolites, soluble ligands [10,80]) and/or by the engagement of ICs expressed by NK cells. Some studies have shown that NK cells infiltrating CRC can express multiple ICs, including both inhibitory receptors specific for HLA class I molecules (NKG2A and KIRs) and those recognizing non-HLA class I ligands (e.g., PD-1, TIM-3, LAG3, TIGIT) [54,81,82,83]. Further studies investigating IC expression and function in CRC-infiltrating NK cells are needed; however, the use of different ICIs that block the interactions between ICs and their ligands expressed on tumor cells is a promising immunotherapeutic approach that could be capable not only of restoring T cell immunity, but also of unleashing NK cell anti-tumor potential (Figure 2b).
A large number of clinical trials in metastatic or advanced MSI and dMMR CRC have been based on the administration of anti-PD-1 and/or anti-PD-L1 mAbs. Interestingly, recent data reported that MSS patients with proficient MMR but harboring the POLE mutation (pole encodes the DNA polymerase responsible for lead strand DNA replication) that favors high neoantigens generation, high TMB, and recruitment of TILs, including NK cells, can also benefit from anti-IC therapies [7,84]. Indeed, with the aim of optimizing/enlarging the ICI potential for MSS patients or ICI refractory/resistant MSI patients, several currently active (or still recruiting) clinical trials are exploring the effect of anti-PD-1 mAbs in combination with chemotherapy or with kinase inhibitors or with other mAbs, such as anti-VEGF, both in MSI and in MSS CRC at advanced stages. Other trials are aimed at simultaneously blocking multiple ICs in combination with conventional chemotherapies that could contribute to converting “cold” tumors to immune-active tumors sensitive to ICI therapies [85]. Although the PD-1-PD-L1 axis may actually contribute to hamper NK cell function in CRC, a major role is likely played by the IC NKG2A, which is consistently expressed by both NK and T cells in CRC TILs [54,81,86]. NKG2A recognizes the non-classical HLA class I molecule HLA-E, which results as overexpressed in a fraction of CRC, preferentially in MSI compared to MSS, and whose expression is associated with a worse prognosis [81,87,88]. Indeed, a combination of anti-NKG2A (Monalizumab or IPH2202) and anti-PD-L1 (Durvalumab) mAbs, offered to patients with metastatic MSS CRC, showed promising activity in a recent clinical trial (NCT02671435) [89]. An additional promising strategy is based on combining the blockade of inhibitory signals with the delivery of activating signals. In this context, in vitro data demonstrated that NKG2A blockade with Monalizumab boosts NK cell-mediated ADCC against Cetuximab-coated tumor targets [81]. Indeed, this combination was effective in a phase 1–2 trial and a phase 3 trial (NCT04590963) is ongoing in a Squamous Cell Carcinoma of Head and Neck (SCCHN) cohort. Along this line, a phase 1 trial (NCT05162755) that combines an NKG2A and PD-1 blockade with anti-EGFR is currently recruiting patients with metastatic gastric tumor and CRC. A similar strategy can be pursued by novel immune tools designed to stimulate ADCC via CD16 and simultaneously block PD-1/PD-L1 interactions. These molecules have been engineered to incorporate an IL-15 moiety with the aim of promoting NK cell activation, in vivo persistence and proliferation and have shown promising results in vitro and in preclinical models [90,91].
Finally, the effects of other ICI on NK cell function, such as those blocking LAG3 and TIGIT that can be expressed by NK cells in CRC, deserve to be examined [59,79].
This entry is adapted from the peer-reviewed paper 10.3390/vaccines10071033
This entry is offline, you can click here to edit this entry!
