1. Early Triggers of Neurodegeneration in Preterm Infants: Protective Roles of Lactoferrin
Preterm delivery is associated with a high burden of neurodevelopmental impairments and lifelong functional disabilities in distinct cognitive and motor domains [
107]. Preterm infants are not adapted to the ex-utero environment and experience reduced access to nutrition, higher amounts of oxygen exposure, blood pressure fluctuations, immune system challenges, and exposure to pro-inflammatory and stressful environment with increased levels of light, sounds, tactile stimulus, olfaction, oxygen and nutrients which are different from those they would have experienced in utero [
108]. The developing brain is particularly sensitive to appropriate levels of such factors and preclinical data has shown that alterations in any of these variables can result in abnormal structural and functional development of the brain [
109,
110]. The CNS has unique characteristics that contribute to the vulnerability of the preterm brain to injury; during this highly active phase, initiation of myelination, axonal and dendritic growth, synaptogenesis and proliferation of microglia and astrocytes are still in progress. Injuries at this stage of development have a profound impact on oligodendrocyte precursors (pre-oligodendrocytes) which are susceptible to injury and cell death, resulting in hypomyelination [
111]. The relative vulnerability of this cell lineage is especially mediated by its high susceptibility to oxidative stress and decreased antioxidant defense capacity [
78]. In a period in which increased oxygen demand and rate of production of reactive oxygen species is high, oxidative stress and lipid peroxidation are observed in the placenta of women experiencing pre-eclampsia. Newborns also have low levels of endogenous antioxidant capacity, including lower levels of plasma antioxidants, which increase their vulnerability to elevated levels of reactive species. In addition, the preterm brain has comparatively underdeveloped vasculature, which matures relatively late in gestation, thus favoring the occurrence of periventricular hemorrhagic infarction, parenchymal bleeding, with ventricular bleeding and subsequent dilation or hydrocephalus leading to periventricular leukomalacia, poor myelination and cortical development alterations which are associated with significant cognitive and/or motor abnormalities in survivors [
112].
Preterm babies are commonly exposed to infections/inflammation, poor respiratory function after birth, ventilation-induced lung and brain injury and a variety of treatments with potential side-effects, including glucocorticoids [
113]. The nature of preterm brain injury presents many challenges in the quest to develop treatment strategies for these infants—not only the acute damage should be targeted but supporting subsequent brain development should also be part of therapeutic strategies. It is likely that different treatment strategies may be more effective in treating brain damage stemming from insults such as oxidative stress, ischemia and, particularly, inflammation. Microglial cells undergo critical stages in development throughout the early life period, and, therefore, inflammation can have severe consequences on the proper development of the CNS [
114]. Apart from the risk of infections and other specific inflammatory events during pregnancy, delivery and prematurity, other factors may also be related to neurodevelopmental problems, such as obesity, insulin resistance (gestational diabetes) and poor diet; it is well established that insulin resistance is caused by a chronic inflammatory response, and that poor quality diets are major contributors to neurodevelopmental delay in preterm infants [
113,
115].
A major concern for optimal development and lifelong wellbeing is that of perinatal infections; neonatal sepsis causes 13% of infant deaths and is responsible for 42% of deaths in the first week of life [
116]. Even with advances in healthcare reducing the rates of death, infections can still have lifelong consequences; 40% of survivors from neonatal sepsis and meningitis present some form of neurodevelopmental sequelae [
117,
118]. Necrotizing enterocolitis (NEC) has a mortality rate of 20% to 30% and a quarter of survivors present with microcephaly [
117]. Recently, a 17-year study in Denmark and The Netherlands [
116] observed that neonates exposed to Group B
Streptococcus disease (iGBS) had increased risk of neurodevelopmental impairment (RR 1.77 and 2.28 respectively). iGBS poses an even greater burden on developing countries, since not all have similar conditions for prophylactic treatment with antimicrobials. These neonatal pathologies contribute significantly to global inflammation in preterm infants, representing risk factors for brain damage and neurodevelopmental delay. In a complimentary way to human cohorts, animal models enable strengthening of the causal link between perinatal inflammation and neurodevelopmental impairment [
119,
120,
121]. The administration of immunostimulants, such as lipopolysaccharide (LPS) and poly I:C, to pregnant dams has been used to model congenital neurological disorders showing social abnormalities associated with autism spectrum disorder [
119,
122], cerebral palsy [
123,
124], white matter injury [
78], and a large array of behavioral abnormalities, according to the gestational age and regimen used [
125,
126]. Postnatal administration of LPS is also often used to model abnormalities related to neurodevelopment [
119,
122], with outcomes being dependent on postnatal age, dose and regimen. It is important to point out that lipopolysaccharide and poly I:C are molecules targeted by the immune system as pathogen signals, but do not present infectious potential to the organism—LPS is extracted from gram-negative bacterial membranes and poly I:C is a synthetic molecule that resembles viral double-stranded DNA. Both are recognized by toll-like receptors, TLR4 and TLR3, respectively, and elicit innate immune responses [
127,
128]. Therefore, the effect observed in the offspring is a product of the mother’s or their own immune response rather than microbial damage. This is an important distinction that can help understanding of the implications of the immune system during development, which can be affected by factors other than infections, such as maternal obesity and diabetes, autoimmune diseases, and environmental stresses from drugs, pollutants or malnutrition.
2. Lactoferrin and the Development of Infant Microbiome
In recent decades, there has been increasing interest in understanding the importance of commensal bacteria as a major environmental component that promotes healthy development, and is not only related to infectious diseases [
127]. The gut microbiota is essential in shaping the development of both the immune and the nervous system [
129] through a symbiotic interaction with the host [
130], providing environmental “clues” that influence immune system development, including pathogen-associated molecular patterns (PAMPs), such as LPS, MDP-2 (muramyl dipeptide 2) and LPA (lysophosphatidic acid), that activate mucosal TLRs, which drive immune system development [
131,
132,
133]. Indeed, it has been proposed that newborns present a reduced immunological response compared to adults because their gut may be sterile during gestation, and therefore “naïve” to pathogens [
134]. Animal models of germ-free mice (GF), which are delivered under fully aseptic conditions, are more susceptible to infections, having a reduced number of Th17 cells which are particularly important for immunomodulatory function in the lamina propria. Moreover, GF mice present abnormal behavior [
135,
136], immature microglia [
130,
137,
138] and abnormal intestine sizes. Since microglia are responsible for synaptic pruning during brain maturation, such hypofunction has been linked to future psychiatric diseases [
131,
132,
133]. The gut microbiome also provides the host with metabolites which would otherwise be unavailable, such as short-chain fatty acids (SCFA) (e.g., butyrate, propionate and acetate) [
139]. These molecules are an exclusive product of fiber digestion by these bacteria since humans lack cellulase enzymes. These molecules can be further oxidized for energy production, but also have signaling properties that modulate immune system development [
140], especially because these molecules can easily cross the intestinal barriers and diffuse across the body, regulating inflammation and immune responses. Their impact is highlighted by the number of recent studies investigating SCFA administration to treat diseases [
140], and how this links fiber-rich diets and human health [
141,
142].
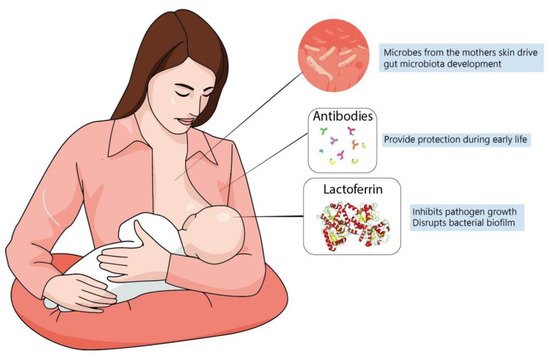
A newborn’s own microbiome development is started from their own mother’s; vaginal delivery is a major event in this process of microbial transfer. Babies delivered by cesarean section miss this step and may be more prone to have allergies, asthma and slower immune development [
143,
144]. Interestingly, it has been observed that the vaginal microbiome of preterm mothers differs from that of term mothers, with a reduced abundance of beneficial microbes, such as
Lactobacillus [
140]; it is possible that such imbalances contribute to proinflammatory cytokines and could increase preterm delivery probability [
140]. It has been speculated that breastfeeding is another important process shaping the newborn microbiome since skin-to-mouth contact during suckling allows the entry of beneficial microbes derived from the mother. Furthermore, the composition of colostrum and milk have a determinant role, since diet is the main driver in microbiome formation and maternal milk likely has a unique balance between lipids, proteins and oligosaccharides that contribute to microbiome development [
145] (
Figure 2). In fact, evidence suggests that formula-fed infants are more susceptible to allergies and have lower microbiome diversity. This is probably because formulas up to this time are more focused on macronutrient composition but do not fulfill the possible “functional” roles of maternal milk [
125]. In this context, lactoferrin has been shown to have many roles in regulating immune function, whether endogenously produced or from the diet.
Figure 2. Maternal milk is the optimal source of nutrients for newborns, even in the absence of consensus about its ideal composition. Dietary interventions during pregnancy can alter maternal milk composition and alter the infant’s immune system, playing a major role during brain development. The best understanding of molecules present in maternal milk is important not only for improving recommendation directives, but also for defining better formulas to be administered for infants at risk.
It is well known that nutrition is the main determinant in the establishment and maintenance of the gut microbiota [
146]. Gut maturation is directly associated with commensal microbes. Mucus, which provides a protective barrier against pathogens, is also dependent on the microbiome, which provides the building blocks for mucus production. Mucus also participates in intestinal transit, allowing better absorption of nutrients that are important not only to the organism but to the enterocytes themselves. The maternal vaginal microbiota has been associated with recurrent and increased risk of premature delivery [
140,
147], and the newborn microbiome seems to develop differently according to gestational maturity at birth [
148] predicting the risk of brain damage in preterm infants [
146,
149]. However, the exact mechanisms connecting the microbiome to brain health are largely unknown. In the following postnatal weeks, the microbial population develops in diversity as well as abundance and is mostly determined by nutrition. Breastfeeding has been associated with greater bacterial diversity in the gut and protection from allergies, colic and autoimmune conditions [
146,
150]. Interestingly, one of the major factors associated with NEC is the use of enteral formula instead of human milk [
151]. It is possible that, besides the mother’s own antibodies transferred to the infant, some macromolecules present in milk are not meant for the infant, but are to develop certain bacterial genera that can produce their own beneficial metabolites for the host, such as SCFA, amino acids and vitamins [
146,
149]. SCFA, such as butyrate and propionate, are the most studied bacterial metabolites, and have been demonstrated to be neuroprotective in neonatal ischemia [
152,
153] and to promote microglial maturation [
130,
154]. They are mostly produced by bacteria from oligosaccharides that are not digestible by human gut enzymes [
35].
Lactoferrin, as well as being present in milk and secretions, is also found in the granules of polymorphonuclear neutrophils [
155] which are recruited to the small intestine during gut inflammation and can release Lf as well as other iron-sequestering proteins, such as NGAL (lipocalin 2) [
156,
157,
158]. Iron sequestration from the milieu is a conserved strategy for fighting bacterial infections since most bacteria depend on Fe3+ as a cofactor for energy production and DNA replication [
156]. Therefore, iron depletion from the media hampers bacterial survival. Lactoferrin is specially designed for this function since its affinity for iron can be 300 times higher than that of serum transferrin [
159], and its affinity for iron is maintained even at low pH, such as in the small intestine [
159,
160]. Lf iron-scavenging activity is not its only role against bacterial infections, especially since some bacteria have evolved mechanisms to use other metals, such as zinc, instead of iron, or to secrete iron siderophores that have higher affinity than those of transferrins for bacterial uptake. It has been demonstrated that Lf can disrupt the formation of bacterial biofilms by
Pseudomonas aeruginosa, which is a key mechanism by which the bacteria resist antibiotic treatment [
158,
161,
162]. Another major role of Lf as an antimicrobial agent is its ability to bind to LPS [
92,
96], which has two important implications: it can disrupt bacterial membranes and also reduce LPS binding to the toll-like receptor 4 (TLR4) [
96,
163]. This latter property is important since it has been demonstrated that TLR4 is a major component in NEC-associated neurological sequelae [
164]. Therefore, it is possible that Lf can also prevent exacerbated inflammatory responses to bacterial infections.
In addition to its role as an antimicrobial agent, it has been demonstrated that Lf also has a role in immune function and has anti-inflammatory properties [
76,
88]. In mouse models it has been observed that oral administration of Lf increased cell counts of CD4 CD8 T-cells and NK cells in the lamina propria of the small intestine [
165,
166]. In addition, lactoferrin receptor expression has been observed in several subsets of immune cells, although its role is still unclear [
167,
168] due to the capacity of Lf to bind to a multitude of molecules. In vitro studies have demonstrated that Lf likely reduces the release of pro-inflammatory cytokines [
169]; however, it is necessary to investigate further what causes such effects, since Lf may be binding to immunostimulants, such as LPS and Poly I:C—in this scenario, such effects would only apply to that specific model and could not be extrapolated to other situations. When observing in vivo responses to lactoferrin, it is often difficult to differentiate its direct effect on immune response and its indirect effect through antimicrobial properties. Even if it is accepted that bacteria/viruses are not really involved in neuropathology in preterm infants as a primary cause of damage, many inflammatory complications due to hypoxic-ischemic events, and, in the most severe cases, neonatal sepsis and NEC, can present concomitant infections; thus, naturally, the effect of Lf on pathogenic bacteria will also modulate the inflammatory response. Another important consideration is the ability of Lf to bind to the Lipid A fraction of LPS, thus impairing its ability to bind to the CD14/TLR4 complex which promotes microbial pattern recognition and the inflammatory response [
93,
170]. Although TLR4 is an important element in the immune response for the detection of bacterial pathogens, its persistent stimulation by endotoxins can promote tissue damage through an exacerbated immune response. This has been demonstrated in NEC, in which TLR4 stimulation has been linked to both intestinal and cerebral damage in affected preterm infants [
164]. TLR4 activation by commensal microbes is a necessary process in the development of the immune system but is also involved in the pathology of infections and it is likely that Lf present in milk is particularly adapted to allowing LPS-TLR4 binding in the physiological range.
It was discussed previously how the gut microbiome can be important for neurodevelopment, and how dietary lactoferrin present in a mother’s milk could be helpful in shaping a beneficial microbiota, which in turn can prevent neurodegeneration; however, such a relationship still needs further study. AD patients present alterations in the gut microbiome when compared to controlled pairs; however it is important to point out that there is far from a consensus on what constitutes a “normal” microbiome. Case studies of fecal microbiota transplantation to patients with
Clostridium difficile gut infection that also show AD suggest that microbiome transplantation of a “healthy” form could improve cognitive symptoms [
171,
172]. Moreover, mouse models of AD, when colonized with the microbiome from their wild type controls, show alleviation of disease progression [
173]. Gastrointestinal symptoms, such as constipation, occur in Parkinson’s disease patients and probiotics are prescribed to alleviate those symptoms [
174]. It has also been suggested that the gut microbiome could be involved in the progression of the disease [
175,
176]. If so, lactoferrin could represent a dietary approach for treatment in these patients, by playing an active role in the gut, regulating iron homeostasis and acting as a source of dietary protein.
3. Lactoferrin for Preventing Neurodegeneration: A Promising Molecule?
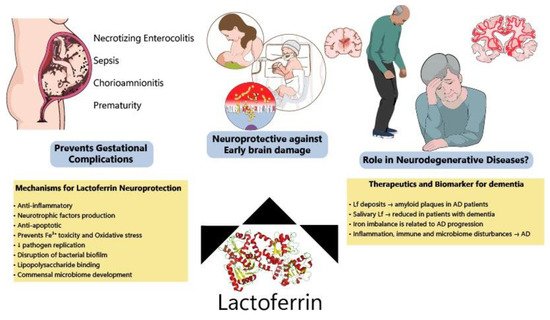
Although most Lf research is focused on the neonatal period due to the presence of Lf in mothers’ milk, new evidence has shown benefits of Lf in neurodegenerative diseases (
Figure 3). Lf is present in the CNS and can easily cross the blood-brain barrier from the peripheral circulation [
177,
178] since some of its putative receptors are found in the brain endothelium, such as LRP1 [
179,
180] (LDL receptor-related protein 1) and intelectin-1[
160], facilitating transcytosis into the brain parenchyma. For this reason, Lf structural motifs are being studied for application in liposomes for targeted CNS delivery of drugs, and these receptors could be used for mediating Lf-bound drugs entry into the CNS [
181,
182].
Figure 3. Protective effects of lactoferrin through the lifespan. Lf has been shown to prevent disturbances during pregnancy, decreasing the incidence of NEC, sepsis and prematurity among other complications. Allied to this, clinical and preclinical data have indicated that Lf modulates several pathways (ion scavenger, anti-inflammatory, anti-apoptotic and improving microbiome) protecting the developing brain. Lf has also been considered a potential biomarker of neurodegeneration and its mechanisms of action make it a good candidate to be tested for the prevention of neurodegenerative diseases.
Concerning its role in neurodegeneration, Lf seems to be increased around amyloid plaques in post-mortem Alzheimer’s disease (AD) brains [
171] and in APP-transgenic mice (specially in animals older than 20 months), which resemble the later stages of AD [
172]. The purpose of this accumulation around amyloid deposits remains unknown; recently, the APOE ε4 allele [
183], which is strongly related to the onset of AD, has been related to abnormalities in brain iron homeostasis and the induction of ferroptosis [
183,
184]. In this paper, although lactoferrin was not mentioned, other transferrins were studied. It is possible that early data regarding Lf around plaques could reflect the process of iron dysregulation, but this mechanism is still unknown. Recently, the neuroprotective effects of intranasal administration of lactoferrin have been tested in an APPswe/PS1DE9 transgenic mouse model of AD [
185]. Intranasal human Lf (hLf) reduced β-amyloid (Aβ) deposition and ameliorated cognitive decline in AD animals through ERK1/2-CREB and HIF-1α signaling pathways activation and consequent ADAM10 expression. The intranasal route is a relatively new and very interesting approach due to increased and target-specific availability in the CNS [
186].
Recently, Lf has been emerging as a putative biomarker for the early diagnosis of neurodegenerative diseases. Salivary Lf was reduced in patients with AD and MCI, as confirmed by PET imaging of amyloid using ([
18F]-florbentapir) [
187], and one study has suggested a negative association with amyloid load [
188,
189,
190]. However, there is far from a consensus on this, since another study failed to observe such a relationship [
191], due, at least partially, to more heterogeneous samples and the diagnostic criteria used in the different studies. The causal relationship between salivary lactoferrin (sLf) and AD is still unknown; however, it has been hypothesized that reduced sLf may reflect immune system abnormalities, which are commonly observed in AD patients [
188,
192]. A major caveat in these correlations is that immunological disturbances are observed across most neurodegenerative diseases [
192] and even chronic diseases that are not directly associated with the CNS show immunological alterations during their onset [
193]. In this sense, Gonzales–Sanchez data suggests that reduced sLf is specific to AD pathology; therefore, there must be a specific mechanism linking peripheral levels of Lf and AD physiopathology [
194].
In Parkinson’s disease models, Lf is increased in rat brains after MPTP administration [
195], which was suggested to be a protective tissue response, since iron dysregulation is observed in PD [
195,
196]. Iron can cause oxidative stress through the Fenton reaction, and it is possible that one of the Lf neuroprotective mechanisms is related to iron chelation. Treatment with oral hLf has been demonstrated to be neuroprotective in an MPTP rodent model of PD, as well as in MPP+ in vivo and in vitro models [
195,
197,
198]. Interestingly, these studies used both apo and holo forms of Lf and observed no difference between them. Thus, it is reasonable to assume that some benefits of Lf are independent of its iron scavenging properties [
186]. Data is still scarce in humans, with a limited number of studies using post-mortem tissue. Alzheimer’s [
199] and Parkinson’s disease mice models presented abnormal microbiomes when compared to wild type and improved their symptoms following antibiotic-induced elimination of the gut microbiome [
175,
200]; this new evidence suggests lactoferrin is a promising candidate for preventing the evolution of neurodegenerative diseases.
This entry is adapted from the peer-reviewed paper 10.3390/nu14142923