A key aspect of the inflammatory phenomenon is the involvement of costimulatory molecules expressed by antigen-presenting cells (APCs) and their ability to secrete cytokines to set instructions for adaptive immune response and to generate tolerance or inflammation. In a novel integrative approach, we present the evaluation of the kinetic expression of the membrane and soluble B7 costimulatory molecules CD86, ICOS-L, PDL1, PDL2, the transcription factor Interferon Regulatory Factor 4 (IRF4), and the cytokines produced by monocyte-derived dendritic cells (Mo-DCs) after challenging them with different concentrations of stimulation with E. coli lipopolysaccharide (LPS) for various lengths of time. The evaluation showed that the stimuli concentration and time of exposure to LPS are critical factors in modulating the dynamic expression pattern of membrane and soluble B7 molecules and cytokines.
- B7 molecules
- Dendritic cells
- Cytokines
- IRF4
1. Introduction
Dendritic cells (DCs) are the primary antigen-presenting cells (APCs) due to their ability to sense, capture, process, and present antigens to T cells. The initiation and polarization of adaptative immune responses are well orchestrated by a display of signals, including the expression of membrane-bound costimulatory molecules, soluble costimulatory molecules, and the secretion of cytokines by DCs.[1][2]
The best known is the B7 costimulatory family, belonging to the immunoglobulin superfamily (IgSF).[3] This family comprises costimulatory molecules that promote activation in T cells, such as CD86 and ICOS-L, and coinhibitory molecules that regulate the tolerance and suppression of functions, such as PDL1 and PDL2.[4] Interestingly, previous studies have proven that soluble forms of B7 molecules (sB7) can be detected in different tissues and supernatants of cell cultures.[5][6][7][8] This finding improves our understanding of the mechanism of action independently of cell contact interactions. However, it is still unclear whether a costimulatory or coinhibitory profile of sB7 molecules prevails under the steady or activation states of dendritic cells and what signals are conveyed to the T cells.
Critical regulators at the transcriptional level for B7 molecules expression have been reported. For example, CD80, CD86, and ICOS-L transcription factors include NF-kB and PU.1.[9][10][11] Recently, a novel transcription factor, the Interferon Regulatory Factor 4 (IRF4), was reported to be related to the expression of PDL1 and PDL2.[12][13][14]
B7 costimulatory molecules have received attention due to their relevance in clinical conditions such as allergies, autoimmunity, cancer, and transplantation.[15] Nevertheless, little is known about the behavior of these molecules in terms of both counterparts (stimulation and inhibition) in physiological conditions since most in vitro studies have evaluated only single molecules and single time points or have focused on samples of patients with specific clinical conditions. [16][17][18]
In addition to the expression of B7 molecules, autocrine-secreted cytokines play a relevant role in the expression of costimulatory molecules. Previous studies have suggested that IL-6 downregulates CD86- and HLA-associated molecules, impairing T-cell activation capacity in DCs;[19] meanwhile, TNF-α favors the expression of these molecules (in a viral context).[20] On the other hand, IFN-γ has been shown to directly regulate PDL1 and PDL2 expression in melanoma cells.[21] However, no studies have evaluated the profiles of cytokine and costimulatory molecules integrally.
2. Results
After a challenge with bacterial lipopolysaccharide (LPS), DCs begin B7 costimulatory molecules expression. PDL2 expression is regulated by transcription factor IRF4 favoring its expression at 12 and 48h, ICOSL reaches a maximum peak of expression at 24 h, and CD86 and PDL1 at 48h. Cytokines TNF-α, IL-6, and IL-10 secretion begin at early stimulation times; IL-2 and IFN-у reach a production peak in late times. Regarding soluble costimulatory molecules, sPDL1 and sPDL2 prevail over sCD86 at any evaluated condition. (See Figure 1)
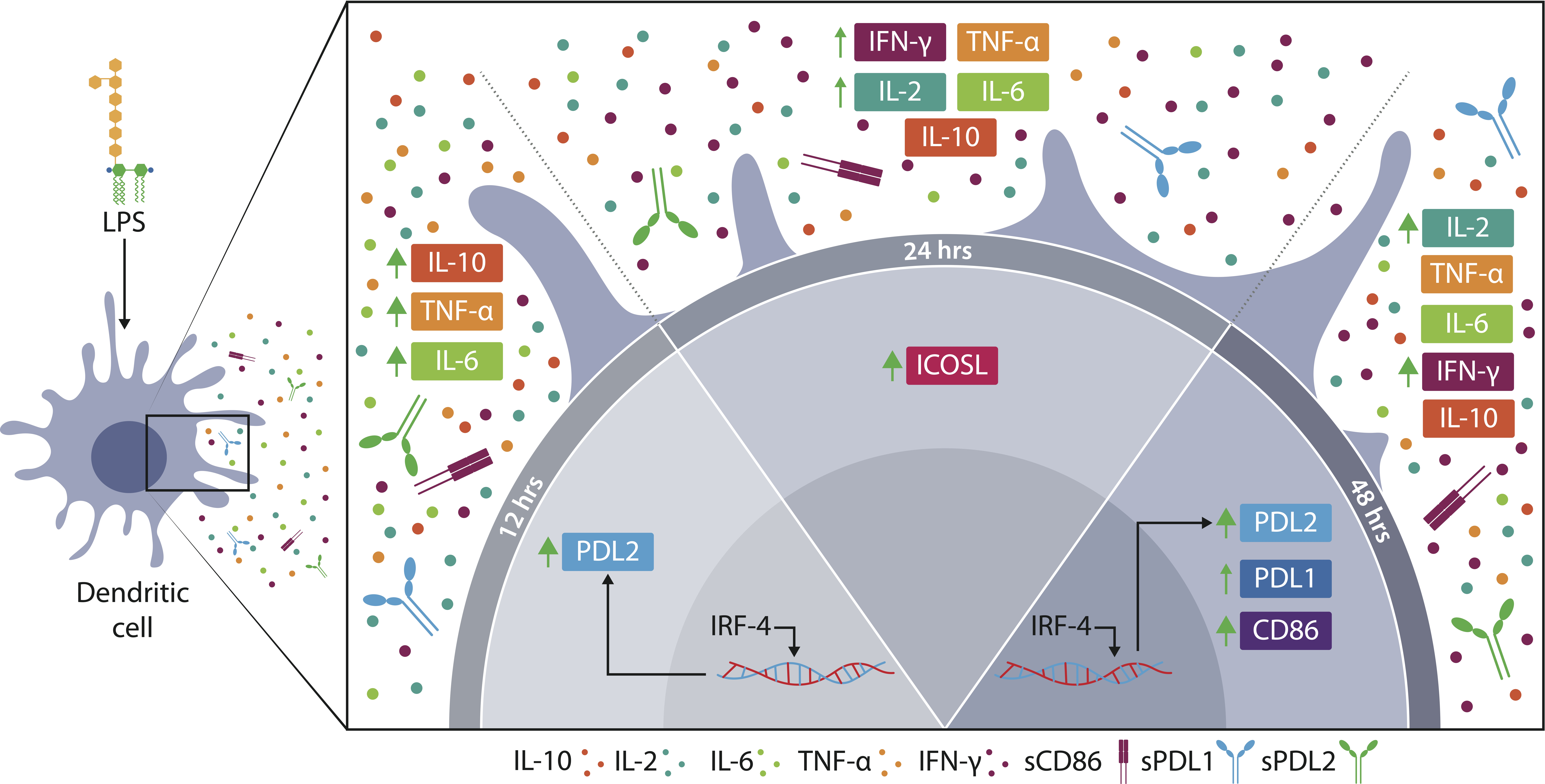
Figure 1. Graphical results showing the behavior of B7 costimulatory molecules. Costimulatory molecules express in a differential fashion after LPS stimulation. Dendritic cells tend to increase PDL2 during the early stages of antigen exposition favored by IRF-4; ICOS-L expression is notable at 24 h. At 48 h, peak expression of molecules CD86 and PDL1 is observed. PDL2 and IRF-4 expression increase again at 48h. Regarding cytokines, TNF-α, IL-6, and IL-10 were found to increase at early stages; meanwhile, IFN-γ and IL-2 had a production peak at 48 h. Finally, soluble costimulatory molecules exhibited constant behavior regardless of the time of exposition or antigen concentration. sPDL1 and sPDL2 had twice the concentration of sCD86
3. Conclusions
The data showed that Mo-DCs might be predisposed to respond silently and prone to initiating tolerogenic responses unless a threshold antigen concentration and stimulation time are reached, promoting B7 molecule activation and cytokine expression. The findings provide novel insights into dendritic cell function and allow a better understanding of its role in promoting or halting adaptative immune responses. Additionally, the knowledge of transcription factors, cytokines, and costimulatory molecules also provides a framework for future research in various clinical settings and the design of treatment strategies.
The entry is from https://doi.org/10.3390/biom12070955
References
- Xiangyun Yin; Shuting Chen; Stephanie C. Eisenbarth; Dendritic Cell Regulation of T Helper Cells. Annual Review of Immunology 2021, 39, 759-790, 10.1146/annurev-immunol-101819-025146.
- Mar Cabeza-Cabrerizo; Ana Cardoso; Carlos M. Minutti; Mariana Pereira da Costa; Caetano Reis e Sousa; Dendritic Cells Revisited. Annual Review of Immunology 2021, 39, 131-166, 10.1146/annurev-immunol-061020-053707.
- Karl S. Peggs; James P. Allison; Co-stimulatory pathways in lymphocyte regulation: the immunoglobulin superfamily. British Journal of Haematology 2005, 130, 809-824, 10.1111/j.1365-2141.2005.05627.x.
- Mary Collins; Vincent Ling; Beatriz M Carreno; The B7 family of immune-regulatory ligands. Genome Biology 2004, 6, 223-223, 10.1186/gb-2005-6-6-223.
- Minyoung Her; D Kim; M Oh; H Jeong; I Choi; Increased expression of soluble inducible costimulator ligand (ICOSL) in patients with systemic lupus erythematosus. Lupus 2009, 18, 501-507, 10.1177/0961203308099176.
- Christopher Montemagno; Anais Hagege; Delphine Borchiellini; Brice Thamphya; Olivia Rastoin; Damien Ambrosetti; Juan Iovanna; Nathalie Rioux-Leclercq; Camillio Porta; Sylvie Negrier; et al. Soluble forms of PD-L1 and PD-1 as prognostic and predictive markers of sunitinib efficacy in patients with metastatic clear cell renal cell carcinoma. OncoImmunology 2019, 9, 1846901, 10.1080/2162402x.2020.1846901.
- Takemichi Fukasawa; Ayumi Yoshizaki; Satoshi Ebata; Kouki Nakamura; Ryosuke Saigusa; Shunsuke Miura; Takashi Yamashita; Megumi Hirabayashi; Yohei Ichimura; Takashi Taniguchi; et al. Contribution of Soluble Forms of Programmed Death 1 and Programmed Death Ligand 2 to Disease Severity and Progression in Systemic Sclerosis. Arthritis & Rheumatology 2017, 69, 1879-1890, 10.1002/art.40164.
- Pascale Jeannin; Giovanni Magistrelli; Jean-Pierre Aubry; Gersende Caron; Jean-François Gauchat; Toufic Renno; Nathalie Herbault; Liliane Goetsch; Aline Blaecke; Pierre-Yves Dietrich; et al. Soluble CD86 Is a Costimulatory Molecule for Human T Lymphocytes. Immunity 2000, 13, 303-312, 10.1016/s1074-7613(00)00030-3.
- Deena M. Maurer; Juraj Adamik; Patricia M. Santos; Jian Shi; Michael R. Shurin; John M. Kirkwood; Walter J. Storkus; Lisa H. Butterfield; Dysregulated NF-κB–Dependent ICOSL Expression in Human Dendritic Cell Vaccines Impairs T-cell Responses in Patients with Melanoma. Cancer Immunology Research 2020, 8, 1554-1567, 10.1158/2326-6066.cir-20-0274.
- Shunsuke Kanada; Chiharu Nishiyama; Nobuhiro Nakano; Ryuyo Suzuki; Keiko Maeda; Mutsuko Hara; Nao Kitamura; Hideoki Ogawa; Ko Okumura; Critical role of transcription factor PU.1 in the expression of CD80 and CD86 on dendritic cells. Blood 2011, 117, 2211-2222, 10.1182/blood-2010-06-291898.
- Gang-Ming Zou; Wen-Yang Hu; LIGHT regulates CD86 expression on dendritic cells through NF-κB, but not JNK/AP-1 signal transduction pathway. Journal of Cellular Physiology 2005, 205, 437-443, 10.1002/jcp.20420.
- Yan Gao; Simone A. Nish; Ruoyi Jiang; Lin Hou; Paula Licona-Limón; Jason S. Weinstein; Hongyu Zhao; Ruslan Medzhitov; Control of T Helper 2 Responses by Transcription Factor IRF4-Dependent Dendritic Cells. Immunity 2013, 39, 722-732, 10.1016/j.immuni.2013.08.028.
- Bryan Vander Lugt; Jeremy Riddell; Aly A. Khan; Jason A. Hackney; Justin Lesch; Jason Devoss; Matthew T. Weirauch; Harinder Singh; Ira Mellman; Transcriptional determinants of tolerogenic and immunogenic states during dendritic cell maturation. Journal of Cell Biology 2017, 216, 779-792, 10.1083/jcb.201512012.
- Jing-Ping Zhang; Zhihui Song; Hong-Bo Wang; Lang Lang; Yuan-Zhong Yang; Wenming Xiao; Daniel E. Webster; Wei Wei; Stefan K. Barta; Marshall E. Kadin; et al. A novel model of controlling PD-L1 expression in ALK+ anaplastic large cell lymphoma revealed by CRISPR screening. Blood 2019, 134, 171-185, 10.1182/blood.2019001043.
- Henry Velazquez-Soto; Fernanda Real; Maria C Jiménez-Martínez; Historical evolution, overview, and therapeutic manipulation of co-stimulatory molecules. World Journal of Immunology 2022, 12, 1-8, 10.5411/wji.v12.i1.1.
- Alexandra Aicher; Martha Hayden-Ledbetter; William A. Brady; Antonio Pezzutto; Guenther Richter; Dario Magaletti; Sonya Buckwalter; Jeffrey A. Ledbetter; Edward A. Clark; Characterization of Human Inducible Costimulator Ligand Expression and Function. The Journal of Immunology 2000, 164, 4689-4696, 10.4049/jimmunol.164.9.4689.
- Janine Obendorf; Pablo Renner Viveros; Michael Fehlings; Christian Klotz; Toni Aebischer; Ralf Ignatius; Increased expression of CD25, CD83, and CD86, and secretion of IL-12, IL-23, and IL-10 by human dendritic cells incubated in the presence of Toll-like receptor 2 ligands and Giardia duodenalis. Parasites & Vectors 2013, 6, 317-317, 10.1186/1756-3305-6-317.
- Marta Rodríguez-García; Filippos Porichis; Olivier G. De Jong; Karen Levi; Thomas J. Diefenbach; Jeffrey D. Lifson; Gordon J. Freeman; Bruce D. Walker; Daniel E. Kaufmann; Daniel G. Kavanagh; et al. Expression of PD-L1 and PD-L2 on human macrophages is up-regulated by HIV-1 and differentially modulated by IL-10. Journal of Leukocyte Biology 2010, 89, 507-515, 10.1189/jlb.0610327.
- Yosuke Ohno; Hidemitsu Kitamura; Norihiko Takahashi; Junya Ohtake; Shun Kaneumi; Kentaro Sumida; Shigenori Homma; Hideki Kawamura; Nozomi Minagawa; Susumu Shibasaki; et al. IL-6 down-regulates HLA class II expression and IL-12 production of human dendritic cells to impair activation of antigen-specific CD4+ T cells. Cancer Immunology, Immunotherapy 2016, 65, 193-204, 10.1007/s00262-015-1791-4.
- Jose M. Trevejo; Michael W. Marino; Nicola Philpott; Regis Josien; Elizabeth C. Richards; Keith B. Elkon; Erik Falck-Pedersen; TNF-α-dependent maturation of local dendritic cells is critical for activating the adaptive immune response to virus infection. Proceedings of the National Academy of Sciences 2001, 98, 12162-12167, 10.1073/pnas.211423598.
- Angel Garcia-Diaz; Daniel Sanghoon Shin; Blanca Homet Moreno; Justin Saco; Helena Escuin-Ordinas; Gabriel Abril Rodriguez; Jesse M. Zaretsky; Lu Sun; Willy Hugo; Xiaoyan Wang; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Reports 2017, 19, 1189-1201, 10.1016/j.celrep.2017.04.031.
