Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Glioblastoma (GBM) is an aggressive primary brain tumor that is associated with a poor prognosis and quality of life. The standard of care consists of surgery followed by radiotherapy (RT), concomitant and adjuvant temozolomide (TMZ), and tumor treating fields (TTF). Factors such as tumor hypoxia and the presence of glioma stem cells contribute to the radioresistant nature of GBM. Small molecules and immunotherapy agents that have been studied in conjunction with RT in clinical trials are presented herein.
- glioblastoma
- radioresistance
- radiosensitizer
- glioma stem cell
- tumor hypoxia
1. Introduction
The rationale behind combining radiation and chemotherapy originates from the Steel paradigm [1]. Steel et al. proposed that synergy is driven by (1) spatial cooperation, (2) toxicity independence, (3) protection of normal tissues, and (4) enhancement of tumor response. The enhancement effect can be driven by inhibiting radiation-induced damage, reoxygenation following treatment, and/or improved drug access following radiotherapy (RT).
Early studies demonstrated some chemotherapeutics such as cisplatin have the ability to sensitize tumor cells to RT, leading to greater radiation efficacy [2]. More recently, radiosensitizers have been developed that work through a variety of mechanisms: (1) Suppression of intracellular thiols or other radioprotective substances, (2) radiation-induced formation of cytotoxic substances via radiolysis of the sensitizer, (3) inhibition of the post-radiation cellular repair processes, (4) structural incorporation of thymine analogues into intracellular DNA, and (5) oxygen mimetic sensitizers [3][4].
Although other disease sites have found success with radiosensitizers, GBM has been particularly challenging due to its anatomic location (e.g., located beyond the blood–brain barrier), cell heterogeneity (e.g., cancer stem cells, tumor microtubes), and increased proliferation rate [5]. To date, temozolomide (TMZ) is the most effective and widely used radiosensitizer in the treatment of GBM. TMZ increases the number of RT-induced double-strand DNA breaks as a result of a decrease in DNA repair capacity [6][7]. The content herein will focus on other small molecule and immunotherapy agents that have shown preclinical promise.
2. Pyrmidine Analogues
Gemcitabine is a difluoro-pyrimidine analog that is phosphorylated and incorporated into the DNA and RNA of cancer cells, leading to chain termination (Figure 1) [8]. The radiosensitizing effects of gemcitabine result from the depletion of phosphorylated deoxynucleotides and cell-cycle redistribution into the S-phase [9][10][11]. To date, gemcitabine has demonstrated activity in breast, ovarian, non-small cell lung, pancreatic, and bladder cancers [12].
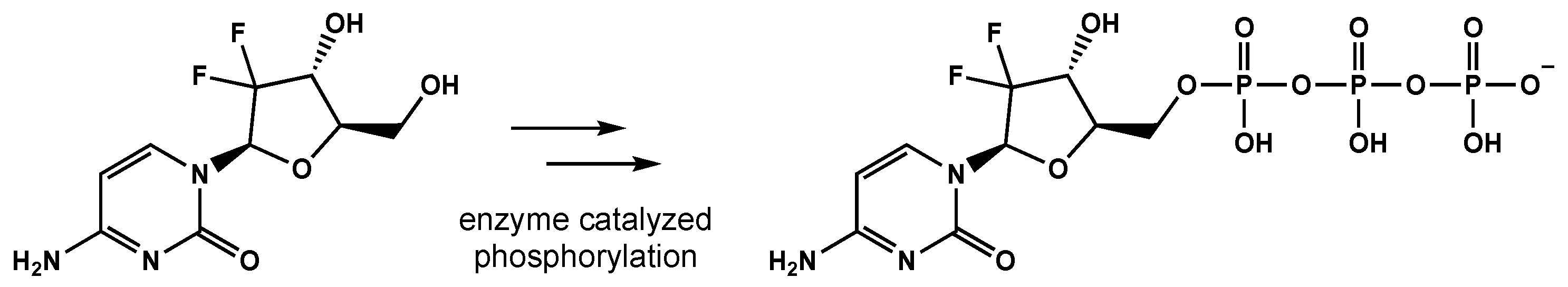
Figure 1. The conversion of gemcitabine to gemcitabine-5′-triphosphate before being incorporated into DNA and RNA, eventually leading to strand termination.
In vitro studies have determined the gemcitabine administration schedule is essential for maximal radiosensitization. Gemcitabine achieved radiosensitization with long exposure (24 h) to low gemcitabine concentrations or brief treatments with increased concentrations [13]. Maraveyas et al conducted a phase I study in brain metastases patients evaluating the maximum tolerated dose of concomitant gemcitabine and RT [14]. A phase I study then evaluated gemcitabine with concomitant RT in newly diagnosed GBM patients [15]. In this study, gemcitabine was delivered at 10 mg/m2/min on a weekly basis for 6 weeks 24 to 72 h prior to concomitant RT (60 Gy in 30 fractions) with the identification of dose-limiting toxicity and maximum tolerated dose as the primary end-points. Based on this study, 175 mg/m2/weekly was recommended for further evaluation in a phase II study. Twenty-three patients were enrolled in their phase II study and found concomitant RT and gemcitabine were well-tolerated with few severe adverse events [16]. Additionally, disease control was observed in both methylated and unmethylated MGMT promoter tumors (91% and 77.5%, respectively).
To date, there is evidence gemcitabine has the ability to cross the blood–brain barrier [17], but some drawbacks include its short plasma half-life, adverse effects related to high drug doses (e.g., myelosuppression, thrombocytopenia, edema), and resistance related to altered expression of nucleoside transporters, kinases, and enzymes [5]. Researchers are currently exploring various delivery strategies for overcoming these limitations (e.g., encapsulation, conjugation, and convention-enhanced delivery) [18][19][20]. For example, Guo et al. surmised gemcitabine coupled to a peripheral benzodiazepine receptor ligand may enhance brain tumor uptake [19]. In their xenograft model, the conjugated agent resulted in a two-fold enhancement in brain tumor selectively compared with gemcitabine alone.
3. Kinase Inhibitors
3.1. Tyrosine Kinase Inhibitors
Tyrosine kinase inhibitors (TKIs) block receptor signaling, inhibiting cell growth and proliferation. Since the approval of imatinib in 2001 for the treatment of chronic myeloid leukemia, there has been an explosion of TKI utilization in multiple types of cancer [21]. TKIs have incredible potential for treating GBM considering their ability to block cell signaling pathways such as EGFR, PDGFR, and VEGF/VEGFR.
EGFR amplification is seen in approximately 40% of GBM cases, correlating with decreased apoptosis, increased cellular proliferation, tumorigenesis, and radioresistance [22][23][24]. Erlotinib is a TKI that has demonstrated activity against the EGFRvIII mutant receptor in preclinical models [25]. Erlotinib is a quinazoline derivative that reversibly inhibits autophosphorylation of EGFR (Figure 2) [26]. Various phase II studies have evaluated the efficacy of erlotinib with concurrent RT and TMZ, but a range of survival and toxicity outcomes have been reported. The first trial included 97 GBM patients who were given erlotinib alone for 1 week followed by concurrent erlotinib, TMZ (75 mg/m2 daily), and RT (60 Gy total) [27]. Patients had a median survival time of 15.3 months, but there was no significant benefit compared to RT/TMZ arm of the European Organization for Research and Treatment of Cancer/National Cancer Institute of Canada trial 26981/22981. Furthermore, molecular subset analysis did not reveal that EGFR amplification was predictive of survival. Another phase II trial included 27 newly diagnosed GBM patients [28]. In this trial, erlotinib was determined to be not efficacious with unacceptable toxicity (grade 3 and 4 toxicities including thrombocytopenia, anemia, lymphopenia, fatigue, and febrile neutropenia). Numerous clinical trials have evaluated other EGFR TKIs (e.g., gefitinib, afatinib) in GBM patients [28][29][30]. Unfortunately, all EGFR TKIs to date have failed to show efficacy in GBM. Researchers hypothesize the lack of efficacy may be due to poor blood–brain barrier penetration, altered signaling pathways, and/or genetic heterogeneity [31].
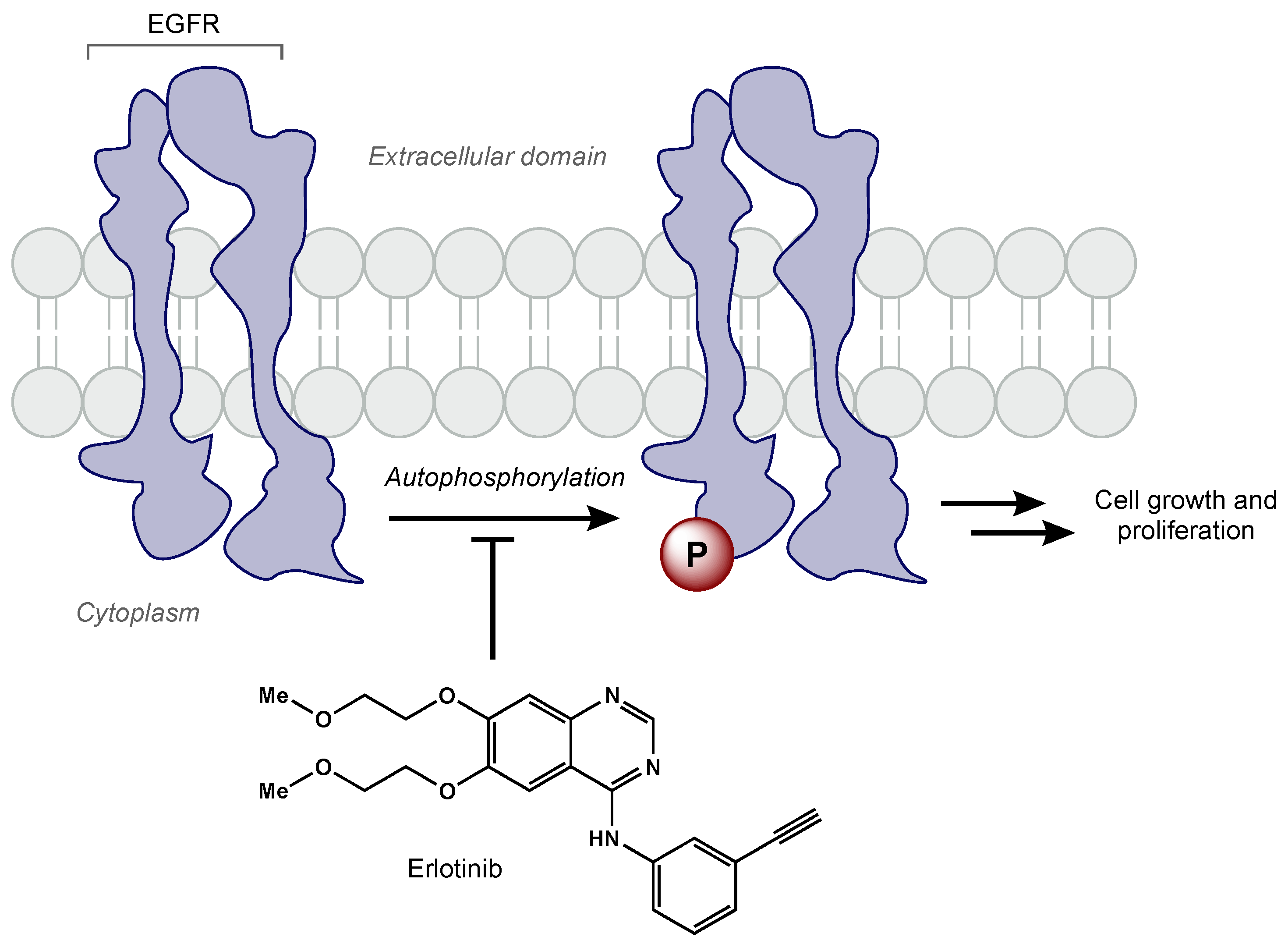
Figure 2. Erlotinib reversibly inhibits EGFR tyrosine kinase activity, which prevents cell growth and proliferation of cancer cells.
Recently, preclinical studies have tested osimertinib, a third-generation EGFR TKI, in various GBM cell lines and mice [32]. Liu et al. showed osimertinib inhibited GBM cell growth ten-fold higher than first-generation EGFR inhibitors and prolonged survival in GBM-bearing mice.
3.2. mTOR Inhibitors
Rapamycin (mTOR) is a protein kinase that is an important regulator of cell survival and proliferation [33]. mTOR is localized in two distinct multi-protein complexes called mTORC1 and mTORC2 [34]. Previous research efforts have uncovered the critical role of mTOR in GBM pathogenesis [35][36]. Recent studies have shown glioma stem cells (GSCs) can activate the mTOR pathway in microglia, creating an immunosuppressive microenvironment that promotes GBM proliferation [37].
Temsirolimus was the first mTORC1 inhibitor investigated in clinical trials (Figure 3). Temsirolimus has been shown to target GICs in preclinical studies, but has failed to demonstrate clinical benefit [38]. Sirolimus, another mTOR inhibitor, also had promising preclinical results, but failed to improve survival, despite being well tolerated [39]. Everolimus, another rapamycin derivative, is a downstream regulator of the EGFR/phosphatidylinositol-3 kinase (PI3K) pathway that has demonstrated radiosensitization in preclinical studies [40]. The North Central Cancer Treatment Group (NCCTG) conducted a phase II trial where weekly everolimus was given concurrently with RT plus TMZ. Ma et al. reported moderate toxicity and survival rates similar to historical phase II trials [41]. The RTOG 0913 trial randomized 171 GBM patients to receive RT with concurrent and adjuvant TMZ with or without daily everolimus (10 mg) [42]. Chinnaiyan and colleagues reported no significant difference in PFS and inferior OS for the patients that received everolimus. There was a significant increase in treatment-related toxicity in patients that received everolimus compared with the control arm; in the experimental arm, there were greater grade 4 and 5 events (30.6% and 11.8%, respectively) than in the control arm (17.9% and 1.3%, respectively).
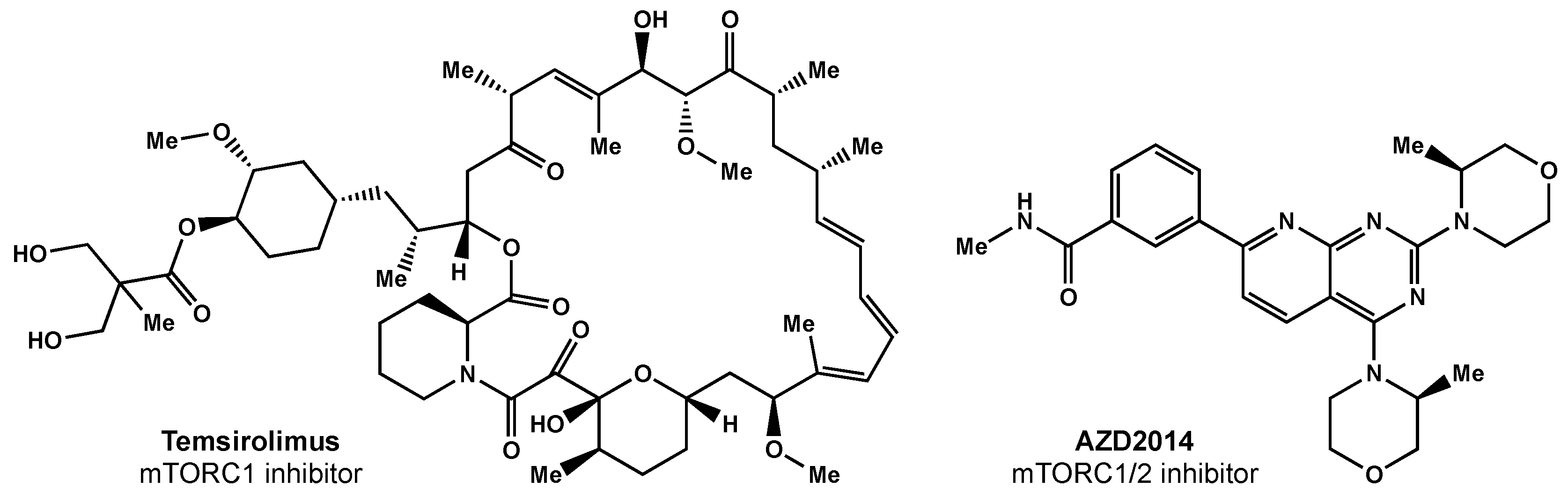
Figure 3. Small molecule inhibitors of mTOR.
Researchers surmise that the lack of efficacy may be related to everolimus only selectively inhibiting mTORC1 alone; studies have shown this inhibition can result in increased AKT activation via the activation of mTORC2 [43]. There are ongoing efforts focused on designing a suitable mTORC1/2 inhibitor [44]. AZD2014 is an inhibitor of mTORC1 and mTORC2 (Figure 3) that has shown radiosensitivity in preclinical studies [44] and is being evaluated in a phase I trial (NCT02619864).
4. Oxygen Mimetics
Conventional RT induces DNA damage via the formation of free radicals generated from the radiolysis of water. Reductants such as glutathione are able to neutralize the radical-induced damage within the cells, but if oxygen is present, this process is prevented, and the damage becomes irreversible. Hypoxic areas of solid tumors greatly hamper the effects of RT, leading researchers to seek oxygen mimetics [45].
Small molecules have been utilized as oxygen mimetics for decades [46] and have historically contained nitro groups that act as electron acceptors [47]. One of the earlier compounds that demonstrated radiosensitizing effects is misonidazole. Although imidazole showed radiosensitizing effects in murine tumors, its lipophilic properties prevented successful translation into clinical trials [48]. Derivatives of misonidazole were tested, and etanidazole had superior hydrophilicity due to the addition of an amide and hydroxyl group [49]. RRx-001 is a dinitro compound originally used as an ingredient in rocket fuel that has demonstrated radiosensitization properties with low toxicity [50]. Currently, RRx-001 is being evaluated in a phase I trial for patients with newly diagnosed glioblastoma (NCT02871843).
Hydrogen peroxide has been explored as a route for enhancing the efficacy of RT [51] and has been evaluated in a phase I/II trial (NCT02757651). Several studies also explored nicotinamide in combination with carbogen breathing in accelerated RT (ARCON) for various tumor types, including laryngeal, bladder, and head and neck [52][53][54][55]. Nicotinamide is a vasoactive agent that decreases perfusion-limited hypoxia, and carbogen (98% oxygen and 2% CO2) decreases diffusion-limited hypoxia [56]. Transfusion with red blood cells, in theory, should increase the oxygen supply of tumor cells, but this has failed to demonstrate benefit [57].
5. Reductive Agents
Bioreductive agents such as quinones and transition metal complexes have garnered attention due to their synergistic effects with RT and their preferential cytotoxicity towards hypoxic cells. Tirapazamine is a pro-drug that can be reduced to a free radical, leading to single- and double-strand DNA breaks under hypoxic environments (Figure 4) [58]. Del Rowe et al conducted a phase II study with RT plus tirapazamine [59]. Although toxicity was acceptable, tirapazamine demonstrated no survival benefit.
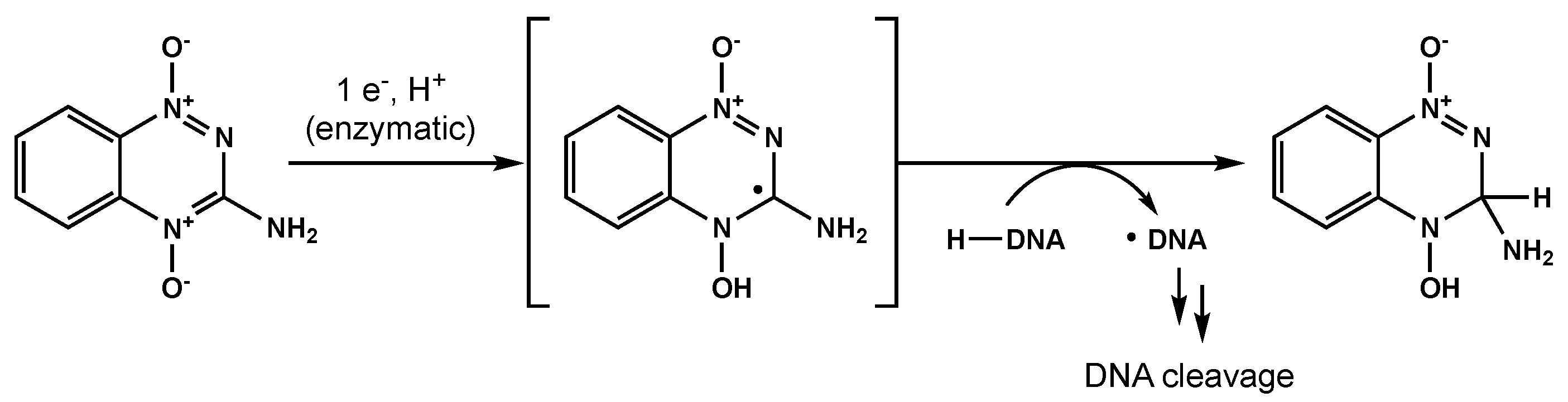
Figure 4. One proposed mechanism for tirapazamine-mediated DNA cleavage under hypoxic conditions.
An analogue of tirapazamine is SN30000 with more favorable diffusion properties and is currently under development [60][61]. Other analogues such as nimorazole demonstrated efficacy in several trials and are currently used in the treatment of head and neck cancers in Denmark [62].
6. Histone Deactylase Inhibitors
Histone deacetylases (HDACs) are enzymes that regulate chromatin structure and gene expression via deacetylation of histones and other cytoplasmic and nuclear proteins [63]. Valproic acid, an HDAC inhibitor, has demonstrated increased RT sensitivity in vitro and in vivo. Although the mechanism is unclear, researchers have proposed radiosensitization may be due to the inhibition of chromatin remodeling [64]. Krauze and colleagues conducted a phase II study evaluating the addition of valproic acid to RT plus TMZ [65]. Median OS was 29.6 months (range, 21–63.8 months), PFS was 10.5 months (range, 6.8–51.2 months), and the addition of valproic acid was generally well tolerated. The utilization of valproic acid remains controversial, though, after a pooled analysis found valproic acid at antiepilepsy doses was not associated with improved PFS or OS [66]. Vorinostat is another HDAC inhibitor that has been explored in one phase I/II trial, but failed to meet its primary efficacy end point [67].
7. Targeting DNA Repair Pathways
Ataxia-telangiectasia-mutated (ATM) serine/threonine protein kinase plays a role in the repair of DNA double-strand breaks [68]. ATM activation is induced within minutes of irradiation, and GSCs are particularly resistant following increased activation of ATM [69][70]. Carruthers et al. demonstrated GSCs display a robust intrinsic phosphor-ATM signal that is further enhanced following irradiation [70]. Other studies have found GBM cell lines and GSCs are radiosensitized by ATM inhibition [71].
Recently, medicinal chemists have developed a novel series of ATM inhibitors that demonstrate excellent efficacy and good pharmacokinetic properties [72]. AZD0156 was selected as a suitable candidate for clinical trials (NCT02588105). Further structure–activity relationship lead optimization led to the development of AZD1390, an orally bioavailable inhibitor with greater blood–brain barrier penetrance (Figure 5) [68]. A phase I clinical trial (NCT03423628) is currently recruiting GBM patients for the evaluation of AZD1390 in combination with RT.
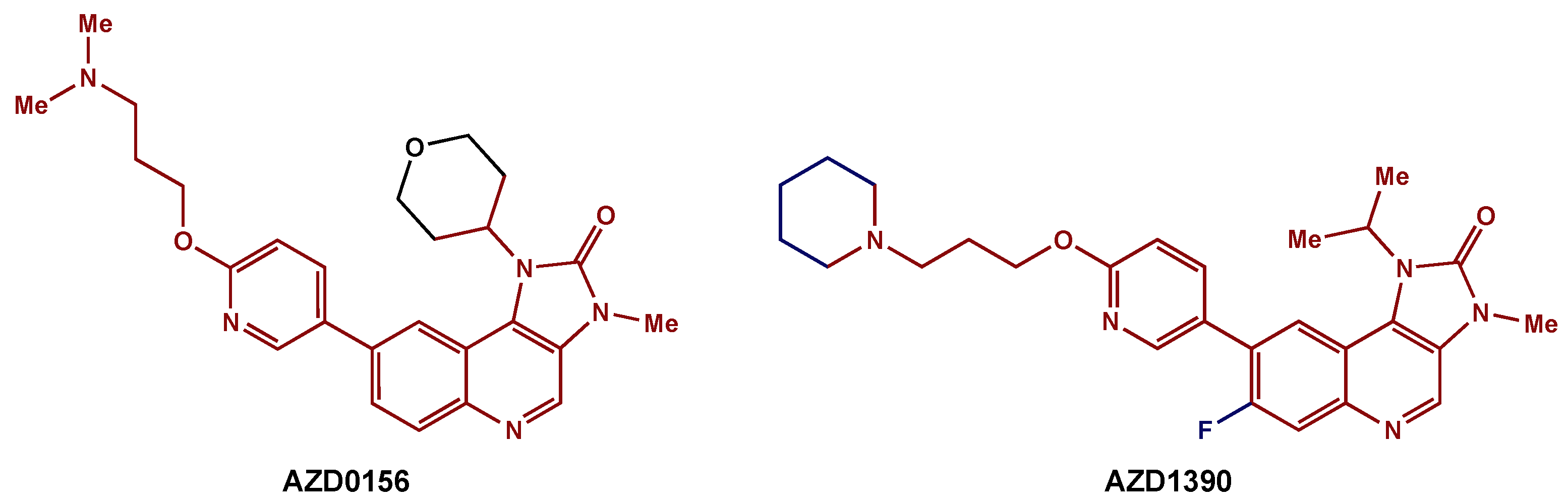
Figure 5. ATM inhibitors: AZD0156 was modified to AZD1390, an orally available compound with greater blood–brain barrier penetrance. The preserved core is highlighted in red.
8. Allosteric Modifiers of Hemoglobin
Phenoxyacetic acid compounds were initially utilized as lipid-lowering drugs but later were found to stabilize the T state of hemoglobin [73]. In a phase III trial, efaproxiral, a phenoxyacetic acid analogue, was found to enhance the effect of RT in patients with advanced lung cancer [74]. Kleinberg et al. then surmised GBM patients may benefit from the radio-enhancing effects of efaproxiral because GBM tumors are known to be hypoxic [75] and radioresistant [76]. Although the results were promising, a large dose was needed to reach a therapeutic effect, and long-term dose-related side effects are a concern [77].
9. Immunotherapy
9.1. Anti-Angiogenic Therapy
VEGF inhibitors such as bevacizumab have been explored with the hope of targeting angiogenesis [78]. Chinot et al conducted a phase III trial evaluating the addition of bevacizumab to RT (2 Gy per fraction, total of 60 Gy) plus TMZ (75 mg/m2/day for 6 weeks) in patients with newly diagnosed GBM [79]. Although there was increased PFS in the bevacizumab group vs. placebo (10.6 months vs. 6.2 months), there was not a significant difference in OS. Furthermore, there were higher rates of adverse events with bevacizumab than with the placebo. Gilbert et al. also conducted a phase III randomized trial investigating the addition of bevacizumab to RT and TMZ [80]. Their study also demonstrated improved PFS (10.7 months vs. 7.3), although the difference was not significant according to the pre-specified alpha level (p < 0.004). The authors also noted a slight increase in adverse events and, over time, a decreased quality of life and neurocognitive function in the bevacizumab group.
9.2. Immune Checkpoint Inhibitors
Cancer immunotherapy is based on the concept of immunosurveillance where the immune system can actively detect and eliminate cancer cells, but some tumor cells are able to develop the ability to evade the immune system through immunoediting [81]. Immunoediting is a process where the immune system can both constrain and promote tumor progression [82]. Researchers propose this complex dynamic occurs in three phases: Elimination (the immune system can recognize and kill transformed cells), equilibrium (tumor growth is limited), and escape (edited tumors can grow, unrestrained) [83].
Immunotherapy aims to overcome this immunoresistance with immune checkpoint inhibitors (ICIs) [84]. Immune checkpoints are crucial for self-tolerance, and cancer cells exploit this feature via the upregulation of various pathways (e.g., PD-1/PD-L1, CTLA-4) [85]. Over the past decade, ICIs have revolutionized the treatment of solid tumors and have created renewed excitement within the field of cancer immunotherapy [86].
Although radiation is known to create DNA damage, several studies have suggested the immune system may impact the efficacy of radiation [87]. The exact mechanisms dictating how radiation and the immune system interact are still unclear, but data have revealed CD8 T cells play a key role [88][89]. In theory, combining RT and checkpoint blockage immunotherapy should increase radiosensitization.
Immune checkpoint inhibitors were believed to affect the tumor microenvironment by enhancing the expression of cytokine and chemokine release, which increases immune cell infiltration [90][91]. Anti-PD-1 monoclonal antibodies have had success in the setting of hepatocellular carcinoma, non-small cell lung cancer, renal cell carcinoma, melanoma, and a variety of other solid tumors [85]. Anti-CTLA-4 monoclonal antibodies have also demonstrated a survival benefit for metastatic melanoma [92].
Unfortunately, the addition of ICIs to GBM treatment has led to disappointing initial results.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10071763
References
- Gordon Steel, G.; Peckham, M.J. Exploitable Mechanisms in Combined Radiotherapy-Chemotherapy: The Concept of Additivity. Int. J. Radiat. Oncol. 1979, 5, 85–91.
- Gill, M.R.; Vallis, K.A. Transition Metal Compounds as Cancer Radiosensitizers. Chem. Soc. Rev. 2019, 48, 540–557.
- Fowler, J.F.; Adams, G.E.; Denekamp, J. Radiosensitizers of Hypoxic Cells in Solid Tumours. Cancer Treat. Rev. 1976, 3, 227–256.
- Adams, G.E. Chemical radiosensitization of hypoxic cells. Br. Med. Bull. 1973, 29, 48–53.
- Bastiancich, C.; Bastiat, G.; Lagarce, F. Gemcitabine and Glioblastoma: Challenges and Current Perspectives. Drug Discov. Today 2018, 23, 416–423.
- Mrugala, M.M.; Chamberlain, M.C. Mechanisms of Disease: Temozolomide and Glioblastoma—Look to the Future. Nat. Clin. Pract. Oncol. 2008, 5, 476–486.
- Chakravarti, A.; Erkkinen, M.G.; Nestler, U.; Stupp, R.; Mehta, M.; Aldape, K.; Gilbert, M.R.; Black, P.M.L.; Loeffler, J.S. Temozolomide-Mediated Radiation Enhancement in Glioblastoma: A Report on Underlying Mechanisms. Clin. Cancer Res. 2006, 12, 4738–4746.
- Lund, B.; Kristjansen, P.E.G.; Hansen, H.H. Clinical and Preclinical Activity of 2′,2′- Difluorodeoxycytidine (Gemcitabine). Cancer Treat. Rev. 1993, 19, 45–55.
- Lawrence, T.S.; Blackstock, A.W.; McGinn, C. The Mechanism of Action of Radiosensitization of Conventional Chemotherapeutic Agents. Semin. Radiat. Oncol. 2003, 13, 13–21.
- Pauwels, B.; Korst, A.E.C.; Pattyn, G.G.O.; Lambrechts, H.A.J.; Van Bockstaele, D.R.; Vermeulen, K.; Lenjou, M.; de Pooter, C.M.J.; Vermorken, J.B.; Lardon, F. Cell Cycle Effect of Gemcitabine and Its Role in the Radiosensitizing Mechanism in Vitro. Int. J. Radiat. Oncol. 2003, 57, 1075–1083.
- Latz, D.; Fleckenstein, K.; Eble, M.; Blatter, J.; Wannenmacher, M.; Weber, K.J. Radiosensitizing Potential of Gemcitabine (2′,2′-Difluoro-2′-Deoxycytidine) within the Cell Cycle in Vitro. Int. J. Radiat. Oncol. 1998, 41, 875–882.
- Ciccolini, J.; Serdjebi, C.; Peters, G.J.; Giovannetti, E. Pharmacokinetics and Pharmacogenetics of Gemcitabine as a Mainstay in Adult and Pediatric Oncology: An EORTC-PAMM Perspective. Cancer Chemother. Pharmacol. 2016, 78, 1–12.
- Lawrence, T.; Eisbruch, A.; Shewach, D. Gemcitabine-Mediated Radiosensitization. Semin. Oncol. 1997, 24 (Suppl. 7), 24–28.
- Maraveyas, A.; Sgouros, J.; Upadhyay, S.; Abdel-Hamid, A.-H.; Holmes, M.; Lind, M. Gemcitabine Twice Weekly as a Radiosensitiser for the Treatment of Brain Metastases in Patients with Carcinoma: A Phase I Study. Br. J. Cancer 2005, 92, 815–819.
- Fabi, A.; Mirri, A.; Felici, A.; Vidiri, A.; Pace, A.; Occhipinti, E.; Cognetti, F.; Arcangeli, G.; Iandolo, B.; Carosi, M.A.; et al. Fixed Dose-Rate Gemcitabine as Radiosensitizer for Newly Diagnosed Glioblastoma: A Dose-Finding Study. J. Neurooncol. 2008, 87, 79–84.
- Metro, G.; Fabi, A.; Mirri, M.A.; Vidiri, A.; Pace, A.; Carosi, M.; Russillo, M.; Maschio, M.; Giannarelli, D.; Pellegrini, D.; et al. Phase II Study of Fixed Dose Rate Gemcitabine as Radiosensitizer for Newly Diagnosed Glioblastoma Multiforme. Cancer Chemother. Pharmacol. 2009, 65, 391.
- Sigmond, J.; Honeywell, R.J.; Postma, T.J.; Dirven, C.M.F.; de Lange, S.M.; van der Born, K.; Laan, A.C.; Baayen, J.C.A.; Van Groeningen, C.J.; Bergman, A.M.; et al. Gemcitabine Uptake in Glioblastoma Multiforme: Potential as a Radiosensitizer. Ann. Oncol. 2009, 20, 182–187.
- Degen, J.W.; Walbridge, S.; Vortmeyer, A.O.; Oldfield, E.H.; Lonser, R.R. Safety and Efficacy of Convection-Enhanced Delivery of Gemcitabine or Carboplatin in a Malignant Glioma Model in Rats. J. Neurosurg. 2003, 99, 893–898.
- Guo, P.; Ma, J.; Li, S.; Guo, Z.; Adams, A.L.; Gallo, J.M. Targeted Delivery of a Peripheral Benzodiazepine Receptor Ligand-Gemcitabine Conjugate to Brain Tumors in a Xenograft Model. Cancer Chemother. Pharmacol. 2001, 48, 169–176.
- Wang, C.-X.; Huang, L.-S.; Hou, L.-B.; Jiang, L.; Yan, Z.-T.; Wang, Y.-L.; Chen, Z.-L. Antitumor Effects of Polysorbate-80 Coated Gemcitabine Polybutylcyanoacrylate Nanoparticles in Vitro and Its Pharmacodynamics in Vivo on C6 Glioma Cells of a Brain Tumor Model. Brain Res. 2009, 1261, 91–99.
- Kim, G.; Ko, Y.T. Small Molecule Tyrosine Kinase Inhibitors in Glioblastoma. Arch. Pharm. Res. 2020, 43, 385–394.
- Libermann, T.A.; Nusbaum, H.R.; Razon, N.; Kris, R.; Lax, I.; Soreq, H.; Whittle, N.; Waterfield, M.D.; Ullrich, A.; Schlessinger, J. Amplification, Enhanced Expression and Possible Rearrangement of EGF Receptor Gene in Primary Human Brain Tumours of Glial Origin. Nature 1985, 313, 144–147.
- Chakravarti, A.; Chakladar, A.; Delaney, M.A.; Latham, D.E.; Loeffler, J.S. The Epidermal Growth Factor Receptor Pathway Mediates Resistance to Sequential Administration of Radiation and Chemotherapy in Primary Human Glioblastoma Cells in a RAS-Dependent Manner. Cancer Res. 2002, 62, 4307–4315.
- Barker, F.G.; Simmons, M.L.; Chang, S.M.; Prados, M.D.; Larson, D.A.; Sneed, P.K.; Wara, W.M.; Berger, M.S.; Chen, P.; Israel, M.A.; et al. EGFR Overexpression and Radiation Response in Glioblastoma Multiforme. Int. J. Radiat. Oncol. 2001, 51, 410–418.
- Prados, M.D.; Lamborn, K.R.; Chang, S.; Burton, E.; Butowski, N.; Malec, M.; Kapadia, A.; Rabbitt, J.; Page, M.S.; Fedoroff, A.; et al. Phase 1 Study of Erlotinib HCl Alone and Combined with Temozolomide in Patients with Stable or Recurrent Malignant Glioma. Neuro-Oncology 2006, 8, 67–78.
- Schettino, C.; Bareschino, M.A.; Ricci, V.; Ciardiello, F. Erlotinib: An EGF Receptor Tyrosine Kinase Inhibitor in Non-Small-Cell Lung Cancer Treatment. Expert Rev. Respir. Med. 2008, 2, 167–178.
- Brown, P.D.; Krishnan, S.; Sarkaria, J.N.; Wu, W.; Jaeckle, K.A.; Uhm, J.H.; Geoffroy, F.J.; Arusell, R.; Kitange, G.; Jenkins, R.B.; et al. Phase I/II Trial of Erlotinib and Temozolomide With Radiation Therapy in the Treatment of Newly Diagnosed Glioblastoma Multiforme: North Central Cancer Treatment Group Study N0177. J. Clin. Oncol. 2008, 26, 5603–5609.
- Peereboom, D.M.; Shepard, D.R.; Ahluwalia, M.S.; Brewer, C.J.; Agarwal, N.; Stevens, G.H.J.; Suh, J.H.; Toms, S.A.; Vogelbaum, M.A.; Weil, R.J.; et al. Phase II Trial of Erlotinib with Temozolomide and Radiation in Patients with Newly Diagnosed Glioblastoma Multiforme. J. Neurooncol. 2010, 98, 93–99.
- Uhm, J.H.; Ballman, K.V.; Wu, W.; Giannini, C.; Krauss, J.C.; Buckner, J.C.; James, C.D.; Scheithauer, B.W.; Behrens, R.J.; Flynn, P.J.; et al. Phase II Evaluation of Gefitinib in Patients With Newly Diagnosed Grade 4 Astrocytoma: Mayo/North Central Cancer Treatment Group Study N0074. Int. J. Radiat. Oncol. 2011, 80, 347–353.
- Reardon, D.A.; Nabors, L.B.; Mason, W.P.; Perry, J.R.; Shapiro, W.; Kavan, P.; Mathieu, D.; Phuphanich, S.; Cseh, A.; Fu, Y.; et al. Phase I/Randomized Phase II Study of Afatinib, an Irreversible ErbB Family Blocker, with or without Protracted Temozolomide in Adults with Recurrent Glioblastoma. Neuro-Oncology 2015, 17, 430–439.
- Brown, N.; McBain, C.; Nash, S.; Hopkins, K.; Sanghera, P.; Saran, F.; Phillips, M.; Dungey, F.; Clifton-Hadley, L.; Wanek, K.; et al. Multi-Center Randomized Phase II Study Comparing Cediranib plus Gefitinib with Cediranib plus Placebo in Subjects with Recurrent/Progressive Glioblastoma. PLoS ONE 2016, 11, e0156369.
- Liu, X.; Chen, X.; Shi, L.; Shan, Q.; Cao, Q.; Yue, C.; Li, H.; Li, S.; Wang, J.; Gao, S.; et al. The Third-Generation EGFR Inhibitor AZD9291 Overcomes Primary Resistance by Continuously Blocking ERK Signaling in Glioblastoma. J. Exp. Clin. Cancer Res. 2019, 38, 219.
- Li, X.; Wu, C.; Chen, N.; Gu, H.; Yen, A.; Cao, L.; Wang, E.; Wang, L. PI3K/Akt/MTOR Signaling Pathway and Targeted Therapy for Glioblastoma. Oncotarget 2016, 7, 22.
- Laplante, M.; Sabatini, D.M. MTOR Signaling in Growth Control and Disease. Cell 2012, 149, 274–293.
- Jhanwar-Uniyal, M.; Gillick, J.L.; Neil, J.; Tobias, M.; Thwing, Z.E.; Murali, R. Distinct Signaling Mechanisms of MTORC1 and MTORC2 in Glioblastoma Multiforme: A Tale of Two Complexes. Adv. Biol. Regul. 2015, 57, 64–74.
- Jhanwar-Uniyal, M.; Wainwright, J.V.; Mohan, A.L.; Tobias, M.E.; Murali, R.; Gandhi, C.D.; Schmidt, M.H. Diverse Signaling Mechanisms of MTOR Complexes: MTORC1 and MTORC2 in Forming a Formidable Relationship. Adv. Biol. Regul. 2019, 72, 51–62.
- Dumas, A.A.; Pomella, N.; Rosser, G.; Guglielmi, L.; Vinel, C.; Millner, T.O.; Rees, J.; Aley, N.; Sheer, D.; Wei, J.; et al. Microglia Promote Glioblastoma via MTOR-Mediated Immunosuppression of the Tumour Microenvironment. EMBO J. 2020, 39, e103790.
- Wick, W.; Gorlia, T.; Bady, P.; Platten, M.; van den Bent, M.J.; Taphoorn, M.J.B.; Steuve, J.; Brandes, A.A.; Hamou, M.-F.; Wick, A.; et al. Phase II Study of Radiotherapy and Temsirolimus versus Radiochemotherapy with Temozolomide in Patients with Newly Diagnosed Glioblastoma without MGMT Promoter Hypermethylation (EORTC 26082). Clin. Cancer Res. 2016, 22, 4797–4806.
- Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; Gururangan, S.; Friedman, A.H.; Herndon, J.E.; Marcello, J.; Norfleet, J.A.; McLendon, R.E.; Sampson, J.H.; et al. Phase 2 Trial of Erlotinib plus Sirolimus in Adults with Recurrent Glioblastoma. J. Neurooncol. 2010, 96, 219–230.
- Rao, R.D.; Mladek, A.C.; Lamont, J.D.; Goble, J.M.; Erlichman, C.; James, C.D.; Sarkaria, J.N. Disruption of Parallel and Converging Signaling Pathways Contributes to the Synergistic Antitumor Effects of Simultaneous MTOR and EGFR Inhibition in GBM Cells. Neoplasia 2005, 7, 921–929.
- Ma, D.J.; Galanis, E.; Anderson, S.K.; Schiff, D.; Kaufmann, T.J.; Peller, P.J.; Giannini, C.; Brown, P.D.; Uhm, J.H.; McGraw, S.; et al. A Phase II Trial of Everolimus, Temozolomide, and Radiotherapy in Patients with Newly Diagnosed Glioblastoma: NCCTG N057K. Neuro-Oncology 2015, 17, 1261–1269.
- Chinnaiyan, P.; Won, M.; Wen, P.Y.; Rojiani, A.M.; Werner-Wasik, M.; Shih, H.A.; Ashby, L.S.; Michael Yu, H.-H.; Stieber, V.W.; Malone, S.C.; et al. A Randomized Phase II Study of Everolimus in Combination with Chemoradiation in Newly Diagnosed Glioblastoma: Results of NRG Oncology RTOG 0913. Neuro-Oncology 2018, 20, 666–673.
- O’Reilly, K.E.; Rojo, F.; She, Q.-B.; Solit, D.; Mills, G.B.; Smith, D.; Lane, H.; Hofmann, F.; Hicklin, D.J.; Ludwig, D.L.; et al. MTOR Inhibition Induces Upstream Receptor Tyrosine Kinase Signaling and Activates Akt. Cancer Res. 2006, 66, 1500–1508.
- Kahn, J.; Hayman, T.J.; Jamal, M.; Rath, B.H.; Kramp, T.; Camphausen, K.; Tofilon, P.J. The MTORC1/MTORC2 Inhibitor AZD2014 Enhances the Radiosensitivity of Glioblastoma Stem-like Cells. Neuro-Oncology 2014, 16, 29–37.
- Wang, H.; Mu, X.; He, H.; Zhang, X.-D. Cancer Radiosensitizers. Trends Pharmacol. Sci. 2018, 39, 24–48.
- Adams, G.E.; Asquith, J.C.; Dewey, D.L.; Foster, J.L.; Michael, B.D.; Willson, R.L. Electron Affinic Sensitization. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1971, 19, 575–585.
- De, P.; Roy, K. Nitroaromatics as Hypoxic Cell Radiosensitizers: A 2D-QSAR Approach to Explore Structural Features Contributing to Radiosensitization Effectiveness. Eur. J. Med. Chem. Rep. 2022, 4, 100035.
- Overgaard, J.; Sand Hansen, H.; Andersen, A.P.; Hjelm-Hansen, M.; Jørgensen, K.; Sandberg, E.; Berthelsen, A.; Hammer, R.; Pedersen, M. Misonidazole Combined with Split-Course Radiotherapy in the Treatment of Invasive Carcinoma of Larynx and Pharynx: Report from the DAHANCA 2 Study. Int. J. Radiat. Oncol. 1989, 16, 1065–1068.
- Coleman, C.N.; Wasserman, T.H.; Urtasun, R.C.; Halsey, J.; Noll, L.; Hancock, S.; Phillips, T.L. Final Report of the Phase i Trial of the Hypoxic Cell Radiosensitizer SR 2508 (Etanidazole) Radiation Therapy Oncology Group 83-03. Int. J. Radiat. Oncol. 1990, 18, 389–393.
- Oronsky, B.; Scicinski, J.; Ning, S.; Peehl, D.; Oronsky, A.; Cabrales, P.; Bednarski, M.; Knox, S. RRx-001, A Novel Dinitroazetidine Radiosensitizer. Investig. New Drugs 2016, 34, 371–377.
- Takaoka, T.; Shibamoto, Y.; Matsuo, M.; Sugie, C.; Murai, T.; Ogawa, Y.; Miyakawa, A.; Manabe, Y.; Kondo, T.; Nakajima, K.; et al. Biological Effects of Hydrogen Peroxide Administered Intratumorally with or without Irradiation in Murine Tumors. Cancer Sci. 2017, 108, 1787–1792.
- Janssens, G.O.; Rademakers, S.E.; Terhaard, C.H.; Doornaert, P.A.; Bijl, H.P.; van den Ende, P.; Chin, A.; Marres, H.A.; de Bree, R.; van der Kogel, A.J.; et al. Accelerated Radiotherapy With Carbogen and Nicotinamide for Laryngeal Cancer: Results of a Phase III Randomized Trial. J. Clin. Oncol. 2012, 30, 1777–1783.
- Yang, L.; Taylor, J.; Eustace, A.; Irlam, J.J.; Denley, H.; Hoskin, P.J.; Alsner, J.; Buffa, F.M.; Harris, A.L.; Choudhury, A.; et al. A Gene Signature for Selecting Benefit from Hypoxia Modification of Radiotherapy for High-Risk Bladder Cancer Patients. Clin. Cancer Res. 2017, 23, 4761–4768.
- Rademakers, S.E.; Hoogsteen, I.J.; Rijken, P.F.; Terhaard, C.H.; Doornaert, P.A.; Langendijk, J.A.; van den Ende, P.; van der Kogel, A.J.; Bussink, J.; Kaanders, J.H. Prognostic Value of the Proliferation Marker Ki-67 in Laryngeal Carcinoma: Results of the Accelerated Radiotherapy with Carbogen Breathing and Nicotinamide Phase III Randomized Trial. Head Neck 2015, 37, 171–176.
- Hoskin, P.J.; Rojas, A.M.; Saunders, M.I.; Bentzen, S.M.; Motohashi, K.J. Carbogen and Nicotinamide in Locally Advanced Bladder Cancer: Early Results of a Phase-III Randomized Trial. Radiother. Oncol. 2009, 91, 120–125.
- Horsman, M.R.; Overgaard, J.; Chaplin, D.J. Combination of Nicotinamide and Hyperthermia to Eliminate Radioresistant Chronically and Acutely Hypoxic Tumor Cells. Cancer Res. 1990, 50, 7430–7436.
- Welsh, L.; Panek, R.; Riddell, A.; Wong, K.; Leach, M.O.; Tavassoli, M.; Rahman, D.; Schmidt, M.; Hurley, T.; Grove, L.; et al. Blood Transfusion during Radical Chemo-Radiotherapy Does Not Reduce Tumour Hypoxia in Squamous Cell Cancer of the Head and Neck. Br. J. Cancer 2017, 116, 28–35.
- Daniels, J.S.; Gates, K.S. DNA Cleavage by the Antitumor Agent 3-Amino-1,2,4-Benzotriazine 1,4-Dioxide (SR4233): Evidence for Involvement of Hydroxyl Radical. J. Am. Chem. Soc. 1996, 118, 3380–3385.
- Del Rowe, J.; Scott, C.; Werner-Wasik, M.; Bahary, J.P.; Curran, W.J.; Urtasun, R.C.; Fisher, B. Single-Arm, Open-Label Phase II Study of Intravenously Administered Tirapazamine and Radiation Therapy for Glioblastoma Multiforme. J. Clin. Oncol. 2000, 18, 1254–1259.
- Wang, J.; Guise, C.P.; Dachs, G.U.; Phung, Y.; Hsu, A.H.-L.; Lambie, N.K.; Patterson, A.V.; Wilson, W.R. Identification of One-Electron Reductases That Activate Both the Hypoxia Prodrug SN30000 and Diagnostic Probe EF5. Biochem. Pharmacol. 2014, 91, 436–446.
- Mistry, I.N.; Thomas, M.; Calder, E.D.D.; Conway, S.J.; Hammond, E.M. Clinical Advances of Hypoxia-Activated Prodrugs in Combination with Radiation Therapy. Int. J. Radiat. Oncol. 2017, 98, 1183–1196.
- Overgaard, J.; Sand Hansen, H.; Overgaard, M.; Bastholt, L.; Berthelsen, A.; Specht, L.; Lindeløv, B.; Jørgensen, K. A Randomized Double-Blind Phase III Study of Nimorazole as a Hypoxic Radiosensitizer of Primary Radiotherapy in Supraglottic Larynx and Pharynx Carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5-85. Radiother. Oncol. 1998, 46, 135–146.
- Cerna, D.; Camphausen, K.; Tofilon, P.J. Histone Deacetylation as a Target for Radiosensitization. In Current Topics in Developmental Biology; Academic Press: Cambridge, MA, USA, 2006; Volume 73, pp. 173–204. ISBN 0070-2153.
- Chinnaiyan, P.; Cerna, D.; Burgan, W.E.; Beam, K.; Williams, E.S.; Camphausen, K.; Tofilon, P.J. Postradiation Sensitization of the Histone Deacetylase Inhibitor Valproic Acid. Clin. Cancer Res. 2008, 14, 5410–5415.
- Krauze, A.V.; Myrehaug, S.D.; Chang, M.G.; Holdford, D.J.; Smith, S.; Shih, J.; Tofilon, P.J.; Fine, H.A.; Camphausen, K. A Phase 2 Study of Concurrent Radiation Therapy, Temozolomide, and the Histone Deacetylase Inhibitor Valproic Acid for Patients With Glioblastoma. Int. J. Radiat. Oncol. 2015, 92, 986–992.
- Happold, C.; Gorlia, T.; Chinot, O.; Gilbert, M.R.; Nabors, L.B.; Wick, W.; Pugh, S.L.; Hegi, M.; Cloughesy, T.; Roth, P.; et al. Does Valproic Acid or Levetiracetam Improve Survival in Glioblastoma? A Pooled Analysis of Prospective Clinical Trials in Newly Diagnosed Glioblastoma. J. Clin. Oncol. 2016, 34, 731–739.
- Galanis, E.; Anderson, S.K.; Miller, C.R.; Sarkaria, J.N.; Jaeckle, K.; Buckner, J.C.; Ligon, K.L.; Ballman, K.V.; Moore, D.F., Jr.; Nebozhyn, M.; et al. Phase I/II Trial of Vorinostat Combined with Temozolomide and Radiation Therapy for Newly Diagnosed Glioblastoma: Results of Alliance N0874/ABTC 02. Neuro-Oncology 2018, 20, 546–556.
- Durant, S.T.; Zheng, L.; Wang, Y.; Chen, K.; Zhang, L.; Zhang, T.; Yang, Z.; Riches, L.; Trinidad, A.G.; Fok, J.H.; et al. The Brain-Penetrant Clinical ATM Inhibitor AZD1390 Radiosensitizes and Improves Survival of Preclinical Brain Tumor Models. Sci. Adv. 2018, 4, eaat1719.
- Bakkenist, C.J.; Kastan, M.B. DNA Damage Activates ATM through Intermolecular Autophosphorylation and Dimer Dissociation. Nature 2003, 421, 499–506.
- Carruthers, R.; Ahmed, S.U.; Strathdee, K.; Gomez-Roman, N.; Amoah-Buahin, E.; Watts, C.; Chalmers, A.J. Abrogation of Radioresistance in Glioblastoma Stem-like Cells by Inhibition of ATM Kinase. Mol. Oncol. 2015, 9, 192–203.
- Golding, S.E.; Rosenberg, E.; Valerie, N.; Hussaini, I.; Frigerio, M.; Cockcroft, X.F.; Chong, W.Y.; Hummersone, M.; Rigoreau, L.; Menear, K.A.; et al. Improved ATM Kinase Inhibitor KU-60019 Radiosensitizes Glioma Cells, Compromises Insulin, AKT and ERK Prosurvival Signaling, and Inhibits Migration and Invasion. Mol. Cancer Ther. 2009, 8, 2894–2902.
- Pike, K.G.; Barlaam, B.; Cadogan, E.; Campbell, A.; Chen, Y.; Colclough, N.; Davies, N.L.; de-Almeida, C.; Degorce, S.L.; Didelot, M.; et al. The Identification of Potent, Selective, and Orally Available Inhibitors of Ataxia Telangiectasia Mutated (ATM) Kinase: The Discovery of AZD0156 (8--3-Methyl-1-(Tetrahydro-2H-Pyran-4-Yl)-1,3-Dihydro-2H-ImidazoQuinolin-2-One). J. Med. Chem. 2018, 61, 3823–3841.
- Abraham, D.J.; Kennedy, P.E.; Mehanna, A.S.; Patwa, D.C.; Williams, F.L. Design, Synthesis, and Testing of Potential Antisickling Agents. 4. Structure-Activity Relationships of Benzyloxy and Phenoxy Acids. J. Med. Chem. 1984, 27, 967–978.
- Suh, J.H.; Stea, B.; Nabid, A.; Kresl, J.J.; Fortin, A.; Mercier, J.-P.; Senzer, N.; Chang, E.L.; Boyd, A.P.; Cagnoni, P.J.; et al. Phase III Study of Efaproxiral As an Adjunct to Whole-Brain Radiation Therapy for Brain Metastases. J. Clin. Oncol. 2006, 24, 106–114.
- Rampling, R.; Cruickshank, G.; Lewis, A.D.; Fitzsimmons, S.A.; Workman, P. Direct Measurement of PO2 Distribution and Bioreductive Enzymes in Human Malignant Brain Tumors. Int. J. Radiat. Oncol. 1994, 29, 427–431.
- Taghian, A.; duBois, W.; Budach, W.; Baumann, M.; Freeman, J.; Suit, H. In Vivo Radiation Sensitivity of Glioblastoma Multiforme. Int. J. Radiat. Oncol. 1995, 32, 99–104.
- Kleinberg, L.; Grossman, S.A.; Carson, K.; Lesser, G.; O’Neill, A.; Pearlman, J.; Phillips, P.; Herman, T.; Gerber, M. Survival of Patients With Newly Diagnosed Glioblastoma Multiforme Treated With RSR13 and Radiotherapy: Results of a Phase II New Approaches to Brain Tumor Therapy CNS Consortium Safety and Efficacy Study. J. Clin. Oncol. 2002, 20, 3149–3155.
- Jain, R.K.; di Tomaso, E.; Duda, D.G.; Loeffler, J.S.; Sorensen, A.G.; Batchelor, T.T. Angiogenesis in Brain Tumours. Nat. Rev. Neurosci. 2007, 8, 610–622.
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus Radiotherapy–Temozolomide for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 709–722.
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 699–708.
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer Immunoediting: From Immunosurveillance to Tumor Escape. Nat. Immunol. 2002, 3, 991–998.
- Vesely, M.D.; Schreiber, R.D. Cancer Immunoediting: Antigens, Mechanisms, and Implications to Cancer Immunotherapy. Ann. N. Y. Acad. Sci. 2013, 1284, 1–5.
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer Immunoediting and Resistance to T Cell-Based Immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167.
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355.
- Medikonda, R.; Dunn, G.; Rahman, M.; Fecci, P.; Lim, M. A Review of Glioblastoma Immunotherapy. J. Neurooncol. 2021, 151, 41–53.
- Robert, C. A Decade of Immune-Checkpoint Inhibitors in Cancer Therapy. Nat. Commun. 2020, 11, 3801.
- Sharabi, A.B.; Lim, M.; DeWeese, T.L.; Drake, C.G. Radiation and Checkpoint Blockade Immunotherapy: Radiosensitisation and Potential Mechanisms of Synergy. Lancet Oncol. 2015, 16, e498–e509.
- Lee, Y.; Auh, S.L.; Wang, Y.; Burnette, B.; Wang, Y.; Meng, Y.; Beckett, M.; Sharma, R.; Chin, R.; Tu, T.; et al. Therapeutic Effects of Ablative Radiation on Local Tumor Require CD8+ T Cells: Changing Strategies for Cancer Treatment. Blood 2009, 114, 589–595.
- Lugade, A.A.; Moran, J.P.; Gerber, S.A.; Rose, R.C.; Frelinger, J.G.; Lord, E.M. Local Radiation Therapy of B16 Melanoma Tumors Increases the Generation of Tumor Antigen-Specific Effector Cells That Traffic to the Tumor. J. Immunol. 2005, 174, 7516.
- Schalper, K.A.; Rodriguez-Ruiz, M.E.; Diez-Valle, R.; López-Janeiro, A.; Porciuncula, A.; Idoate, M.A.; Inogés, S.; de Andrea, C.; López-Diaz de Cerio, A.; Tejada, S.; et al. Neoadjuvant Nivolumab Modifies the Tumor Immune Microenvironment in Resectable Glioblastoma. Nat. Med. 2019, 25, 470–476.
- Zhao, J.; Chen, A.X.; Gartrell, R.D.; Silverman, A.M.; Aparicio, L.; Chu, T.; Bordbar, D.; Shan, D.; Samanamud, J.; Mahajan, A.; et al. Immune and Genomic Correlates of Response to Anti-PD-1 Immunotherapy in Glioblastoma. Nat. Med. 2019, 25, 462–469.
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723.
This entry is offline, you can click here to edit this entry!
