Biosensors are ubiquitous in a variety of disciplines, such as biochemical, electrochemical, agricultural, and biomedical areas. They can integrate various point-of-care applications, such as in the food, healthcare, environmental monitoring, water quality, forensics, drug development, and biological domains. Multiple strategies have been employed to develop and fabricate miniaturized biosensors, including design, optimization, characterization, and testing. In view of their interactions with high-affinity biomolecules, they find application in the sensitive detection of analytes, even in small sample volumes. Among the many developed techniques, microfluidics have been widely explored; these use fluid mechanics to operate miniaturized biosensors. The currently used commercial devices are bulky, slow in operation, expensive, and require human intervention; thus, it is difficult to automate, integrate, and miniaturize the existing conventional devices for multi-faceted applications. Microfluidic biosensors have the advantages of mobility, operational transparency, controllability, and stability with a small reaction volume for sensing.
- biosensor
- electrochemical
- miniaturization
- microfluidics
- fabrication
- nanomaterials
- point of care (POC)
1. Evolution of Biosensors
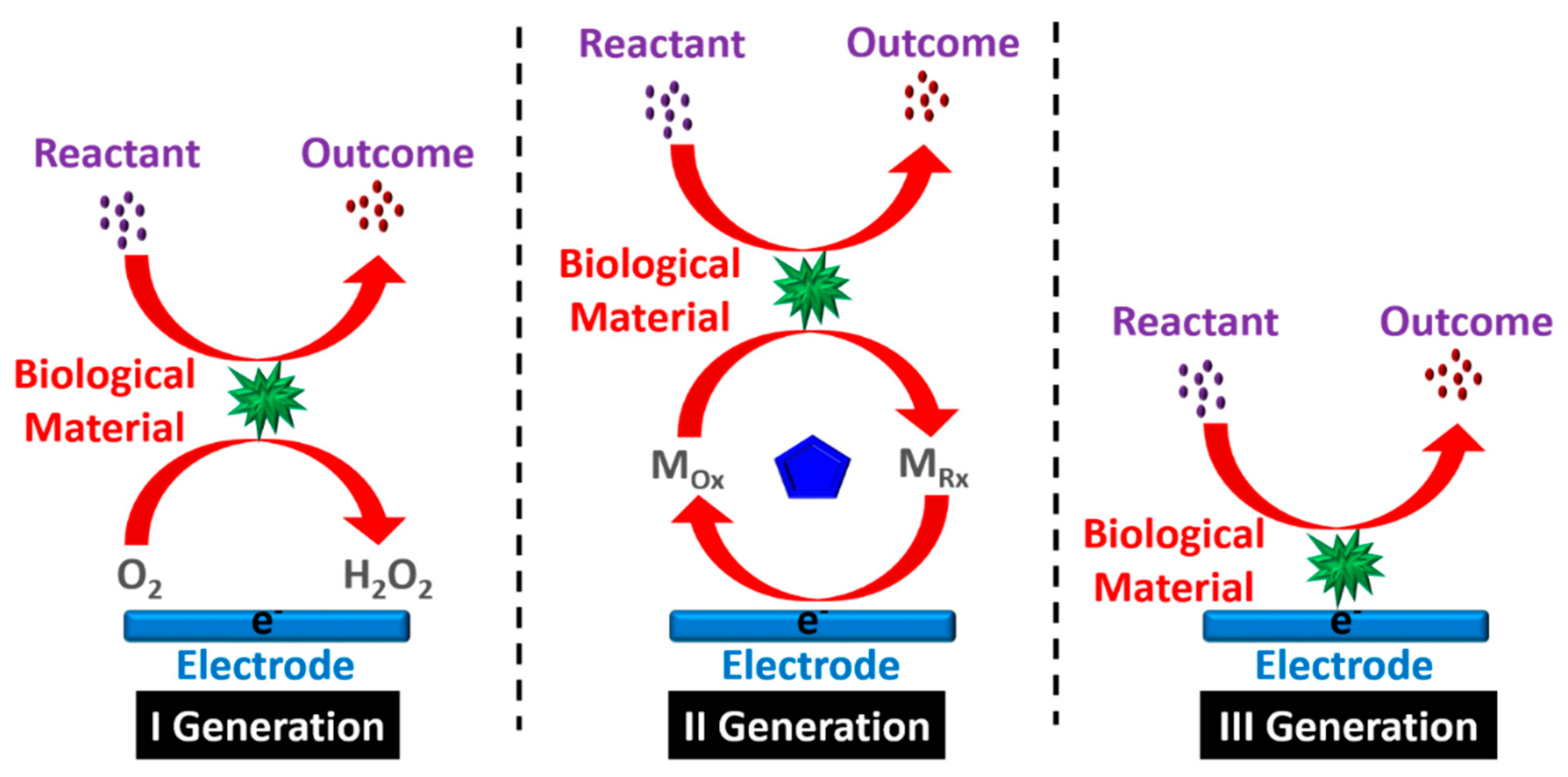
| Year | Generation | Development Phases of Biosensor |
|---|---|---|
| 1906 | First | M. Cramer noticed voltage difference generating between parts of the electrolyte. |
| 1909 | Sorensen described the idea of pH and pH sensors. | |
| 1909–1922 | Nelson and Griffin were the first to discover that enzyme invertase could be immobilized on charcoal aluminium hydroxide [2][3]. | |
| 1922 | Hughes observed a pH determination electrode [4]. | |
| 1956 | Clark first discovered the biosensor electrode that is capable of determining blood oxygen levels [5]. | |
| 1962 | Clark also demonstrated the use of an amperometric enzyme electrode for glucose sensing [6]. | |
| 1967 | Hicks et al. [7] enhanced Clark’s work; glucose oxidase was immobilized using an enzyme-based working electrode with an oxygen sensor. | |
| 1969 | The first potentiometric enzyme electrode-based urea detection sensor was reported by Montalvo and Guilbault. | |
| 1970 | Bergveld discovered ion-sensitive field-effect transistors (ISFET) [8]. | |
| 1973 | Lubrano and Guilbault demonstrated glucose and lactate enzyme platinum electrode to detect hydrogen peroxide (H2O2) [9]. | |
| 1974 | Klaus Mosbach group developed a thermistor sensor based on a heat-sensitive enzyme [10]. | |
| 1975 | Opitz and Lubbers developed an optical biosensor for alcohol detection [11]. | |
| 1976 | Second | Clemens et al. [12] integrated an electrochemical biosensor for glucose detection into an artificial bedside pancreas. A unique semi-continuous catheter-based blood glucose analyzer was also demonstrated using VIA-based technology. |
| 1977 | La Roche introduced the lactate analyzer LA 640, which was utilized to transmit an electron from dehydrogenase to an electrode [13]. | |
| 1980 | Peterson was the first to perform in vivo blood gas analysis to create a fiber-optic pH sensor [14]. | |
| 1982 | Schultz detected glucose by using the fiber-optic biosensor [15]. | |
| 1983 | Third | Liedberg discovered the reliance-based reactions in real time using the surface plasmon resonance (SPR) method in real time [16]. |
| 1984 | For glucose detection, the first mediated amperometric biosensor was constructed using ferrocene and glucose oxidase [17]. | |
| 1987 | University of Cambridge created a pen-sized detector for assessing blood glucose levels. | |
| 1990 | Pharmacia Biacore proposed an SPR-based biosensor [18]. | |
| 1992 | i-STAT developed a handheld blood biosensor [19]. | |
| 2018 | Girbi designed a neuron-on-chip biosensor to measure the nerve impulse conduction [20]. | |
| 2021 | Kulkarni et al. [21] described an Al-foil-based electrode for sensing cysteine. |
2. Miniaturized Microfluidic-Based Biosensors: Design and Fabrication
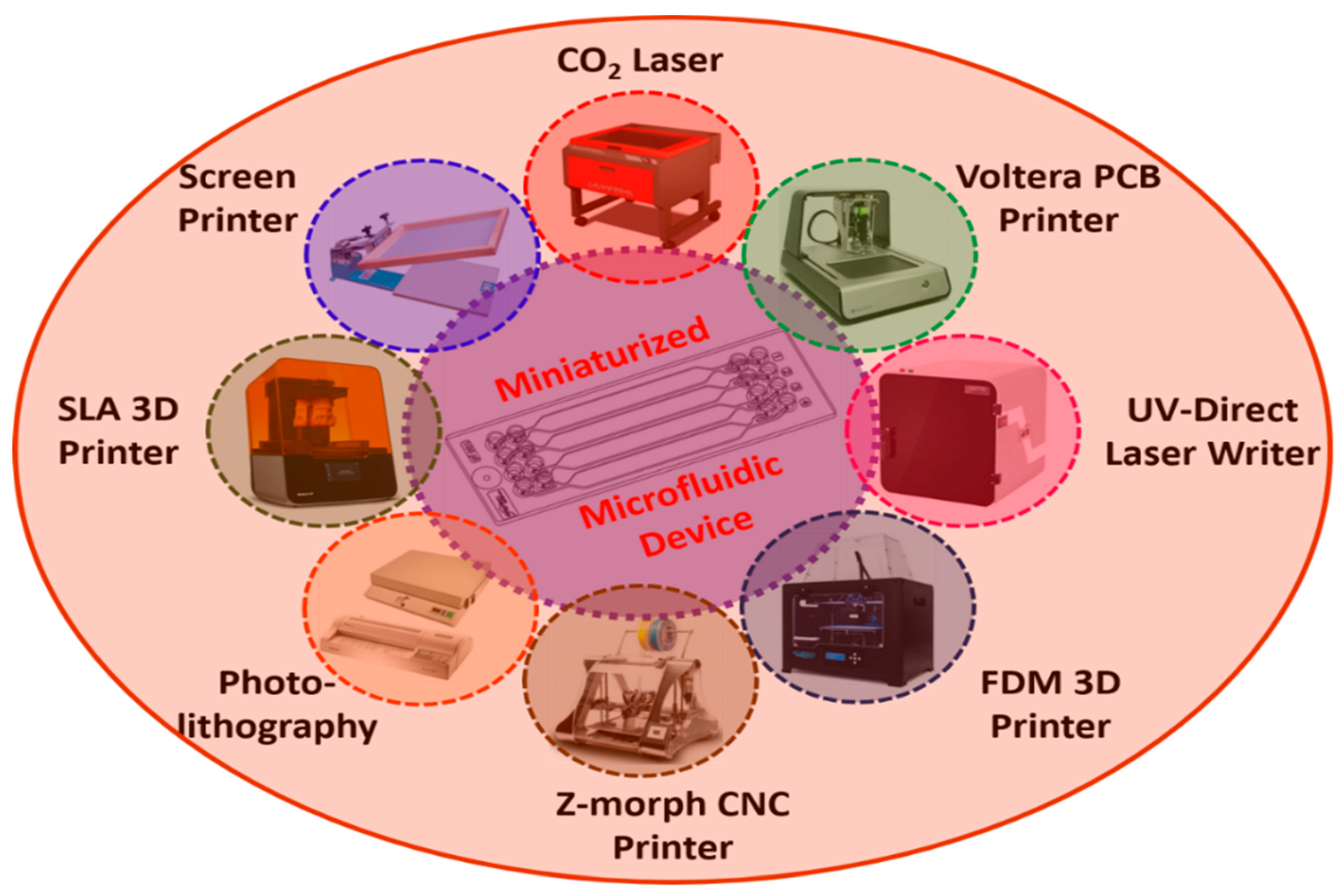
| Fabrication Instruments [Ref] | Materials | Specifications | Advantages | Disadvantages |
|---|---|---|---|---|
| CO2 Laser Ablation [23] | PMMA, polyimide | IR source, λ = 10.6 µm | Precise dissection, good efficiency | Expensive instrument |
| Voltera Ink-jet Printer [24] | Paper, PCB, polyimide | Minimum trace width = 0.2 mm | Flexible substrates | Refilling of conductive ink |
| UV-Direct Laser writer (DLW) [25] | Glass, silicon wafer | GaN laser diode, λ = 405 nm | Better resolution | Expensive instrument |
| FDM 3D printer [26] | ABS, PLA, PCL | Filament Diameter = 1.75 mm, accuracy = 100 µm | Easily scaled to any size | Less throughput, low speed, low resolution |
| Z-morph 3D printer [27] | Paper, wood, PMMA | Blue laser, λ = 420 nm | Multipurpose tool with interchangeable tool heads capable of FDM 3D printing (50 µm accuracy), CNC cutting/drilling, and PCB engraving | Slow process |
| Photolithography [28] | Dry film photoresist (DFR) | Max width = 325 mm, maximum substrate thickness = 3 mm | Photosensitive polymers are necessary | Mask is expensive |
| SLA 3D printer [26] | Various liquid resins | Layer resolution = 35 microns | Higher resolution and accuracy | Requires post-processing tasks such as cleaning with IPA and ethanol |
| Screen printer [29] | Cloth, paper | Minimum trace width = 0.4 mm | Low cost | Less accurate |
| Sothlithography [30] | PDMS | Silicone elastomer | Transparent | Low thermal conductivity |
3. Applications
3.1. Food Processing and Environmental Monitoring
3.2. Biomedical Domain
3.3. Plant Biology
3.4. Biodefense Sensing
This entry is adapted from the peer-reviewed paper 10.3390/bios12070543
References
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109.
- Nelson, J.M.; Griffin, E.G. The influence of certain substances on the activity of invertase. J. Am. Chem. Soc. 1916, 38, 722–730.
- Nelson, J.M.; Griffin, E.G. Adsorption of invertase. J. Am. Chem. Soc. 1916, 38, 1109–1115.
- Hughes, W.S. The potential difference between glass and electrolytes in contact with the glass. J. Am. Chem. Soc. 1922, 44, 2860–2867.
- Heineman, W.R.; Jensen, W.B. Leland c. Clark Jr. (1918–2005). Biosens. Bioelectron. 2006, 21, 1403–1404.
- Clark, L.C.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45.
- Updike, S.J.; Hicks, G.P. The Enzyme Electrode. Nature 1967, 214, 986–988.
- Bergveld, P. Development of an Ion-Sensitive Solid-State. IEEE Trans. Biomed. Eng. 1970, BME-17, 70–71.
- Guilbault, G.G.; Lubrano, G.J. An enzyme electrode for the amperometric determination of glucose. Anal. Chim. Acta 1973, 64, 439–455.
- Mosbach, K.; Danielsson, B. An enzyme thermistor. Biochim. Biophys. Acta-Enzymol. 1974, 364, 140–145.
- Miller, B.V.; Limes, R.W. Recent Advances in Particle Size Measurements: A Critical Review. Crit. Rev. Anal. Chem. 1988, 20, 75–116.
- Brückel, J.; Zier, H.; Kerner, W.; Pfeiffer, E. Progress in Practical Endocrinology. Horm. Metab. Res. 1990, 22, 382–384.
- Falkowski, P.G.; LaRoche, J. Acclimation to spectral irradiance in algae. J. Phycol. 1991, 27, 8–14.
- Peterson, J.I. Fiber optic pH sensor for gastric measurements-preliminary results. In Proceedings of the Fiber Optic Sensors in Medical Diagnostics, Los Angeles, CA, USA, 21 May 1993; Volume 1886, pp. 109–117.
- Schultz, J.S.; Mansouri, S.; Goldstein, I.J. Affinity sensor: A new technique for developing implantable sensors for glucose and other metabolites. Diabetes Care 1982, 5, 245–253.
- Liedberg, B.; Nylander, C.; Lunström, I. Surface plasmon resonance for gas detection and biosensing. Sens. Actuators 1983, 4, 299–304.
- Roederer, J.E.; Bastiaans, G.J. Microgravimetric Immunoassay with Piezoelectric Crystals. Anal. Chem. 1983, 55, 2333–2336.
- D’Orazio, P. Biosensors in clinical chemistry. Clin. Chim. Acta 2003, 334, 41–69.
- Yoo, E.H.; Lee, S.Y. Glucose biosensors: An overview of use in clinical practice. Sensors 2010, 10, 4558–4576.
- Gribi, S.; du Bois de Dunilac, S.; Ghezzi, D.; Lacour, S.P. A microfabricated nerve-on-a-chip platform for rapid assessment of neural conduction in explanted peripheral nerve fibers. Nat. Commun. 2018, 9, 4403.
- Kulkarni, M.B.; Enaganti, P.K.; Amreen, K.; Goel, S. Integrated Temperature Controlling Platform to Synthesize ZnO Nanoparticles and its Deposition on Al-Foil for Biosensing. IEEE Sens. J. 2021, 21, 9538–9545.
- Pathak, A.K.; Singh, V.K. SPR Based Optical Fiber Refractive Index Sensor Using Silver Nanowire Assisted CSMFC. IEEE Photonics Technol. Lett. 2020, 32, 465–468.
- Annabestani, M. An Intelligent Machine Learning-Based Sheath-free Microfluidic Impedance Flow cytometer. In Proceedings of the 2020 10th International Conference on Computer and Knowledge Engineering (ICCKE), Mashhad, Iran, 29–30 October 2020; pp. 284–288.
- Ali, S.; Hassan, A.; Hassan, G.; Eun, C.H.; Bae, J.; Lee, C.H.; Kim, I.J. Disposable all-printed electronic biosensor for instantaneous detection and classification of pathogens. Sci. Rep. 2018, 8, 5920.
- Kulkarni, M.B.; Goel, S. Miniaturized DNA amplification platform with soft-lithographically fabricated continuous-flow PCR microfluidic device on a portable temperature controller. Microfluid. Nanofluid. 2021, 25, 69.
- Ballacchino, G.; Weaver, E.; Mathew, E.; Dorati, R.; Genta, I.; Conti, B.; Lamprou, D.A. Manufacturing of 3d-printed microfluidic devices for the synthesis of drug-loaded liposomal formulations. Int. J. Mol. Sci. 2021, 22, 8064.
- Kulkarni, M.B.; Goel, S. Recent advancements in integrated microthermofluidic systems for biochemical and biomedical applications—A review. Sensors Actuators A Phys. 2022, 341, 113590.
- Lee, D.S.; Park, S.H.; Chung, K.H.; Pyo, H.B. A disposable plastic-silicon micro PCR chip using flexible printed circuit board protocols and its application to genomic DNA amplification. IEEE Sens. J. 2008, 8, 558–564.
- Felix, F.S.; Baccaro, A.L.B.; Angnes, L. Disposable voltammetric immunosensors integrated with Microfluidic Platforms for Biomedical, Agricultural and Food Analyses: A Review. Sensors 2018, 18, 4124.
- Das, S.; Srivastava, V.C. Microfluidic-based photocatalytic microreactor for environmental application: A review of fabrication substrates and techniques, and operating parameters. Photochem. Photobiol. Sci. 2016, 15, 714–730.
- Holah, J.T. Industrial Monitoring: Hygiene in Food Processing. In Biofilms—Science and Technology; Springer: Dordrecht, The Netherlands, 1992; pp. 645–659.
- McHugh, A.J.; Yap, M.; Crispie, F.; Feehily, C.; Hill, C.; Cotter, P.D. Microbiome-based environmental monitoring of a dairy processing facility highlights the challenges associated with low microbial-load samples. NPJ Sci. Food 2021, 5, 4.
- Beno, S.M.; Stasiewicz, M.J.; Andrus, A.D.; Ralyea, R.D.; Kent, D.J.; Martin, N.H.; Wiedmann, M.; Boor, K.J. Development and validation of pathogen environmental monitoring programs for small cheese processing facilities. J. Food Prot. 2016, 79, 2095–2106.
- Radke, S.M.; Alocilja, E.C. A high density microelectrode array biosensor for detection of E. coli O157:H7. Biosens. Bioelectron. 2005, 20, 1662–1667.
- Leong, D.; Alvarez-Ordóñez, A.; Jordan, K. Monitoring occurrence and persistence of Listeria monocytogenes in foods and food processing environments in the Republic of Ireland. Front. Microbiol. 2014, 5, 436.
- Meshram, B.D.; Agrawal, A.K.; Adil, S.; Ranvir, S.; Sande, K.K. Biosensor and its Application in Food and Dairy Industry: A Review. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3305–3324.
- Rhouati, A.; Hayat, A.; Hernandez, D.B.; Meraihi, Z.; Munoz, R.; Marty, J.L. Development of an automated flow-based electrochemical aptasensor for on-line detection of Ochratoxin A. Sens. Actuators B Chem. 2013, 176, 1160–1166.
- Roisen, F.I.; Murphy, R.A.; Pichichero, M.E.; Braden, W.G. Cyclic adenosine monophosphate stimulation of axonal elongation. Science 1972, 175, 73–74.
- Shokr, A.; Pacheco, L.G.C.; Thirumalaraju, P.; Kanakasabapathy, M.K.; Gandhi, J.; Kartik, D.; Silva, F.S.R.; Erdogmus, E.; Kandula, H.; Luo, S.; et al. Enabled with Adaptive Adversarial Learning. ACS Nano 2020, 15, 665–673.
- Justino, C.I.L.; Duarte, A.C.; Rocha-Santos, T.A.P. Critical overview on the application of sensors and biosensors for clinical analysis. TrAC Trends Anal. Chem. 2016, 85, 36–60.
- Chen, Y.W.; Liu, M.; Kaneko, T.; McIntyre, P.C. Atomic layer deposited hafnium oxide gate dielectrics for charge-based biosensors. Electrochem. Solid-State Lett. 2010, 13, G29.
- Bergveld, P. The development and application of rapid. Biosensors 1986, 2, 15–33.
- Ariffin, S.A.B.; Adam, T.; Hashim, U.; Faridah, S.; Zamri, I.; Uda, M.N.A. Plant diseases detection using nanowire as biosensor transducer. Adv. Mater. Res. 2014, 832, 113–117.
- Giepmans, B.N.G.; Adams, S.R.; Ellisman, M.H.; Tsien, R.Y. The fluorescent toolbox for assessing protein location and function. Science 2006, 312, 217–224.
- Evanko, D.S.; Haydon, P.G. Elimination of environmental sensitivity in a cameleon FRET-based calcium sensor via replacement of the acceptor with Venus. Cell Calcium 2005, 37, 341–348.
- Lee, W.G.; Kim, Y.G.; Chung, B.G.; Demirci, U.; Khademhosseini, A. Nano/Microfluidics for diagnosis of infectious diseases in developing countries. Adv. Drug Deliv. Rev. 2010, 62, 449–457.
- Prakash, R.; Kaler, K.V.I.S. An integrated genetic analysis microfluidic platform with valves and a PCR chip reusability method to avoid contamination. Microfluid. Nanofluid. 2007, 3, 177–187.
