Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Glioblastoma (GBM) is the most common and aggressive primary brain tumor.
- glioblastoma
- diagnosis
- follow-up
1. Introduction
Glioblastoma (GBM) is the most common and malignant primary brain tumor. The cellular origin of GBM is uncertain, but it could arise from neural stem cells or glial precursor cells, which accumulate mutations [1]. GBM account for almost half of malignant Central Nervous System (CNS) tumors and are mainly located in frontal (28.6%), temporal (25%), and parietal (15.3%) lobes [2]. The annual incidence rate is 3.23 per 100,000 population in the United States, with the highest rates between 75 and 84 years [2]. There is no identifiable cause for GBM, but some genetic syndromes predestinate a higher risk [3]. The 2021 World Health Organization (WHO) classification of tumors of the CNS, based on the 2016 updated edition and on the recommendations of the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy—Not Official WHO (cIMPACT-NOW), emphasizes not only histological investigations but also the use of molecular markers in classifying brain tumors [4,5,6]. According to this classification, GBM is defined as isocitrate dehydrogenase (IDH)-wildtype diffuse astrocytic glioma, arising in adults and showing one or more of the following features: microvascular proliferation, necrosis, telomerase reverse transcriptase (TERT) promoter mutation, epidermal growth factor receptor (EGFR) gene amplification, combined gain of entire chromosome 7, and loss of entire chromosome 10 [4]. At present, GBM is mainly diagnosed by neuroimaging techniques and by histopathological and molecular analysis of the resected or biopsied tissue. Both imaging and tissue-based methods have, despite their advantages, some important limitations which will be discussed in detail.
Current treatment for newly diagnosed GBM relies on a combination of maximal safe surgical resection, fractionated radiotherapy, and temozolomide (TMZ) -based alkylating chemotherapy [7]. Despite this multimodal treatment, most of the patients (70%) experienced relapse within one year from diagnosis, median survival is only 15 months, and 5-year survival is only 7% [2,8,9]. The standard technique for patients’ follow-up is brain magnetic resonance imaging (MRI). However, the discrimination between actual relapses and the so-called pseudoprogression (PsP), consisting of treatment-related lesions mimicking a recurrence, is frequently challenging and, therefore, changes induced by, e.g., radiation, may be misinterpreted as tumor progression [10].
In this context, liquid biopsy, which involves the detection and quantification of tumoral content released by tumors into the biofluids, has emerged as a promising and non-invasive diagnostic tool complementary to conventional methods [9].
2. Pitfalls and Limitations of Current Techniques for GBM Diagnosis and Follow-Up
2.1. Neuroimaging
At present, the gold standard for GBM radiological diagnosis is MRI since it overperforms computed tomography (CT) for a number of reasons, such as better anatomical resolution, better capacity to identify GBM features, and the possibility to realize more advanced analyses (i.e., brain tumor spectroscopy) [11,12]. Only in some specific settings, such as MRI not being available, not feasible (i.e., presence of metallic implants), or in urgent situations (i.e., life-threatening brain hemorrhage), brain CT is still used [12]. For this reason, the present review will focus on brain MRI as the technique of choice for the initial diagnosis and follow-up of GBM. Various morphological sequences are used to evaluate tumor lesions on MRI, such as pre- and post-gadolinium contrast-enhanced T1-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR), and others, to characterize GBM morphology [13]. In addition to morphological imaging, advanced MRI techniques have more recently emerged, such as perfusion-weighted imaging, magnetic resonance spectroscopy, diffusion-weighted imaging, and its variants, such as diffusion tensor imaging [14] (Figure 1). These advanced techniques can provide more detailed information on tumor properties, and they can be particularly useful in differential diagnosis given their better sensitivity and specificity compared to conventional MRI, which showed pooled sensitivity and specificity of 68% (95% CI 51–81) and 77% (95% CI 45–93), respectively [14,15].
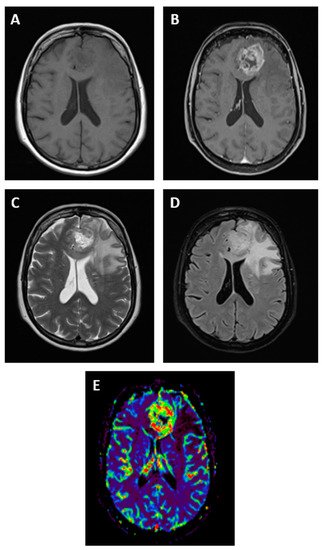
Figure 1. Magnetic resonance imaging features of a patient with GBM in the left frontal lobe. GBM appears as a hypointense or isointense mass on T1-weighted images (A), with a ring pattern of enhancement on gadolinium-enhanced images reflecting the increased blood–brain barrier permeability (B). GBM is typically hyperintense on both T2-weighted (C) and fluid-attenuated inversion recovery (FLAIR, D) images, surrounded by vasogenic edema. Perfusion-weighted imaging is an advanced MRI method useful in GBM for differential diagnosis (E).
Despite the continuous improvement of MRI-based diagnostic techniques, three main limitations can still be identified.
Firstly, MRI does not always discriminate GBM unequivocally from other tumorous or non-tumorous diseases, or it does not clearly discriminate the tumor mass from other concomitant pathological processes implicated in the disease, such as inflammation, edema, scaring, or bleeding, possibly leading to an overestimation of the extension of the tumor mass [16]. As a consequence, the misinterpretation of MRI images can occur, and GBM is therefore confused with other brain tumors such as low-grade gliomas, brain metastases, or primary CNS lymphoma but also with non-neoplastic pathologies such as brain abscess, demyelinating diseases, the hemorrhagic transformation of ischemic strokes, and others [12,16].
Secondly, the exact correlation between MRI features and GBM’s molecular markers, which have become essential in GBM diagnosis, must still be elucidated. At present, radiogenomics, which studies such correlations, has become an active research field [17]. Connections between some molecular alterations found in GBM such as changes in O6-methylguanine-DNA methyltransferase (MGMT), IDH, EGFR, platelet-derived growth factor, vascular endothelial growth factor (VEGF), phosphatase and TENsin homolog, TERT genes, and radiophenotypic manifestations have been shown [17,18]. However, current results are divergent because of the interpatient heterogeneity of GBM, the small size of cohorts that cannot represent this heterogeneity, and the lack of standardized guidelines for systematic image acquisition and analysis [17]. In addition, studies evaluating the link between MRI features and GBM molecular alterations have not been validated prospectively, currently limiting the clinical implementation of radiogenomics to help predict genomic modifications of GBM [18].
Thirdly, the distinction between actual tumor recurrence or progression following treatment and PsP can be challenging in a relatively high number of cases. Radiologically, PsP is defined as a subacute contrast-enhancing lesion that becomes apparent in MRI images within or around the GBM resection cavity [10]. Just like actual recurrences, PsP can be associated with clinical deterioration, or it can be asymptomatic [10]. PsP occurs in 10–30% of GBM patients who undergo their first MRI scan, usually within the first 12 weeks after chemoradiotherapy [10]. It seems to occur more frequently in patients with MGMT promoter methylation (91%) than in patients with unmethylated MGMT promoter (41%) [19]. Overexpression of p53 is also potentially correlated with the development of PsP [20]. Interferon regulatory factor 9 and X-ray repair cross-complementing 1 are highly expressed in patients with PsP [21]. The exact mechanisms underlying PsP are not known. However, the direct destruction of tumor and endothelial cells in the tumor area induced by chemoradiotherapy might lead to inflammation, increased vascular permeability, and edema, resulting in the abnormal contrast enhancement seen in this condition [22]. Furthermore, the expression of hypoxia-related molecules by the tumor and surrounding cells is triggered by treatment-related cellular hypoxia and may play a role in increased endothelial permeability and contrast enhancement in neuroimaging [10]. Contrarily to actual recurrences, PsP resolves spontaneously over time without any change in the current treatment. For obvious reasons, discrimination between an actual recurrence requiring a modification of the therapeutic approach and PsP is of utmost importance for GBM patients [10]. Nevertheless, at present, no biomarker has been clinically validated to better distinguish true progression from PsP [9]. A study by Topkan et al. revealed that, in a series of 28 GBM patients diagnosed with radiological progression at MRI and who were re-operated, 12 (42%) showed pathological features of PsP with no sign of actual recurrence [23]. Advanced MRI techniques could improve the distinction between PsP and true progression. Results of the meta-analysis of Yu et al. revealed that diffusion imaging with apparent diffusion coefficient (ADC) values could discriminate PsP from true progression with higher ADC observed for PsP [24]. The perfusion MRI method could also differentiate PsP from tumor progression with higher relative cerebral blood volume values in true progression compared to treatment effects [25].
Positron emission tomography (PET) is another neuroimaging method that uses radiolabeled molecules to study the biochemical activity of tissues and might provide higher sensitivity compared to MRI in distinguishing between PsP [26]. Many PET tracers can be used; however, in the context of GBM, amino acid analogues are generally preferred, given their relatively low uptake in normal brain and the high uptake in GBM [26]. It has been shown that the amino acid uptake is increased in tumors at progression but not in brain areas of treatment-related changes [26]. For example, 18F-fluoroethyltyrosine (18F-FET) could facilitate the detection of PsP because 18F-FET uptake is significantly lower in patients with PsP than in those with true progression [27].
2.2. Tissue Biopsies
Due to the MRI limitations described above, the histopathological and molecular analysis of the biopsied or resected tissue is still considered the gold-standard method to diagnose GBM [9]. In addition to very rare cases, a surgical approach is always necessary for these patients. The typical histopathological features of GBM include increased cellularity, nuclear atypia, microvascular proliferation, and necrosis [28]. As already discussed, in addition to the histopathological analysis, immunohistochemical staining and molecular tests are usually performed to highlight specific genetic and epigenetic features of GBM such as MGMT promoter methylation, IDH-wildtype, TERT promoter mutation, combined gain of entire chromosome 7 and loss of entire chromosome 10, EGFR amplification, and others [9]. Despite being accurate, direct tissue analysis also has some limitations.
Obviously, tissue analysis requires collecting tumor samples by means of a surgical procedure. At diagnosis, obtaining tumor samples normally does not represent an issue since most GBM patients undergo surgical resection. However, a small proportion of patients is not eligible for surgical resection (old age, deep-seated lesions, etc.), and an image-guided stereotactic biopsy could then be performed. Nevertheless, also for such minimally invasive biopsies, a surgical risk exists and includes hemorrhage or brain swelling within and around the tumor with potential permanent deficits or death [29,30]. After the standard of care treatment, GBM patients usually develop MRI alterations suggestive of recurrence within one year. As already stated, 10–30% of these cases can be PsP: tissue analysis via stereotactic biopsies, for cases where surgery is not indicated, could help distinguish PsP for actual recurrence [10]. However, this could further expose the patients to non-negligible surgical risks. Furthermore, given the risk related to brain tumors biopsies, repeated sampling during tumor progression is usually not performed, thereby limiting the possibility of monitoring the treatment response and identifying the emergence of therapeutic resistance early [29].
Another limitation is that the analysis of tumor tissue is restricted, for technical reasons, to a small part of the whole tumor mass. In the case of stereotactic biopsies, the size of the analyzed tissue is even smaller. Given the increasing evidence of the high degree of spatial heterogeneity in GBM, an analysis conducted on a small fragment might not be representative of the whole tumor [31]. Therefore, critical genomic alterations can possibly be missed, and typical histopathological characteristics of the GBM may be underrepresented [18,32].
At last, the tumor also evolves continuously over time due to treatments, clonal evolution, and hypoxia; thus, tissue biopsies capturing only a static snapshot of the entire tumor cannot evaluate the tumor activity in real-time [33].
This entry is adapted from the peer-reviewed paper 10.3390/cancers14143394
This entry is offline, you can click here to edit this entry!
