Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Erectile dysfunction (ED) is a multi-factorial illness that is characterized by the presence of vascular atherosclerosis and hormonal, lifestyle, age, neurological, and physiological factors, all occurring in a well-coordinated manner. Among all of the listed characteristics, vascular disease is the most common cause of ED. Testosterone levels, psychological concerns, such as performance anxiety, and iatrogenesis are all the variables that contribute to ED development.
- erectile dysfunction
- pathophysiology
- atherosclerosis
- cardiovascular disease
1. Introduction
Erectile dysfunction (ED) is a multi-factorial illness that is characterized by the presence of vascular atherosclerosis and hormonal, lifestyle, age, neurological, and physiological factors, all occurring in a well-coordinated manner [1][2]. Among all of the listed characteristics, vascular disease is the most common cause of ED [3]. Testosterone levels, psychological concerns, such as performance anxiety, and iatrogenesis are all the variables that contribute to ED development [4][5]. According to a variety of demographic studies, ED affects up to 150 million men globally [6][7]. As the world’s population ages, the prevalence of ED is expected to climb to 300 million men by 2025 [8][9]. Males aged 18–75 years in Europe had a prevalence of 19%, but men in the same age range in the UK had a prevalence of 39% for life ED and 26% for current ED [8][10][11].
ED has been linked to future cardiovascular events (CVE) in various studies [12][13], showing a high mortality rate due to cardiovascular disease (CVD) and stroke. Various studies have shown that ED patients had a considerably higher CVD risk than non-ED patients [14][15][16]. The most prominent risk factors associated with ED and CVD are diabetes, dyslipidemia, hypertension, smoking, and obesity, which lead to the development of oxidative stress, the primary cause of endothelial dysfunction [11][17]. Due to the reduction in endothelium-dependent vasodilation, there have been changes in structural vascular abnormalities, such as increased carotid intima-media thickness (cIMT) and the formation of atherosclerotic plaques [18][19][20].
Significantly, the majority of male sexual ED is now recognized to be arterial in origin, with endothelial dysfunction serving as the common link [21][22]. The patient and his spouse are both negatively affected by ED, stressing the need for addressing ED as soon as feasible [23]. Figure 1 indicates the relationship between CVD risk factors and ED. From the above, it can be concluded that “There is a clear correlation between ED and CVD.” A comprehensive investigation of ED and CVD can be beneficial in the early diagnosis of heart attacks, strokes, and other unfavorable CVE [24][25].
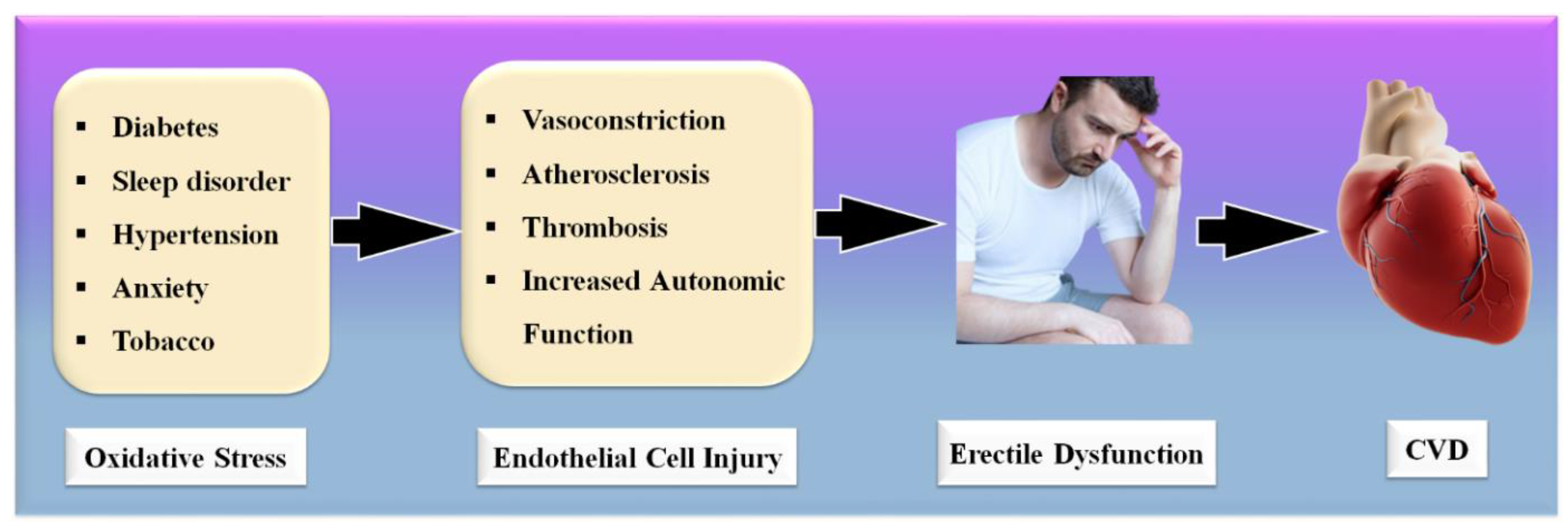
Figure 1. Relationship between CVD risk factors, ED, and CVD.
Several changes occur as a result of the advancement of ED, including the creation of exudates, bleeding, and other symptoms [26]. These modifications have been implicated in the development of CVD [16]. Patients in the more severe phases of ED have a higher risk of CVD, and once a patient has been diagnosed with a CVD risk, coronary imaging is indicated to stratify the risks [13]. Also essential for visualizing the plaque in coronary artery disease (COAD), coronary artery imaging (CAI) is vital [27]. Intravascular ultrasonography and coronary angiography are the most frequently used imaging modalities for the visualization of coronary plaque [28][29].
The imaging modalities are costly and difficult to get one’s hands on, especially in underdeveloped nations [30]. As a result, it seems sensible to explore low-cost alternative imaging technologies that can still monitor carotid artery disease (CTAD) in ED patients and risk-stratify them [20][31]. Vascular imaging technologies are useful for the treatment and can save lives before they become life-threatening [19]. Because the carotid artery and the coronary artery have genetically similar compositions, B-mode carotid ultrasonography is a preferred alternative for CTAD imaging of the carotid artery [32]. Image-based phenotypes such as carotid intima-media thickness and carotid total plaque area can be used as CVD surrogates. Further, accurate and automated carotid plaque burden quantification, risk stratification, and early monitoring of atherosclerotic disease in ED patients is therefore required [33].
Artificial intelligence (AI)-based methods have recently played a vital role in computer-aided diagnosis [34][35], especially in the detection and classification of several diseases [36][37]. Machine learning applications in medical imaging have just lately risen to prominence, such as diabetes [38]; the risk stratification of cancer types such as thyroid [39], liver [37][40], prostate [41][42], and ovarian [43]; vascular screening [44]; coronary artery disease risk characterization [45][46]; and surrogate biomarker CTAD imaging and its risk stratification [47][48]. Previously, machine learning (ML) models were developed to predict CVD, as it contains a variety of features from the CVD datasets [49][50][51]. Recently, the deep learning (DL) algorithms have been used to segment the carotid plaque wall thickness [52][53] for CVD risk assessment. As a result, it may be conceivable to use these AI-based solutions to handle CVD and stroke risk stratification in ED patients.
These contents would introduce (a) the clinical linking between ED and CVD and vice versa, along with the risk factors of CVD in ED patients, and (b) the CVD risk stratification for the severity of heart failure and stroke in ED patients based on AI. One can use the risk factors such as office-based biomarkers (OBBM), laboratory-based biomarkers (LBBM), carotid ultrasound image phenotypes (CUSIP), and medicine usage (MedUSE) combined with ED covariates for designing knowledge-based systems for CVD prediction. Thus, ML and DL solutions can help in establishing the early CVD risk assessment of ED patients who are at a high risk of CVD or ischemic and hemorrhage stroke.
2. Erectile Dysfunction and Cardiovascular Disease Links: Clinical Evidence
The definition of a health risk is “a characteristic or incident that is associated with a higher probability of a certain result, such as the occurrence of a disease.” [54]. The Framingham Heart Study is a major milestone in terms of identifying risk factors for CVD. The FHS’s work has considerably helped preventive medicine. As a result, the focus shifted from treatment to prevention and education [55]. All combined atherosclerotic plaque risk factors should be considered relevant to CVD [56]. Age, gender, a family background of CVD, and ethnicity should be considered as non-modifiable CVD risk factors. Age is an indicator of duration, and it is linked to CVD risk. Age is also the largest indicator affecting cardiovascular outcomes [57][58]. Another well-known CVD risk factor is the male gender. According to the FHS data, women’s CVD mortality is equivalent to that of males 10 years younger [59]. Another well-established, non-modifiable risk factor is a first-degree relative with a history of CVD [60][61]. This link is especially robust in younger people who have a strong family history of premature illness [62][63]. Even though these risk variables are non-modifiable, their identification is important in clinical treatment because it helps in identifying individuals who require more stringent control of modifiable CVD risk factors.
In addition to ischemic heart disease, stroke, and peripheral artery disease, hypertension has been linked to several of the most significant atherosclerotic symptoms, including peripheral artery disease (PAD) [64][65]. In the normal BP range (>115/75 mmHg), there is no solid evidence of a risk threshold for CVD [65]. This link has been seen in people of all ages, and it appears to be greater for systolic BP than diastolic BP [66][67]. Stroke and heart disease fatalities increase more than multiple times for those aged 40–69 years who have an increase in their blood pressure of 20 or 10 mm Hg [65].
Diabetes mellitus (DM) doubles or triples the risk of myocardial infarction or stroke, as well as the risk of CVD mortality [68][69]. This risk rises in proportion to the degree of glycemic change [70]. Intermediate carbohydrate metabolic anomalies have also been linked to a higher CVD risk [71][72]. In contrast to diabetes, diabetic people have a higher risk of CVD due to the existence of additional metabolic abnormalities [73].
ED is generally referred to as a vascular disease, and it is generally known that it shares several health risks with CVD, including obesity [32][74], chronic renal disease [75], poor socioeconomic status [58], low fruit and vegetable consumption [76], inadequate physical activity [77], metabolic syndrome [78][79], and elevated C-reactive protein levels [80], which are all well-known risk factors for CVD. In this context, a large prospective study evaluating the effect of CVD risk variables on ED over 25 years showed that age, BMI, cholesterol, and triglycerides were all highly associated with ED [79]. Smoking, BMI, hypertension, cholesterol dietary consumption, and unsaturated fat intake have all been linked to an increased risk of ED [78][81]. Figure 2 indicates the shared risk factors of ED.
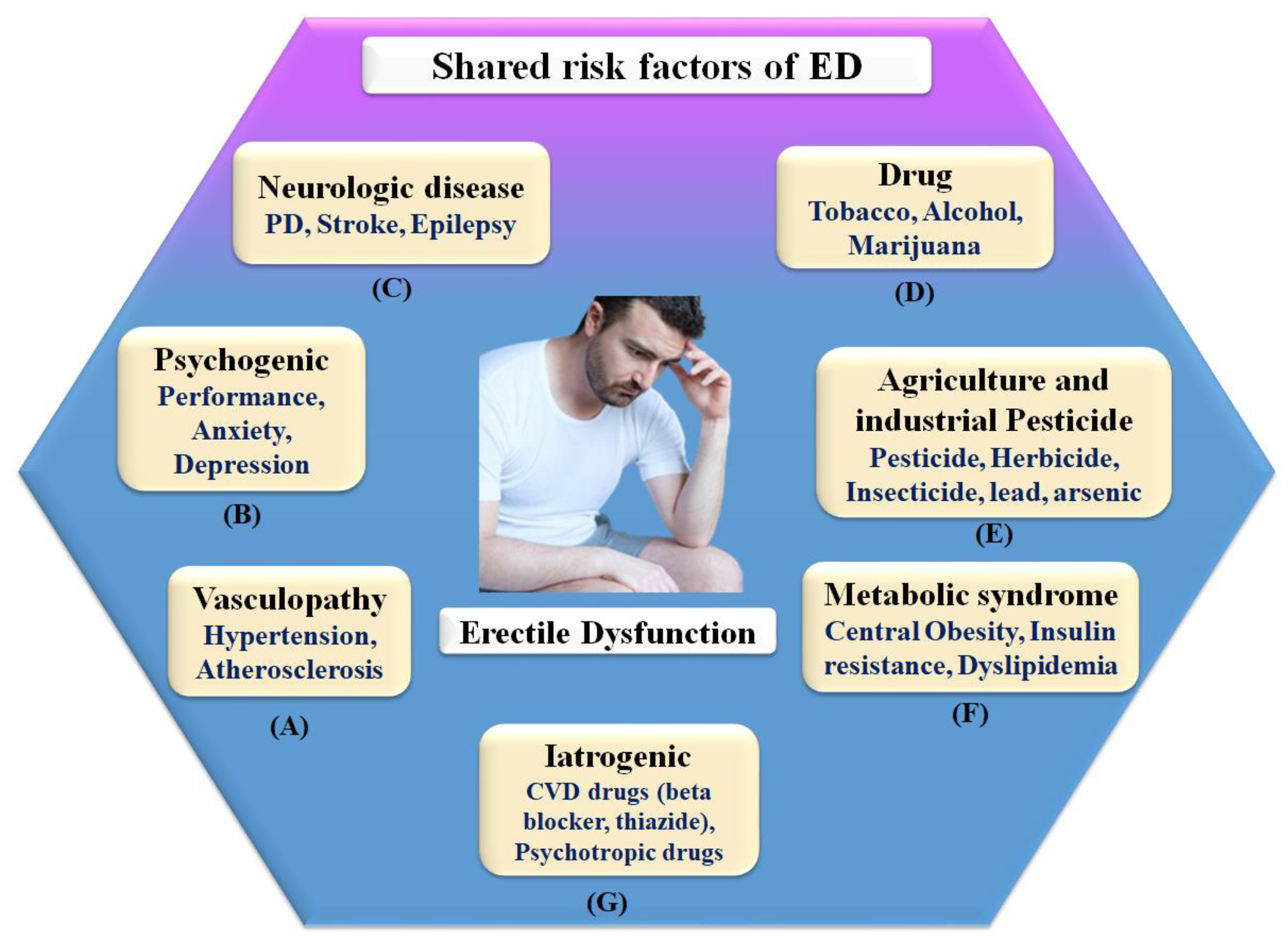
Figure 2. Shared risk factors of ED.
Therefore, in connection, ED affects around 75% of diabetes patients over the age of 60 and grows proportionately with the severity of the condition [82]. It is possible that ED and penile atherosclerosis are the common denominators between ED and diabetes [83]. However, the link between these two clinical diseases is complex, and additional pathophysiologic processes, such as autonomic neuropathy and hormonal abnormalities, may be involved in the development of these two clinical conditions [22][84].
2.1. The Pathophysiologic Link between ED and CVD
The pathophysiology of ED is dependent on the integrity of the endothelium [85][86]. Sexual drive induces the production of NO and other endothelial mediators, resulting in stimulating sympathetic stimulation in the veins feeding penile regions and an enhanced blood flow to the penis while blocking the vein discharge [86][87]. These occurrences cause blood to be trapped within the corpora cavernosa. This increase leads to system pressure and an erection [88]. The carotid arteries hypothesized that ED and COAD have the same involvement in the pathogenesis pathway [89]. ED and circulation stenosis may result from exposure to known risk factors. Due to the systematic character of atherosclerosis, all arterial pathways may be harmed to the same amount, but the onset of signs is linked to arterial size [9][90]. Increased vascular tolerance for the same amount of endothelial dysfunction and/or atherosclerotic burden is observed in bigger vessels when compared to smaller arteries [91]. Alongside the more compact ones, penile veins are smaller than other veins in the body [92]. Compared to coronary arteries, they are tiny, (1–2 mm) to (3–4 mm), with endothelial dysfunction at the very same level, and atherosclerosis may cause a greater decline in blood flow [9].
Consequently, the vascular system of the penile organ may serve as an early warning system for a wide range of vascular conditions [93]. Individuals with chronic coronary syndromes (CCS) are more likely to have ED than those without CCS, according to this hypothesis. In this respect, Montorsi et al. [3] explained that for patients with chronic coronary syndrome, ED is common before CAD symptoms appear. Most patients with CCS begin to have sexual dysfunction three years before any cardiac symptoms appear. This contrasts with the rarity of sexual dysfunction in those suffering from acute coronary syndrome [3]. Appropriate arterial penile lesions were found in only 12.9% of the cases, compared to a high frequency of 87% in the coronary system and 77% in the internal iliac artery area [94]. Figure 3 shows the CVD risk factors linked with inflammation, androgen, and endothelial dysfunction.
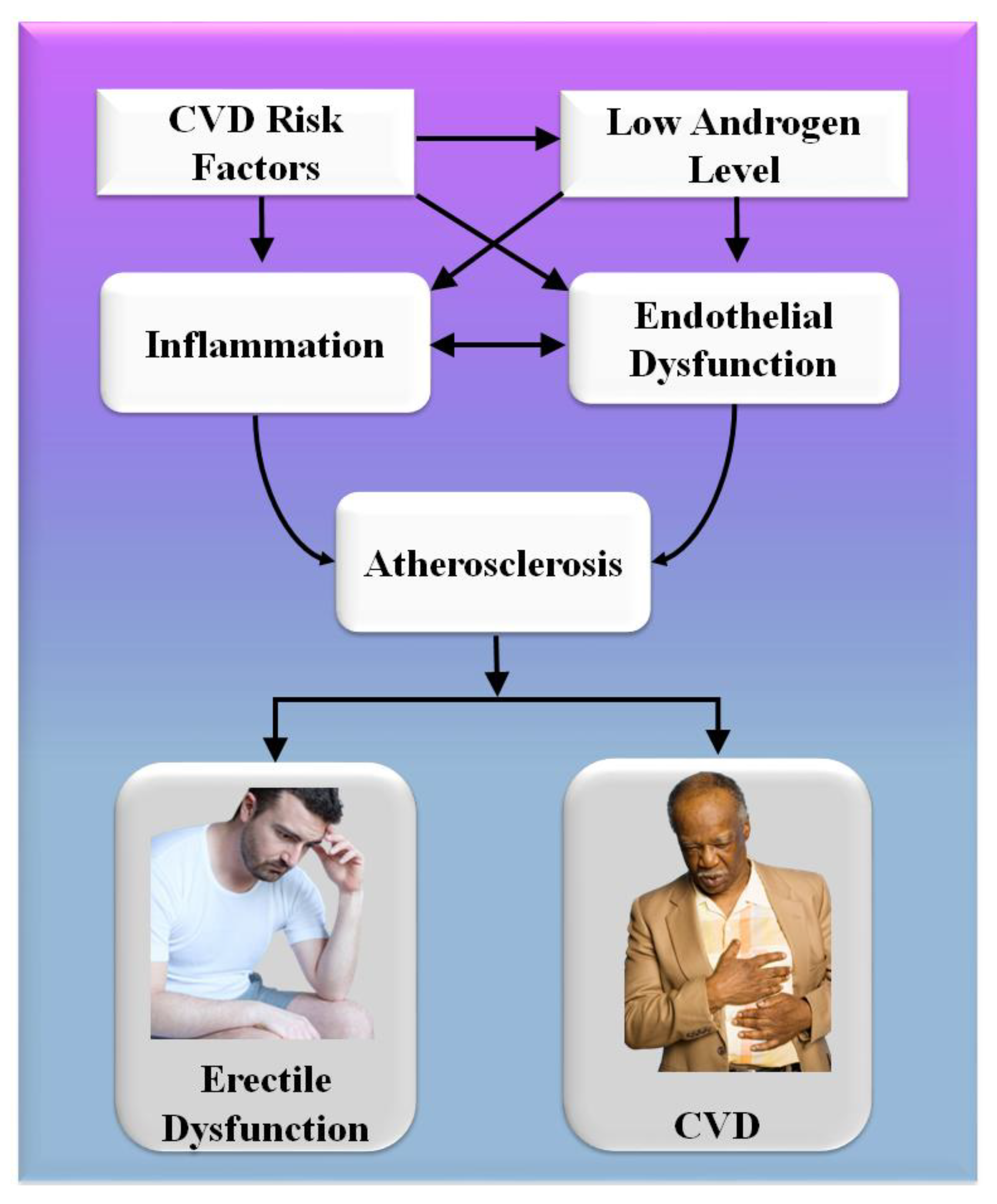
Figure 3. CVD risk factors are linked with inflammation, androgen, and endothelial dysfunction.
A comprehensive reformulation of all available evidence revealed that, while the artery-size theory is crucial to understanding the complicated relationship between ED and COAD, vasculogenic ED is also connected with dynamic, macroscopically intangible irregularities linked to endothelial dysfunction and neurogenic hyperactivity [42]. The usual indications of cardiovascular problems are quite often disguised in diabetics, causing a diagnostic lag of COAD and difficulty in altering the disease’s natural history [95]. In diabetes patients, an individual relationship between ED and asymptomatic COAD has indeed been described [96][97]. Endothelial functioning is affected by low-grade inflammatory cytokines, which can lead to a thrombogenic state [98]. Several studies have linked the development and intensity of ED to the elevated expression of inflammation biomarkers [44][45][46][47]. The major targets for androgen actions inside the penile and cardiovascular pathways are endothelium and sleek cells, and congenital hypothyroidism is associated with an increased risk of arteriosclerotic remodeling [99][100].
As a result, it is found that people who have ED and risk factors for cardiovascular disease are more likely to have a “silent COAD.” They should get a full CVD examination.
Mechanism of Penile Erection
The mechanism of the male penile erection, as well as cross-section, is shown in Figure 4A,B, where the aorta is directly connected to the penal artery. A significant blood input is essential for successful sexual performance [101][102]. As previously stated, normal penile erection is a neurovascular event that causes sexual stimulation and the release of NO hormones from endothelial cells [103][104].
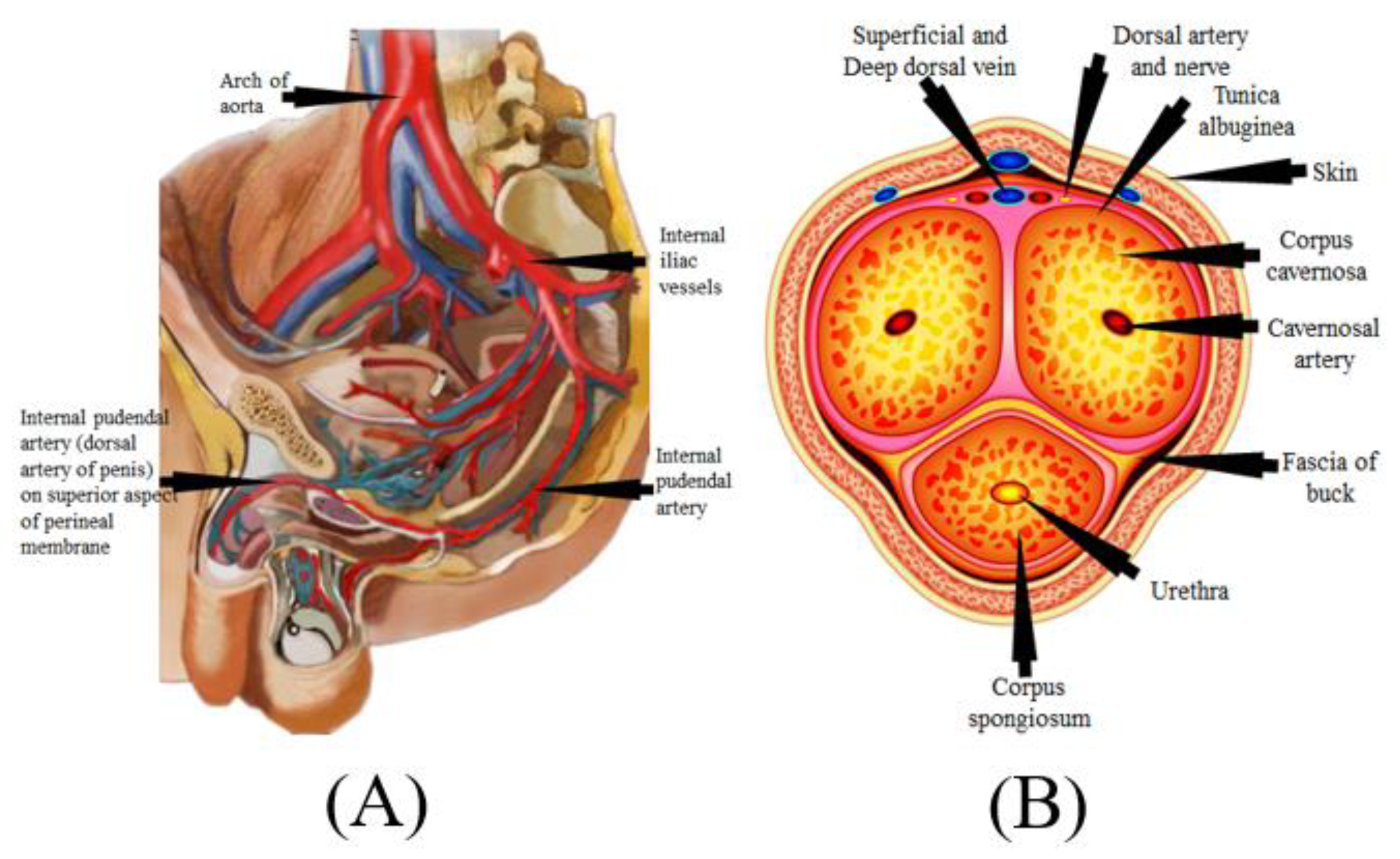
Figure 4. (A) Mechanics of penile erection (courtesy of AtheropointTM, Roseville, CA, USA). (B) Cross-sectional of the penis (courtesy of AtheropointTM, Roseville, CA, USA).
As a result, strong blood flow from the heart to the penal muscle cells is required for a proper erection [105][106]. All these processes cause blood to be caught inside the corpora cavernosa (Figure 4B), resulting in intracavernous pressure and an erection [107].
2.2. The Effect of SARS-CoV-19 on Erectile Dysfunction
SARS-CoV-2, the interaction of the enhanced ACE2 and the transmembrane protease serine 2 with a component of the spike protein, accelerates binding and transit into vascular endothelium cells [108]. According to the studies, endothelial dysfunction is a significant contributor to COVID-19 symptoms [109][110]. The Table 1 show the relationship between ED with CVD or coronary artery disease. Direct viral invasion of testicular tissue via ACE2 receptors, temperature-related testicular injury resulting from sustained high fever, inflammatory and autoimmune responses, and viral infection-related oxidative stress are some of the suggested causes of this damage [111][112]. Figure 5 explains the biological link between ED and CVD/Stroke and Figure 6 validates the biological link between SARS-CoV-19 with ED.
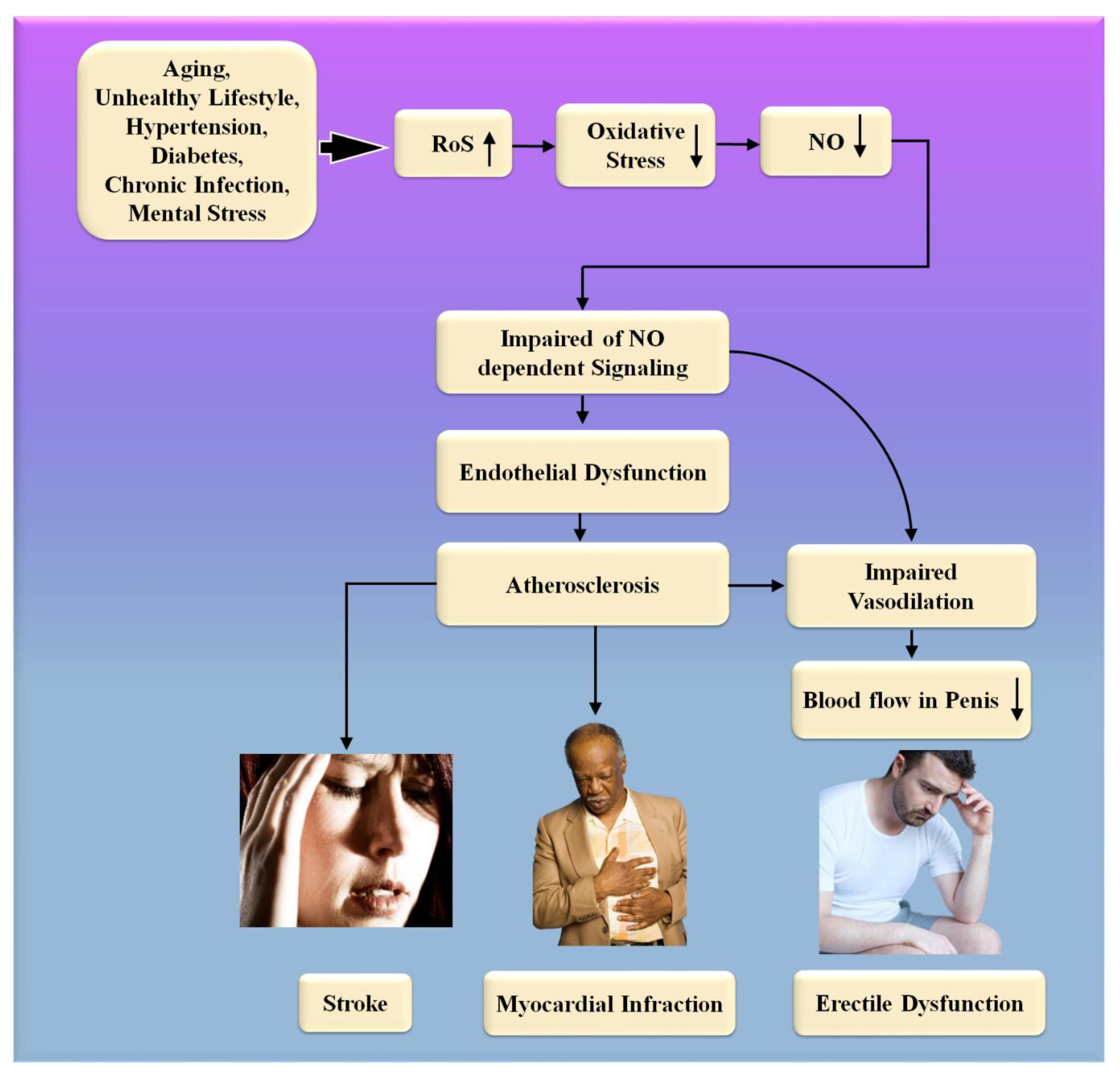
Figure 5. The biological link between ED and CVD/Stroke. RoS: reactive oxides stress, NO: nitric oxide, Up Arrow: depicts increase, Down Arrow: depicts decrease.
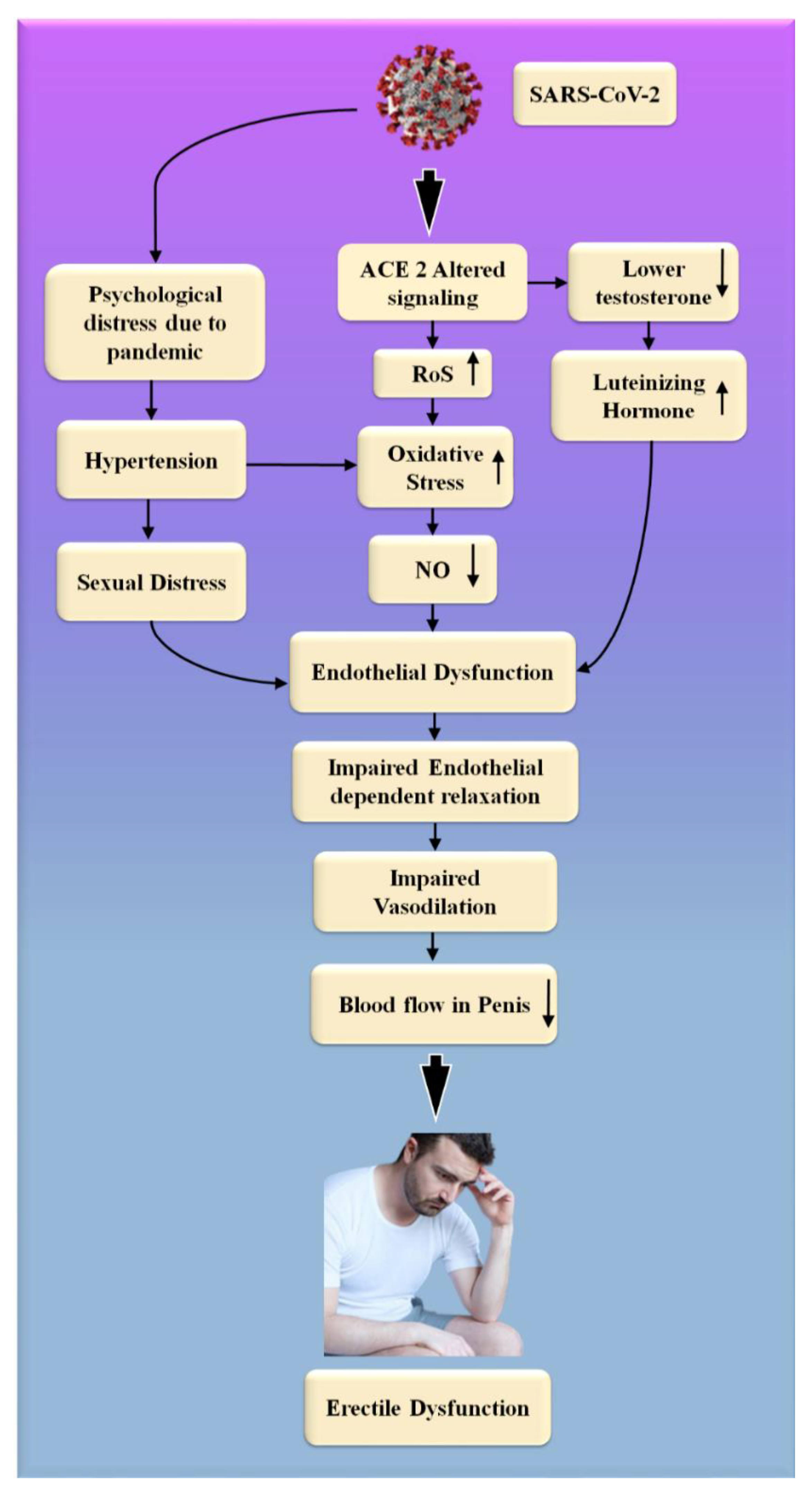
Figure 6. The biological link between COVID-19 and ED. RoS: reactive oxides stress, NO: nitric oxide, Up Arrow: depicts increase, Down Arrow: depicts decrease.
Table 1. The studies show the relationship between ED with CVD or coronary artery disease.
| SN | Citations | Relation * | ME | PS | Outcome | Treatment |
|---|---|---|---|---|---|---|
| 1 | Bonetti et al. [113] (2002) |
ED with CVD | LBBM | 45 | ED is a systemic disease that contributes significantly to the advancement of atherosclerosis and its associated complications. There is a need for direct evidence that therapeutic improvements in endothelial function resulted in decreased CVE rates. | NR |
| 2 | Montorsi et al. [9] (2005) |
ED with CAD | LBBM | 34 | Because of the progressive or simultaneous alterations in microvascular and macrovascular function, ED is fundamentally an atherosclerotic disorder in its origin and progression. | NR |
| 3 | Kirby et al. [16] (2005) |
ED with CAD | OBBM | NR | ED and COAD are two distinct clinical manifestations of the same systemic illness, with pathological causes and risk factors that are quite similar to one another. Because of increased understanding of the emergency department as a barometer for cardiovascular health, it is possible to take early action to reduce future CV risk. | NR |
| 4 | Vlachopoulos et al. [114] (2007) |
ED with CAD | LBBM | NR | ED, inflammation, and low testosterone levels in the bloodstream are all risk factors and pathophysiological links that are shared by cardiovascular disease and erectile dysfunction. | NR |
| 5 | Diaconu et al. [115] (2011) |
ED with CVD | OBBM, LBBM | 231 | Both erectile dysfunction and CVD are symptoms of the same illness. ED symptoms often appear three to five years before the onset of symptoms of coronary artery disease, and they may serve as an early warning indication that CVD is on the verge of manifesting itself. As a result, male patients with CVD risk factors should have their erectile dysfunction checked regularly. | phosphodiesterase-5 inhibitors, alprostadil (prostaglandin E1) intracavernous injections, alternatives for the management of ED. |
| 6 | Yannas et al. [54] (2011) |
ED with CVD | OBBM, LBBM | NR | ED is a sign of cardiovascular disease. As a result, guys with ED should be thoroughly evaluated for cardiovascular risk factors to avoid future CVE (MACE). | NR |
| 7 | Gandaglia et al. [82] (2014) | ED With CVD | LBBM | NR | ED and cardiovascular disease (CVD) are two symptoms of the same systemic illness. Atherosclerosis and blood vessel constriction are caused by the interplay of CV risk factors, androgens, and chronic inflammation in the blood vessels. Endothelial dysfunction and autonomic hyperactivity, for example, are both isotropic alterations in the body. | NR |
| 8 | Lim et al. [13] (2018) |
ED with CVD | OBBM, LBBM | 1757 | Distinguishing between symptoms of ED and cardiovascular disease (CVD) demands a distinct strategy. Atherosclerosis and vascular constriction are associated with each other, and this association is generated by the combination of CV risk factors, androgens, and chronic inflammation. Atherosclerosis and autonomic hyperactivity are both apparent alterations that are isotropic. | NR |
| 9 | Roushias et al. [116] (2018) | ED with CVD | OBBM, LBBM | 1768 | Endothelial dysfunction is a common denominator in the pathophysiology of both erectile dysfunction and cardiovascular disease. ED is a warning symptom of endothelial dysfunction and a risk factor for cardiovascular disease. Early detection and assessment of ED redefines the risk of cardiovascular disease and allows for earlier intervention. Patients with cardiovascular disease should be treated and monitored more closely if they develop erectile dysfunction. | NR |
| 10 | Miner et al. [117] (2019) |
ED with COAD | LBBM | 242 | Angiographic studies show that ED patients under the age of 60 had more severe COAD. This connection is independent of COAD and ED risk factors. | NR |
| 11 | Sayadi et al. [118] (2021) |
ED with COAD | OBBM | 100 | COAD is an indicator of atherosclerosis. As a result, the IIEF questionnaire can help diagnose COAD early on. | NR |
| 12 | Kałka et al. [119] (2021) |
ED with COAD | OBBM, LBBM | 751 | Sexual health concerns are crucial in cardiac patients. ED predicts CVD due to shared risk factors and pathophysiology. Hypertension, dyslipidemia, smoking, diabetes, obesity, and a poor diet all contribute to vascular endothelium dysfunction. | NR |
| 13 | Inman et al. [120] (2021) |
ED with COAD | LBBM | 1402 | ED and CAD may be signs of the same vascular illness. In young men, ED increases the risk of future cardiac incidents, but in older men, it appears to have little predictive value. | NR |
| 14 | Imprialos et al. [121] (2021) | ED with CVD | LBBM | NR | Erectile dysfunction is a major health condition that affects many people, and it is more common in people with cardiovascular risk factors or illnesses. Both ED and CVD share pathophysiological pathways. | Patients with or without cardiovascular illness can use phosphodiesterase type 5 inhibitors as first-line ED treatment. |
| 15 | Rinkūnienė et al. [122] (2021) | ED with CVD | LBBM | 171 | ED is common in guys who have had a MI. Men with a history of MI had greater traditional CVD risk factors. Men with ED who have had a MI are more prone to AH. | NR |
* SN: serial number, RELATION: effect of ED on CVD, ME: method of evaluation, PS: patient size, OE: outcome, TE: treatment, NR: not reported, MI: myocardial interaction, OBBM: office-based biomarker, LBBM: lab-based biomarker, NR: not reported.
Endothelial cells infected with SARS-CoV-2 suffer endothelial damage, which causes thromboembolic vascular lumen alteration in the endothelium, immune thrombosis, and reversal in many organs [123]. These are the ultimate and noticeable consequences of the cells taken by SARS-CoV-2 from the endothelium [124]. ED is one of the most common symptoms of COVID-19, which is caused by endothelial dysfunction [123]. This can result in circulatory problems in numerous organs [109][110]. This includes a reduction in blood supply to the testicles, which can lead to ED. Natural nitric oxide (NO), generated by healthy endothelial cells, is an essential cofactor in the endothelium-dependent phase transition in the corpora cavernosa [125]. Endothelial dysfunction is caused by a decrease in eNOS expression, which results in a decrease in NO production [126][127]. Increased endothelium-bound cavernosal tissue vasodilation is associated with hypertension and diabetes [128].
People were experiencing psychological trauma, as well as the overall feeling of a high degree of uncertainty associated with the COVID-19 global epidemic [129]. The restrictive measures that were implemented during this critical period, in the long term, influenced interpersonal and intimate relationships [130]. Concerns about safe intimate/sexual interplay, the forced separation of intimate partners, the escalation of marital disputes, and degradation in contact are some of the most significant contributors to a person’s experience of sexual troubles and sexual unhappiness at this age [130][131][132].
Sexual desire and expression differences, as well as a lack of privacy while confined, have both been linked to the development of sexual difficulties and dissatisfaction [133][134]. COVID-19 infection, on the other hand, has the potential to negatively impact male sexual function by inducing endothelial damage, which can result in erectile dysfunction, testicular injury, and psychological alterations [134][135].
It can be hypothesized that erectile dysfunction occurs more frequently in the presence of heart issues when the endothelium and smooth muscle are dysfunctional. Endothelial dysfunction impairs blood flow to the heart and the penis, contributing to the development of atherosclerosis.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics12051249
References
- Nguyen, H.M.T.; Gabrielson, A.T.; Hellstrom, W.J. Erectile dysfunction in young men—A review of the prevalence and risk factors. J. Sex. Med. Rev. 2017, 5, 508–520.
- Ludwig, W.; Phillips, M. Organic causes of erectile dysfunction in men under 40. J. Urol. Int. 2014, 92, 1–6.
- Solomon, H.; Man, J.W.; Wierzbicki, A.S.; Jackson, G. Relation of erectile dysfunction to angiographic coronary artery disease. J. Am. J. Cardiol. 2003, 91, 230.
- Cui, X.; Zhou, J.; Qin, Z.; Liu, Z. Acupuncture for erectile dysfunction: A systematic review. J. BioMed. Res. Int. 2016, 2016, 2171923.
- Kouyanou, K.; Pither, C.E.; Wessely, S. Iatrogenic factors and chronic pain. J. Psychosom. Med. 1997, 59, 597–604.
- Johansson, M.; Ehnvall, A.; Friberg, P.; Myredal, A. Arterial baroreflex dysfunction in major depressive disorder. J. Clin. Auton. Res. 2010, 20, 235–240.
- Porst, H.; Montorsi, F.; Rosen, R.C.; Gaynor, L.; Grupe, S.; Alexander, J. The Premature Ejaculation Prevalence and Attitudes (PEPA) survey: Prevalence, comorbidities, and professional help-seeking. J. Eur. Urol. 2007, 51, 816–824.
- Sabino-Carvalho, J.L.; Falquetto, B.; Takakura, A.C.; Vianna, L.C. Baroreflex dysfunction in Parkinson’s disease: Integration of central and peripheral mechanisms. J. Neurophysiol. 2021, 125, 1425–1439.
- Montorsi, P.; Ravagnani, P.M.; Galli, S.; Rotatori, F.; Briganti, A.; Salonia, A.; Rigatti, P.; Montorsi, F. The artery size hypothesis: A macrovascular link between erectile dysfunction and coronary artery disease. J. Am. J. Cardiol. 2005, 96, 19–23.
- Walter, B.L. Cardiovascular autonomic dysfunction in patients with movement disorders. J. Clevel. Clin. J. Med. 2008, 75, S54.
- Tamler, R. Diabetes, obesity, and erectile dysfunction. J. Gend. Med. 2009, 6, 4–16.
- Gowani, Z.; Uddin, S.; Mirbolouk, M.; Ayyaz, D.; Billups, K.L.; Miner, M.; Feldman, D.I.; Blaha, M.J. Vascular erectile dysfunction and subclinical cardiovascular disease. J. Curr. Sex. Health Rep. 2017, 9, 305–312.
- Lim, G.B. Erectile dysfunction predicts CVD events. J. Nat. Rev. Cardiol. 2018, 15, 502.
- Ryan, J.M.; Peterson, M.D.; Ryan, N.; Smith, K.J.; O’connell, N.E.; Liverani, S.; Anokye, N.; Victor, C.; Allen, E. Mortality due to cardiovascular disease, respiratory disease, and cancer in adults with cerebral palsy. J. Dev. Med. Child Neurol. 2019, 61, 924–928.
- Osondu, C.U.; Vo, B.; Oni, E.T.; Blaha, M.J.; Veledar, E.; Feldman, T.; Agatston, A.S.; Nasir, K.; Aneni, E.C. The relationship of erectile dysfunction and subclinical cardiovascular disease: A systematic review and meta-analysis. J. Vasc. Med. 2018, 23, 9–20.
- Kirby, M. The association between erectile dysfunction and CVD. J. Trends Urol. Men’s Health 2019, 10, 11–15.
- Choo, E.H.; Chang, K.; Lee, K.Y.; Lee, D.; Kim, J.G.; Ahn, Y.; Kim, Y.J.; Chae, S.C.; Cho, M.C.; Kim, C.J. Prognosis and predictors of mortality in patients suffering myocardial infarction with non-obstructive coronary arteries. J. Am. Heart Assoc. 2019, 8, e011990.
- Buob, A.; Winter, H.; Kindermann, M.; Becker, G.; Möller, J.; Oertel, W.; Böhm, M. Parasympathetic but not sympathetic cardiac dysfunction at early stages of Parkinson’s disease. J. Clin. Res. Cardiol. 2010, 99, 701–706.
- Shiferaw, W.S.; Akalu, T.Y.; Aynalem, Y.A. Prevalence of erectile dysfunction in patients with diabetes mellitus and its association with body mass index and Glycated hemoglobin in Africa: A systematic review and meta-analysis. Int. J. Endocrinol. 2020, 2020, 5148370.
- Lasker, G.F.; Maley, J.H.; Kadowitz, P.J. A review of the pathophysiology and novel treatments for erectile dysfunction. J. Adv. Pharmacol. Sci. 2010, 2010, 730861.
- Aschenbach, R.; Steiner, T.; Kerl, M.; Zangos, S.; Basche, S.; Vogl, T. Endovascular embolisation therapy in men with erectile impotence due to veno-occlusive dysfunction. Eur. J. Radiol. 2013, 82, 504–507.
- Shamloul, R.; Ghanem, H. Erectile dysfunction. Lancet 2013, 381, 153–165.
- Javaroni, V.; Neves, M.F. Erectile dysfunction and hypertension: Impact on cardiovascular risk and treatment. Int. J. Hypertens. 2012, 2012, 627278.
- Eardley, I. Imaging for erectile dysfunction. J. Curr. Opin. Urol. 2002, 12, 143–147.
- Aversa, A.; Sarteschi, L.M. The role of penile color-duplex ultrasound for the evaluation of erectile dysfunction. J. Sex. Med. 2007, 4, 1437–1447.
- Seftel, A.D. Erectile dysfunction in the elderly: Epidemiology, etiology and approaches to treatment. J. Urol. 2003, 169, 1999–2007.
- Liao, K.P.; Ananthakrishnan, A.N.; Kumar, V.; Xia, Z.; Cagan, A.; Gainer, V.S.; Goryachev, S.; Chen, P.; Savova, G.K.; Agniel, D. Methods to develop an electronic medical record phenotype algorithm to compare the risk of coronary artery disease across 3 chronic disease cohorts. PLoS ONE 2015, 10, e0136651.
- Jamthikar, A.D.; Gupta, D.; Mantella, L.E.; Saba, L.; Laird, J.R.; Johri, A.M.; Suri, J.S. Multiclass machine learning vs. conventional calculators for stroke/CVD risk assessment using carotid plaque predictors with coronary angiography scores as gold standard: A 500 participants study. Int. J. Cardiovasc. Imaging 2021, 37, 1171–1187.
- Rybicki, F.J.; Melchionna, S.; Mitsouras, D.; Coskun, A.U.; Whitmore, A.G.; Steigner, M.; Nallamshetty, L.; Welt, F.G.; Bernaschi, M.; Borkin, M. Prediction of coronary artery plaque progression and potential rupture from 320-detector row prospectively ECG-gated single heart beat CT angiography: Lattice Boltzmann evaluation of endothelial shear stress. Int. J. Cardiovasc. Imaging 2009, 25, 289–299.
- Jamthikar, A.D.; Gupta, D.; Johri, A.M.; Mantella, L.E.; Saba, L.; Kolluri, R.; Sharma, A.M.; Viswanathan, V.; Nicolaides, A.; Suri, J.S. Low-cost office-based cardiovascular risk stratification using machine learning and focused carotid ultrasound in an Asian-Indian cohort. J. Med. Syst. 2020, 44, 1–15.
- Chiurlia, E.; D’Amico, R.; Ratti, C.; Granata, A.R.; Romagnoli, R.; Modena, M.G. Subclinical coronary artery atherosclerosis in patients with erectile dysfunction. J. Am. Coll. Cardiol. 2005, 46, 1503–1506.
- Wilson, P.W.; Bozeman, S.R.; Burton, T.M.; Hoaglin, D.C.; Ben-Joseph, R.; Pashos, C.L. Prediction of first events of coronary heart disease and stroke with consideration of adiposity. J. Circ. 2008, 118, 124–130.
- Jackson, G. Prevention of cardiovascular disease by the early identification of erectile dysfunction. Int. J. Impot. Res. 2008, 20, S9–S14.
- Tandel, G.S.; Biswas, M.; Kakde, O.G.; Tiwari, A.; Suri, H.S.; Turk, M.; Laird, J.R.; Asare, C.K.; Ankrah, A.A.; Khanna, N.J.C. A review on a deep learning perspective in brain cancer classification. Cancers 2019, 11, 111.
- Biswas, M.; Kuppili, V.; Saba, L.; Edla, D.R.; Suri, H.S.; Cuadrado-Godia, E.; Laird, J.R.; Marinhoe, R.T.; Sanches, J.M.; Nicolaides, A.J.F.B. State-of-the-art review on deep learning in medical imaging. Front. Biosci.—Landmark 2019, 24, 392–426.
- Saba, L.; Biswas, M.; Kuppili, V.; Godia, E.C.; Suri, H.S.; Edla, D.R.; Omerzu, T.; Laird, J.R.; Khanna, N.N.; Mavrogeni, S. The present and future of deep learning in radiology. Eur. J. Radiol. 2019, 114, 14–24.
- Kuppili, V.; Biswas, M.; Sreekumar, A.; Suri, H.S.; Saba, L.; Edla, D.R.; Marinhoe, R.T.; Sanches, J.M.; Suri, J.S. Extreme learning machine framework for risk stratification of fatty liver disease using ultrasound tissue characterization. J. Med. Syst. 2017, 41, 152.
- Maniruzzaman, M.; Rahman, M.J.; Al-MehediHasan, M.; Suri, H.S.; Abedin, M.M.; El-Baz, A.; Suri, J.S. Accurate diabetes risk stratification using machine learning: Role of missing value and outliers. J. Med. Syst. 2018, 42, 92.
- Acharya, U.R.; Faust, O.; Sree, S.V.; Molinari, F.; Suri, J.S. ThyroScreen system: High resolution ultrasound thyroid image characterization into benign and malignant classes using novel combination of texture and discrete wavelet transform. J. Comput. Methods Programs Biomed. 2012, 107, 233–241.
- Schneider, D.F.; Chen, H. New developments in the diagnosis and treatment of thyroid cancer. J. CA Cancer J. Clin. 2013, 63, 373–394.
- Pareek, G.; Acharya, U.R.; Sree, S.V.; Swapna, G.; Yantri, R.; Martis, R.J.; Saba, L.; Krishnamurthi, G.; Mallarini, G.; El-Baz, A. Prostate tissue characterization/classification in 144 patient population using wavelet and higher order spectra features from transrectal ultrasound images. J. Technol. Cancer Res. Treat. 2013, 12, 545–557.
- McClure, P.; Elnakib, A.; El-Ghar, M.A.; Khalifa, F.; Soliman, A.; El-Diasty, T.; Suri, J.S.; Elmaghraby, A.; El-Baz, A. In-vitro and in-vivo diagnostic techniques for prostate cancer: A review. J. Biomed. Nanotechnol. 2014, 10, 2747–2777.
- Acharya, U.R.; Saba, L.; Molinari, F.; Guerriero, S.; Suri, J.S. Ovarian tumor characterization and classification: A class of GyneScanTM systems. In 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society; IEEE: Piscataway, NJ, USA, 2012; pp. 4446–4449.
- Liu, K.; Suri, J.S. Automatic Vessel Indentification for Angiographic Screening. U.S. Patent No. 6,845,260, 18 January 2005.
- Suri, J.S.; Bhagawati, M.; Paul, S.; Protogeron, A.; Sfikakis, P.P.; Kitas, G.D.; Khanna, N.N.; Ruzsa, Z.; Sharma, A.M.; Saxena, S.; et al. Understanding the bias in machine learning systems for cardiovascular disease risk assessment: The first of its kind review. Comput. Biol. Med. 2022, 142, 105204.
- Knuuti, J.; Bengel, F.; Bax, J.J.; Kaufmann, P.A.; le Guludec, D.; Filardi, P.P.; Marcassa, C.; Marsan, N.A.; Achenbach, S.; Kitsiou, A. Risks and benefits of cardiac imaging: An analysis of risks related to imaging for coronary artery disease. Eur. Heart J. 2014, 35, 633–638.
- Acharya, U.R.; Faust, O.; Sree, S.V.; Molinari, F.; Saba, L.; Nicolaides, A.; Suri, J.S. An accurate and generalized approach to plaque characterization in 346 carotid ultrasound scans. IEEE Trans. Instrum. Meas. 2011, 61, 1045–1053.
- Soares, H.D.; Lovestone, S. Biomarker utility in Alzheimer’s disease clinical trials. J. Drug Discov. Today Ther. Strateg. 2013, 10, e55–e62.
- Paul, S.; Maindarkar, M.; Saxena, S.; Saba, L.; Turk, M.; Kalra, M.; Krishnan, P.R.; Suri, J.S. Bias Investigation in Artificial Intelligence Systems for Early Detection of Parkinson’sDisease: A Narrative Review. Diagnostics 2022, 12, 166.
- Sibley, K.G.; Girges, C.; Hoque, E.; Foltynie, T. Video-based analyses of Parkinson’s disease severity: A brief review. J. Parkinson’s Dis. 2021, 1–11, preprint.
- Dias, A.E.; Limongi, J.C.; Barbosa, E.R.; Hsing, W.T. Voice telerehabilitation in Parkinson’s disease. J. Codas 2016, 28, 176–181.
- Agarwal, M.; Saba, L.; Gupta, S.K.; Carriero, A.; Falaschi, Z.; Paschè, A.; Danna, P.; El-Baz, A.; Naidu, S.; Suri, J.S. A novel block imaging technique using nine artificial intelligence models for COVID-19 disease classification, characterization and severity measurement in lung computed tomography scans on an Italian cohort. J. Med. Syst. 2021, 45, 1–30.
- Suri, J.S.; Agarwal, S.; Gupta, S.K.; Puvvula, A.; Biswas, M.; Saba, L.; Bit, A.; Tandel, G.S.; Agarwal, M.; Patrick, A. A narrative review on characterization of acute respiratory distress syndrome in COVID-19-infected lungs using artificial intelligence. Comput. Biol. Med. 2021, 130, 104210.
- Yannas, D.; Frizza, F.; Vignozzi, L.; Corona, G.; Maggi, M.; Rastrelli, G. Erectile Dysfunction Is a Hallmark of Cardiovascular Disease: Unavoidable Matter of Fact or Opportunity to Improve Men’s Health? J. Clin. Med. 2021, 10, 2221.
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. J. Lancet 2014, 383, 999–1008.
- Jashari, F.; Ibrahimi, P.; Nicoll, R.; Bajraktari, G.; Wester, P.; Henein, M.Y. Coronary and carotid atherosclerosis: Similarities and differences. J. Atheroscler. 2013, 227, 193–200.
- Anderson, K.M.; Odell, P.M.; Wilson, P.W.; Kannel, W.B. Cardiovascular disease risk profiles. J. Am. Heart J. 1991, 121, 293–298.
- Payne, R.A. Cardiovascular risk. Br. J. Clin. Pharmacol. 2012, 74, 396–410.
- Lerner, D.J.; Kannel, W.B. Patterns of coronary heart disease morbidity and mortality in the sexes: A 26-year follow-up of the Framingham population. Am. Heart J. 1986, 111, 383–390.
- Lloyd-Jones, D.M.; Nam, B.-H.; Sr, R.B.D.; Levy, D.; Murabito, J.M.; Wang, T.J.; Wilson, P.W.; O’Donnell, C. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: A prospective study of parents and offspring. J. JAMA Cardiol. 2004, 291, 2204–2211.
- Bachmann, J.M.; Willis, B.L.; Ayers, C.R.; Khera, A.; Berry, J.D. Association between family history and coronary heart disease death across long-term follow-up in men: The Cooper Center Longitudinal Study. J. Circ. 2012, 125, 3092–3098.
- Sivapalaratnam, S.; Boekholdt, S.M.; Trip, M.D.; Sandhu, M.S.; Luben, R.; Kastelein, J.J.; Wareham, N.J.; Khaw, K.-T. Family history of premature coronary heart disease and risk prediction in the EPIC-Norfolk prospective population study. J. Heart 2010, 96, 1985–1989.
- Hoit, B.D.; Gilpin, E.; Henning, H.; Maisel, A.; Dittrich, H.; Carlisle, J.; Ross, J., Jr. Myocardial infarction in young patients: An analysis by age subsets. J. Circ. 1986, 74, 712–721.
- Bier, A.; Braun, T.; Khasbab, R.; di Segni, A.; Grossman, E.; Haberman, Y.; Leibowitz, A. A high salt diet modulates the gut microbiota and short chain fatty acids production in a salt-sensitive hypertension rat model. J. Nutr. 2018, 10, 1154.
- Collaboration, P.S. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. J. Lancet 2002, 360, 1903–1913.
- Stamler, J.; Stamler, R.; Neaton, J.D. Blood pressure, systolic and diastolic, and cardiovascular risks: US population data. J. Arch. Intern. Med. 1993, 153, 598–615.
- Kannel, W.B. Elevated systolic blood pressure as a cardiovascular risk factor. Am. J. Cardiol. 2000, 85, 251–255.
- Almdal, T.; Scharling, H.; Jensen, J.S.; Vestergaard, H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: A population-based study of 13 000 men and women with 20 years of follow-up. J. Arch. Intern. Med. 2004, 164, 1422–1426.
- Sheth, T.; Nair, C.; Muller, J.; Yusuf, S. Increased winter mortality from acute myocardial infarction and stroke: The effect of age. J. Am. Coll. Cardiol. 1999, 33, 1916–1919.
- Selvin, E.; Marinopoulos, S.; Berkenblit, G.; Rami, T.; Brancati, F.L.; Powe, N.R.; Golden, S.H. Meta-analysis: Glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. J. Ann. Intern. Med. 2004, 141, 421–431.
- Huang, Y.; Cai, X.; Mai, W.; Li, M.; Hu, Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: Systematic review and meta-analysis. J. BMJ 2016, 355, i5953.
- Lakier, J.B. Smoking and cardiovascular disease. Am. J. Med. 1992, 93, S8–S12.
- Prescott, E.; Hippe, M.; Schnohr, P.; Hein, H.O.; Vestbo, J. Smoking and risk of myocardial infarction in women and men: Longitudinal population study. J. BMJ 1998, 316, 1043.
- Manna, P.; Jain, S.K. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: Causes and therapeutic strategies. J. Metab. Syndr. Relat. Disord. 2015, 13, 423–444.
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.L.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. J. Lancet 2013, 382, 339–352.
- Joshipura, K.J.; Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Rimm, E.B.; Speizer, F.E.; Colditz, G.; Ascherio, A.; Rosner, B.; Spiegelman, D. The effect of fruit and vegetable intake on risk for coronary heart disease. J. Ann. Intern. Med. 2001, 134, 1106–1114.
- Powell, K.E.; Thompson, P.D.; Caspersen, C.J.; Kendrick, J.S. Physical activity and the incidence of coronary heart disease. J. Annu. Rev. Public Health 1987, 8, 253–287.
- Feldman, H.A.; Johannes, C.B.; Derby, C.A.; Kleinman, K.P.; Mohr, B.A.; Araujo, A.B.; McKinlay, J.B. Erectile dysfunction and coronary risk factors: Prospective results from the Massachusetts male aging study. J. Prev. Med. 2000, 30, 328–338.
- Fung, M.M.; Bettencourt, R.; Barrett-Connor, E. Heart disease risk factors predict erectile dysfunction 25 years later: The Rancho Bernardo Study. J. Am. Coll. Cardiol. 2004, 43, 1405–1411.
- Ridker, P.M.; Glynn, R.J.; Hennekens, C.H. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. J. Circ. 1998, 97, 2007–2011.
- Hackett, G.; Krychman, M.; Baldwin, D.; Bennett, N.; El-Zawahry, A.; Graziottin, A.; Lukasiewicz, M.; McVary, K.; Sato, Y.; Incrocci, L. Coronary heart disease, diabetes, and sexuality in men. J. Sex. Med. 2016, 13, 887–904.
- Gandaglia, G.; Salonia, A.; Passoni, N.; Montorsi, P.; Briganti, A.; Montorsi, F. Erectile dysfunction as a cardiovascular risk factor in patients with diabetes. J. Endocr. 2013, 43, 285–292.
- Shin, D.; Pregenzer, G., Jr.; Gardin, J.M. Erectile dysfunction: A disease marker for cardiovascular disease. J. Cardiol. Rev. 2011, 19, 5–11.
- Ryan, J.G.; Gajraj, J. Erectile dysfunction and its association with metabolic syndrome and endothelial function among patients with type 2 diabetes mellitus. J. Diabetes Its Complicat. 2012, 26, 141–147.
- Billups, K.; Bank, A.; Padma-Nathan, H.; Katz, S.; Williams, R. Erectile dysfunction as a harbinger for increased cardiometabolic risk. Int. J. Impot. Res. 2008, 20, 236–242.
- Bedir, F.; Kocatürk, H.; Altay, M.S.; Kocaturk, H.; Bedir, B.; Yapanoglu, T. Erektil Disfonksiyon Şikayeti ile Üroloji Polikliniğine Başvuran Hastalarda Kardiyovasküler Hastalıkların Değerlendirilmesi. J. Kocaeli Tıp Derg. 2021, 10 (Suppl. 2), 38–43.
- Marwah, R.S.; Doux, J.D.; Lee, P.Y.; Yun, A.J. Is atherosclerosis a neurogenic phenomenon? J. Med. Hypotheses 2007, 69, 884–887.
- Kendirci, M.; Nowfar, S.; Hellstrom, W.J. The impact of vascular risk factors on erectile function. J. Drugs Today 2005, 41, 65.
- van Dijk, R.A.; Rauwerda, J.A.; Steyn, M.; Twisk, J.W.; Stehouwer, C.D. Long-term homocysteine-lowering treatment with folic acid plus pyridoxine is associated with decreased blood pressure but not with improved brachial artery endothelium-dependent vasodilation or carotid artery stiffness: A 2-year, randomized, placebo-controlled trial. J. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 2072–2079.
- Kirby, M.; Jackson, G.; Betteridge, J.; Friedli, K. Is erectile dysfunction a marker for cardiovascular disease? Int. J. Clin. Pract. 2001, 55, 614–618.
- Blum, A.; Hadas, V.; Burke, M.; Yust, I.; Kessler, A. Viral load of the human immunodeficiency virus could be an independent risk factor for endothelial dysfunction. J. Clin. Cardiol. 2005, 28, 149–153.
- Thum, T.; Tsikas, D.; Frölich, J.C.; Borlak, J. Growth hormone induces eNOS expression and nitric oxide release in a cultured human endothelial cell line. J. FEBS Lett. 2003, 555, 567–571.
- Tycinska, A.; Mroczko, B.; Musial, W.; Sawicki, R.; Kaminski, K.; Borowska, H.; Sobkowicz, B.; Szmitkowski, M. Blood pressure in relation to neurogenic, inflammatory and endothelial dysfunction biomarkers in patients with treated essential arterial hypertension. J. Adv. Med. Sci. 2011, 56, 80–87.
- Ponholzer, A.; Stopfer, J.; Bayer, G.; Susani, M.; Steinbacher, F.; Herbst, F.; Schramek, P.; Madersbacher, S.; Maresch, J. Is penile atherosclerosis the link between erectile dysfunction and cardiovascular risk? An autopsy study. Int. J. Impot. Res. 2012, 24, 137–140.
- Gandaglia, G.; Briganti, A.; Jackson, G.; Kloner, R.A.; Montorsi, F.; Montorsi, P.; Vlachopoulos, C. A systematic review of the association between erectile dysfunction and cardiovascular disease. J. Eur. Urol. 2014, 65, 968–978.
- Gazzaruso, C.; Giordanetti, S.; de Amici, E.; Bertone, G.; Falcone, C.; Geroldi, D.; Fratino, P.; Solerte, S.B.; Garzaniti, A. Relationship between erectile dysfunction and silent myocardial ischemia in apparently uncomplicated type 2 diabetic patients. J. Circ. 2004, 110, 22–26.
- García-Malpartida, K.; Mármol, R.; Jover, A.; Gómez-Martínez, M.J.; Solá-Izquierdo, E.; Victor, V.M.; Rocha, M.; Sanmiguel, D.; Hernández-Mijares, A. Relationship between erectile dysfunction and silent myocardial ischemia in type 2 diabetic patients with no known macrovascular complications. J. Sex. Med. 2011, 8, 2606–2616.
- Chironi, G.N.; Boulanger, C.M.; Simon, A.; Dignat-George, F.; Freyssinet, J.-M.; Tedgui, A. Endothelial microparticles in diseases. J. Cell Tissue Res. 2009, 335, 143–151.
- Mirone, V.; Imbimbo, C.; Fusco, F.; Verze, P.; Creta, M.; Tajana, G. Androgens and morphologic remodeling at penile and cardiovascular levels: A common piece in complicated puzzles? J. Eur. Urol. 2009, 56, 309–316.
- Rajagopalan, V.; Gerdes, A.M. Role of thyroid hormones in ventricular remodeling. J. Curr. Heart Fail. Rep. 2015, 12, 141–149.
- Kaynar, M.; Gomes, A.L.Q.; Sokolakis, I.; Gül, M. Tip of the iceberg: Erectile dysfunction and COVID-19. Int. J. Impot. Res. 2022, 34, 152–157.
- Dispenzieri, A.; Moreno-Aspitia, A.; Suarez, G.A.; Lacy, M.Q.; Colon-Otero, G.; Tefferi, A.; Litzow, M.R.; Roy, V.; Hogan, W.J.; Kyle, R.A. Peripheral blood stem cell transplantation in 16 patients with POEMS syndrome, and a review of the literature. J. Blood 2004, 104, 3400–3407.
- Hernández-Cerda, J.; Bertomeu-González, V.; Zuazola, P.; Cordero, A. Understanding erectile dysfunction in hypertensive patients: The need for good patient management. J. Vasc. Health Risk Manag. 2020, 16, 231.
- Zeiher, A.M.; Drexler, H.; Saurbier, B.; Just, H. Endothelium-mediated coronary blood flow modulation in humans. Effects of age, atherosclerosis, hypercholesterolemia, and hypertension. J. Clin. Investig. 1993, 92, 652–662.
- Aversa, A.; Isidori, A.; de Martino, M.; Caprio, M.; Fabbrini, E.; Rocchietti-March, M.; Frajese, G.; Fabbri, A. Androgens and penile erection: Evidence for a direct relationship between free testosterone and cavernous vasodilation in men with erectile dysfunction. J. Clin. Endocrinol. 2000, 53, 517–522.
- Lue, T.F. Erectile dysfunction. N. Engl. J. Med. 2000, 342, 1802–1813.
- Jevtich, M.J.; Khawand, N.Y.; Vidic, B. Clinical significance of ultrastructural findings in the corpora cavernosa of normal and impotent men. J. Urol. 1990, 143, 289–293.
- Djomkam, A.L.Z.; Olwal, C.O.; Sala, T.B.; Paemka, L. Commentary: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. J. Front. Oncol. 2020, 1448.
- Jung, F.; Krüger-Genge, A.; Franke, R.-P.; Hufert, F.; Küpper, J.-H. COVID-19 and the endothelium. J. Clin. Hemorheol. Microcirc. 2020, 75, 7–11.
- Pons, S.; Fodil, S.; Azoulay, E.; Zafrani, L. The vascular endothelium: The cornerstone of organ dysfunction in severe SARS-CoV-2 infection. J. Crit. Care 2020, 24, 1–8.
- Fathi, M.; Vakili, K.; Aliaghaei, A.; Nematollahi, S.; Peirouvi, T.; Shalizar-Jalali, A. Coronavirus disease and male fertility: A systematic review. Middle East Fertil. Soc. J. 2021, 26, 1–6.
- Pavone, C.; Giammanco, G.M.; Baiamonte, D.; Pinelli, M.; Bonura, C.; Montalbano, M.; Profeta, G.; Curcurù, L.; Bonura, F. Italian males recovering from mild COVID-19 show no evidence of SARS-CoV-2 in semen despite prolonged nasopharyngeal swab positivity. Int. J. Impot. Res. 2020, 32, 560–562.
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction: A marker of atherosclerotic risk. J. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175.
- Vlachopoulos, C.; Aznaouridis, K.; Ioakeimidis, N.; Rokkas, K.; Vasiliadou, C.; Alexopoulos, N.; Stefanadi, E.; Askitis, A.; Stefanadis, C. Unfavourable endothelial and inflammatory state in erectile dysfunction patients with or without coronary artery disease. Eur. Heart J. 2006, 27, 2640–2648.
- Diaconu, C.C.; Manea, M.; Marcu, D.R.; Socea, B.; Spinu, A.D.; Bratu, O.G. The erectile dysfunction as a marker of cardiovascular disease: A review. J. Acta Cardiol. 2020, 75, 286–292.
- Roushias, S.; Ossei-Gerning, N. Sexual function and cardiovascular disease: What the general cardiologist needs to know. J. Heart 2019, 105, 160–168.
- Miner, M.; Parish, S.J.; Billups, K.L.; Paulos, M.; Sigman, M.; Blaha, M.J. Erectile dysfunction and subclinical cardiovascular disease. J. Sex. Med. Rev. 2019, 7, 455–463.
- Sayadi, M.; Elmafshar, R.; Razeghian-Jahromi, I.; Zibaeenezhad, M.J. Detection of Coronary Artery Disease by an Erectile Dysfunction Questionnaire. J. Cardiol. Res. Pract. 2021, 2021, 6647995.
- Kałka, D.; Gebala, J.; Biernikiewicz, M.; Mrozek-Szetela, A.; Rożek-Piechura, K.; Sobieszczańska, M.; Szuster, E.; Majchrowska, M.; Miętka, A.; Rusiecka, A. Erectile Dysfunction in Men Burdened with the Familial Occurrence of Coronary Artery Disease. J. Clin. Med. 2021, 10, 4046.
- Inman, B.A.; Sauver, J.L.S.; Jacobson, D.J.; McGree, M.E.; Nehra, A.; Lieber, M.M.; Roger, V.L.; Jacobsen, S.J. A population-based, longitudinal study of erectile dysfunction and future coronary artery disease. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2009; Volume 84, pp. 108–113.
- Imprialos, K.; Koutsampasopoulos, K.; Manolis, A.; Doumas, M. Erectile Dysfunction as a Cardiovascular Risk Factor: Time to Step Up? J. Curr. Vasc. Pharmacol. 2021, 19, 301–312.
- Rinkūnienė, E.; Gimžauskaitė, S.; Badarienė, J.; Dženkevičiūtė, V.; Kovaitė, M.; Čypienė, A. The Prevalence of Erectile Dysfunction and Its Association with Cardiovascular Risk Factors in Patients after Myocardial Infarction. J. Med. 2021, 57, 1103.
- Ergül, E.; Yılmaz, A.S.; Öğütveren, M.M.; Emlek, N.; Kostakoğlu, U.; Çetin, M. COVID 19 disease independently predicted endothelial dysfunction measured by flow-mediated dilatation. Int. J. Cardiovasc. Imaging 2022, 38, 25–32.
- Zhou, L.; Xu, Z.; Castiglione, G.M.; Soiberman, U.S.; Eberhart, C.G.; Duh, E.J. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. J. Ocul. Surf. 2020, 18, 537–544.
- Gur, S.; Alzweri, L.; Yilmaz-Oral, D.; Kaya-Sezginer, E.; Abdel-Mageed, A.B.; Dick, B.; Sikka, S.C.; Oztekin, C.V.; Hellstrom, W.J. Testosterone positively regulates functional responses and nitric oxide expression in the isolated human corpus cavernosum. J. Androl. 2020, 8, 1824–1833.
- Aydinoglu, F.; Ogulener, N. The relaxant mechanisms of hydrogen sulfide in corpus cavernosum. In Vascular Effects of Hydrogen Sulfide; Springer: Berlin/Heidelberg, Germany, 2019; pp. 137–150.
- Bertolo, R.; Cipriani, C.; Bove, P. Anosmia and ageusia: A piece of the puzzle in the etiology of COVID-19-related transitory erectile dysfunction. J. Endocrinol. Investig. 2021, 44, 1123–1124.
- Musicki, B.; Burnett, A. Endothelial dysfunction in diabetic erectile dysfunction. Int. J. Impot. Res. 2007, 19, 129–138.
- Aksoy, Y.E.; Koçak, V. Psychological effects of nurses and midwives due to COVID-19 outbreak: The case of Turkey. J. Arch. Psychiatr. Nurs. 2020, 34, 427–433.
- Carvalho, J.; Pascoal, P.M. Challenges in the practice of sexual medicine in the time of COVID-19 in Portugal. J. Sex. Med. 2020, 17, 1212–1215.
- Karagöz, M.A.; Gül, A.; Borg, C.; Erihan, İ.B.; Uslu, M.; Ezer, M.; Erbağcı, A.; Çatak, B.; Bağcıoğlu, M. Influence of COVID-19 pandemic on sexuality: A cross-sectional study among couples in Turkey. Int. J. Impot. Res. 2020, 33, 815–823.
- Culha, M.G.; Demir, O.; Sahin, O.; Altunrende, F. Sexual attitudes of healthcare professionals during the COVID-19 outbreak. Int. J. Impot. Res. 2021, 33, 102–109.
- de Oliveira, L.; Carvalho, J. Women’s Sexual Health During the Pandemic of COVID-19: Declines in Sexual Function and Sexual Pleasure. J. Curr. Sex. Health Rep. 2021, 13, 76–88.
- Healy, E.; McGuire, B.; Evans, D.; Carley, S. Sexuality and personal relationships for people with an intellectual disability. Part I: Service-user perspectives. J. Intellect. Disabil. Res. 2009, 53, 905–912.
- Evans, P.C.; Rainger, G.E.; Mason, J.C.; Guzik, T.J.; Osto, E.; Stamataki, Z.; Neil, D.; Hoefer, I.E.; Fragiadaki, M.; Waltenberger, J. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. J. Cardiovasc. Res. 2020, 116, 2177–2184.
This entry is offline, you can click here to edit this entry!
