Light increases the germinability of positively photoblastic seeds and inhibits the germination of negative ones. In an area where plant-generated smoke from fire is a periodically occurring environmental factor, smoke chemicals can affect the germination of seeds, including those that are photoblastically sensitive. In general, germination is under control of inhibitors involved in seed dormancy (mostly abscisic acid, ABA, and auxin, IAA), while gibberellic acid (GA) stimulates the process. Light, via the phytochrome system positively affects GA and decreases ABA and IAA levels. Similarly, karrikin1 (KAR1), physiologically active smoke compound, regulates some light-induced genes which results in germination of positively photoblastic seeds in darkness.
- seed germination
- smoke compounds
- karrikin
- photoblastism
- smoke formulations
1. Introduction
2. The Impact of Smoke Formulations and Isolated Smoke Compounds on the Germination of Photoblastic Seeds
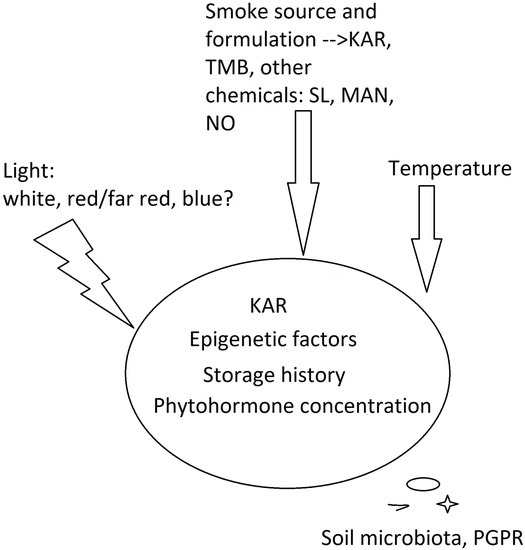
3. Physiological Window of KAR1 Perception by Seeds
This entry is adapted from the peer-reviewed paper 10.3390/plants11131773
References
- Milberg, P.; Andersson, L.; Thompson, K. Large-seeded spices are less dependent on light for germination than small-seeded ones. Seed Sci. Res. 2000, 10, 99–104.
- Xia, Q.; Ando, M.; Seiwa, K. Interaction of seed size with light quality and temperature regimes as germination cues in 10 temperate pioneer tree species. Funct. Ecol. 2016, 30, 866–874.
- Luna, B.; Moreno, J.M. Range-size, local abundance and germination niche-breadth in Mediterranean plants of two life-forms. Plant Ecol. 2010, 210, 85–95.
- Donohue, K.; Heschel, M.S.; Chiang, G.C.; Butler, C.M.; Barua, D. Phytochrome mediates germination responses to multiple seasonal cues. Plant Cell Environ. 2007, 30, 202–212.
- Nelson, D.C.; Risenborough, J.-A.; Flematti, G.R.; Stevens, J.; Ghisalberti, E.L.; Dixon, K.W.; Smith, S.M. Karrikins Discovered in Smoke Trigger Arabidopsis Seed Germination by a Mechanism Requiring Gibberellic Acid Synthesis and Light. Plant Physiol. 2009, 149, 863–873.
- Tognacca, R.S.; Botto, J.F. Post-transcriptional regulation of seed dormancy and germination: Current understanding and future directions. Plant Commun. 2021, 2, 100169.
- Gubler, F.; Hughes, T.; Waterhouse, P.; Jacobsen, J. Regulation of dormancy in barley by blue light and after-ripening: Effects on abscisic acid and gibberellin metabolism. Plant Physiol. 2008, 147, 886–896.
- Nemahunguni, N.K.; Gupta, S.; Kulkarni, M.; Finnie, J.F.; van Staden, J. The effect of biostimulants and light wavelengths on the physiology of Cleome gynandra seeds. Plant Growth Regul. 2020, 90, 467–474.
- Yang, L.; Liu, S.; Lin, R. The role of light in regulating seed dormancy and germination. J. Integr. Plant Biol. 2020, 62, 1310–1326.
- Drewes, F.E.; Smith, M.T.; Van Staden, J. The effect of a plant derived smoke extract on the germination of light-sensitive lettuce seed. Plant Growth Regul. 1995, 16, 205–209.
- Jäger, A.K.; Light, M.E.; van Staden, J. Effects of source of plant material and temperature on the production of smoke extracts that promote germination of light-sensitive lettuce seeds. Environ. Exp. Bot. 1996, 36, 421–429.
- Keeley, J.E.; Babr-Keeley, M. Role of charred wood, heat-shock, and light in germination of postfire phrygana species from the eastern Mediterranean basin. Israel J. Plant Sci. 1999, 47, 11–16.
- Van Staden, J.; Jäger, A.K.; Light, M.E.; Burger, B.V. Isolation of the major germination cue from plant-derived smoke. S. Afr. J. Bot. 2004, 70, 654–659.
- Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Trengove, R.D. A compound from smoke that promotes seed germination. Science 2004, 305, 977.
- Flematti, G.R.; Merritt, D.J.; Piggott, M.J.; Trengove, R.D.; Smith, S.M.; Dixon, K.W.; Ghisalberti, E.L. Burning vegetation produces cyanohydrins that liberate cyanide and stimulate seed germination. Nat. Commun. 2011, 2, 360.
- Cao, D.; Schöttner, M.; Halitschke, R.; Baldwin, I.T. Syringaldehyde is a novel smoke-derived germination cue for the native fire-chasing tobacco, Nicotiana attenuata. Seed Sci. Res. 2021, 31, 292–299.
- Light, M.E.; Burger, B.V.; Staerk, D.; Kohout, L.; Van Staden, J. Butenolides from plant-derived smoke: Natural plant-growth regulators with antagonistic actions on seed germination. J. Nat. Prod. 2010, 73, 267–269.
- Baldos, O.C.; DeFrank, J.; Sakamoto, G.S. Germination response of dormant tanglehead (Heteropogon contortus) seeds to smoke-infused water and the smoke-associated stimulatory compounds, karrikinolide and cyanide. HortScience 2015, 50, 421–429.
- Kamran, M.; Khan, A.L.; Ali, L.; Hussain, J.; Waqas, M.; Al-Harrasi, A.; Lee, I.J. Hydroquinone; a novel bioactive compound from plant-derived smoke can cue seed germination of lettuce. Front. Chem. 2017, 5, 30.
- Reynolds, C.J.; Long, R.L.; Flematti, G.R.; Cherry, H.; Turner, S.R. Karrikins promote germination of physiologically dormant seeds of Chrysanthemoides monilifera ssp. monilifera (boneseed). Weed Res. 2013, 54, 48–57.
- Tavşanoğlu, Ç.; Ergan, G.; Çatav, Ş.S.; Zar, G.; Küçükakyüz, K.; Özüdoğru, B. Multiple fire-related cues stimulate germination in Chaenorhinum rubrifolium (Plantaginaceae), a rare annual in the Mediterranean Basin. Seed Sci. Res. 2017, 27, 26–38.
- Çatav, Ş.S.; Küçükakyüz, K.; Tavşanoğlu, Ç.; Pausas, J.G. Effect of fire-derived chemicals on germination and seedling growth in Mediterranean plant species. Basic Appl. Ecol. 2018, 30, 65–75.
- Papenfus, H.B.; Naidoo, D.; Pošta, M.; Finnie, J.F.; Van Staden, J. The effects of smoke derivatives on in vitro seed germination and development of the leopard orchid Ansellia africana. Plant Biol. 2016, 18, 289–294.
- Pošta, M.; Papenfus, H.B.; Light, M.E.; Beier, P.; Van Staden, J. Structure–activity relationships of N- and S-analogs of the seed germination inhibitor (3,4,5-trimethylfuran-2(5H)-one) for mode of action elucidation. Plant Growth Regul. 2017, 82, 47–53.
- Merritt, D.J.; Kristiansen, M.; Flematti, G.R.; Turner, S.R.; Ghisalberti, E.L.; Trengove, R.D.; Dixon, K.W. Effects of a butenolide present in smoke on light-mediated germination of Australian Asteraceae. Seed Sci. Res. 2006, 16, 29–35.
- Dayamba, S.D.; Sawadogo, L.; Tigabu, M.; Savadogo, P.; Zida, D.; Tiveau, D.; Oden, P.C. Effects of aqueous smoke solutions and heat on seed germination of herbaceous species of the Sudanian savanna-woodland in Burkina Faso. Flora 2010, 205, 319–325.
- Gupta, S.; Plačková, L.; Kulkarni, M.G.; Doležal, K.; Van Staden, J. Role of Smoke Stimulatory and Inhibitory Biomolecules in Phytochrome-Regulated Seed Germination of Lactuca sativa. Plant Physiol. 2019, 181, 458–470.
- Gupta, S.; Hrdlička, J.; Ngoroyemoto, N.; Nemahunguni, N.K.; Gucký, T.; Novák, O.; Kulkarni, M.G.; Doležal, K.; Van Staden, J. Preparation and Standardisation of Smoke-Water for Seed Germination and Plant Growth Stimulation. J. Plant Growth Regul. 2020, 39, 338–345.
- Elsadek, M.A.; Yousef, E.A.A. Smoke-Water Enhances Germination and Seedling Growth of Four Horticultural Crops. Plants 2019, 8, 104.
- Bączek-Kwinta, R. Swailing affects seed germination of plants of European bio-and agricenosis in a different way. Open Life Sci. 2017, 12, 62–75.
- Abenavoli, M.R.; Cacco, G.; Sorgona, A.; Marabottini, R.; Paolacci, A.R.; Ciaffi, M.; Badiani, M. The inhibitory effects of coumarin on the germination of durum wheat (Triticum turgidum ssp. durum, cv. Simeto) seeds. J. Chem. Ecol. 2006, 32, 489–506.
- Kępczyński, J.; Cembrowska, D.; Van Staden, J. Releasing primary dormancy in Avena fatua L. caryopses by smoke-derived butenolide. Plant Growth Regul. 2010, 62, 85–91.
- Long, R.L.; Stevens, J.C.; Griffiths, E.M.; Adamek, M.; Powles, S.B.; Merritt, D.J. Detecting karrikinolide responses in seeds of the Poaceae. Austral J. Bot. 2011, 59, 610–620.
- Dave, G.S.; Galvadiya, B.; Bariya, H.; Vyas, S.R. A dataset of LC-MS QTOF analysis of potato and mustard crop residue smoke water. Data Brief 2018, 21, 343–350.
- Hidayati, S.N.; Walck, J.L.; Merritt, D.J.; Turner, S.R.; Turner, D.W.; Dixon, K.W. Sympatric species of Hibbertia (Dilleniaceae) vary in dormancy break and germination requirements: Implications for classifying morphophysiological dormancy in Mediterranean biomes. Ann. Bot. 2012, 109, 1111–1123.
- Jurado, E.; Márquez-Linares, M.; Flores, J. Effect of cold storage, heat, smoke and charcoal on breaking seed dormancy of Arctostaphylos pungens HBK (Ericaceae). FYTON 2011, 80, 4–11.
- Flores, J.; Jurado, E.; Arredondo, A. Effect of light on germination of seeds of Cactaceae from the Chihuahuan Desert, México. Seed Sci. Res. 2006, 16, 149–155.
- De Cuyper, C.; Struk, S.; Braem, L.; Gevaert, K.; De Jaeger, G.; Goormachtig, S. Strigolactones, karrikins and beyond. Plant Cell Environ. 2017, 40, 1691–1703.
- Nelson, D.C.; Flematti, G.R.; Riseborough, J.A.; Ghisalberti, E.L.; Dixon, K.W.; Smith, S.M. Karrikins enhance light responses during germination and seedling development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 7095–7100.
- Meng, Y.; Chen, F.; Shuai, H.; Luo, X.; Ding, J.; Tang, S.; Xu, S.; Liu, J.; Liu, W.; Du, J.; et al. Karrikins delay soybean seed germination by mediating abscisic acid and gibberellin biogenesis under shaded conditions. Sci. Rep. 2016, 6, 22073.
- Bose, U.; Juhász, A.; Broadbent, J.A.; Komatsu, S.; Colgrave, M.L. Multi-Omics Strategies for Decoding Smoke-Assisted Germination Pathways and Seed Vigour. Int. J. Mol. Sci. 2020, 21, 7512.
- Guercio, A.M.; Torabi, S.; Cornu, D.; Dalmais, M.; Bendahmane, A.; Le Signor, C.; Pillot, J.P.; Le Bris, P.; Boyer, F.D.; Rameau, C.; et al. Structural and functional analyses explain Pea KAI2 receptor diversity and reveal stereoselective catalysis during signal perception. Commun. Biol. 2022, 5, 126.
