Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Immunology
Muramyl peptides (MPs) are part of the peptidoglycan that forms the backbone of the cell walls of bacteria—both Gram-positive and Gram-negative. MPs are formed during the degradation of bacteria and are pathogen-associated molecular patterns (PAMPs) that are recognized by innate immune receptors.
- innate immunity
- NOD2
- muramyl peptide
- MDP
- glucoseeaminylmuramyldipeptide
1. Introduction
Microorganisms that inhabit human skin and mucous membranes take an active part in the formation of the human immune system from the moment of birth. Evolutionarily formed relationships between microorganisms and a macroorganism provide the possibility of sustainable development of the human body in the modern world, which has more than a million lines of bacteria [1]. At the same time, microorganisms that do not have a harmful effect on the human body are commensal. Commensal organisms that inhabit the skin and mucous membranes prevent the colonization of harmful bacteria, they produce substances and vitamins that are vital for humans, and when degraded, they are a source of molecules that keep the human immune system active, providing an adequate response to pathogens not only of a bacterial nature, but also on viruses, fungi and protozoa [2]. Low molecular weight bioregulators of bacterial origin include short chain fatty acids, bile acids, fragments of cell walls—lipopolysaccharide and muramyl peptides [2]. Muramyl peptides (MPs) are part of the peptidoglycan that forms the backbone of the cell walls of bacteria—both Gram-positive and Gram-negative. MPs are formed during the degradation of bacteria and are pathogen-associated molecular patterns (PAMPs) that are recognized by innate immune receptors. For decades, MPs have attracted the attention of researchers as promising drugs and vaccine components for combating infectious and oncological diseases due to their ability to affect innate and acquired immunity [3,4,5].
2. Sources of Muramyl Peptides in the Human’s Body
Muramyl peptides are monomers of peptidoglycan, which forms the cell wall of almost all known bacteria, with the exception of Rickettsia, and protect bacteria from osmotic lysis. Gram-positive bacteria have a thicker peptidoglycan layer than Gram-negative bacteria. N-acetylmuramic acid, which is part of muramyl peptides, is a highly conserved structure, synthesized exclusively in prokaryotic organisms, and which, together with N-acetylglucosamine, forms the backbone of bacterial walls. Monosaccharide-containing muramyl peptides (N-acetylmuramyl-L-alanyl-D-isoglutamine, MDP) and disaccharide-containing glucosaminylmuramyl dipeptides (N-acetylglucosaminyl-N-acetylmuramyl-L-alanyl-D-isoglutamine GMDP) (Figure 1) can have modifications as a sugar residue and the peptide part of the molecule, which differ in different bacteria.
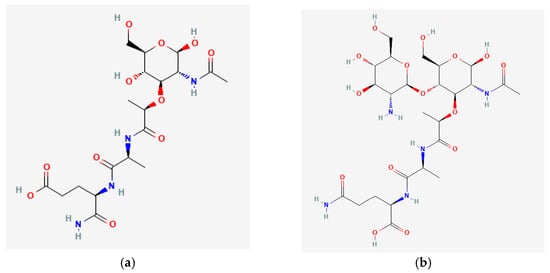
Figure 1. (a) N-Acetylmuramyl-L-Alanyl-D-Isoglutamine (MDP); (b) N-Acetyl-D-Glucosaminyl-(beta1,4)-N Acetylmuramyl-L-Alanyl-D-Isoglutamine (GMDP).
The presence or absence of muramyl peptides (MPs) in the organism of a healthy person was a long time of discussion. Methods of mass chromatography (MC), gas liquid chromatography (GLC), and mass spectrometry (MS) were used to determine MPs in human tissues and fluids in order to identify the relationship with chronic infectious foci [6,7,8].
3. Effect of Muramyl Peptides on Microorganisms
Muramyl peptides, being monomers of peptidoglycan, have a direct effect on microorganisms and their development cycles. Remodeling of peptidoglycan is necessary for bacteria to reproduce; with each division of bacteria, about 40–50% of peptidoglycan breaks down into monomeric units, and can be used to build the cell walls of the next generation of bacteria [27]. When murein hydrolases act on the bacterial cell wall, the muramyl peptides formed in the periplasm are recycled and reused by bacteria to form peptidoglycan [28]. Dozens of murein hydrolases have been described that are characteristic of certain lines of bacteria and bacteriophages, resulting in the formation of a large number of monosaccharide-containing and disaccharide-containing muramyl peptide derivatives that are recycled during bacterial growth [20,29]. Numerous experiments have shown the spontaneous release of peptidoglycan fragments from E. coli [30], Bordetella pertussis [31,32], Neisseria gonorrhoeae [29,32], Neisseria meningitides [33], Bordetella pertussis [34], and Shigella [35].
The direct effect of muramyl peptides and their analogues on five bacterial strains of four species (S. aureus MRSA/MSSA, K. pneumoniae ESBL, P. aeruginosa and E. coli) revealed that the value of the minimum inhibitory concentration (MIC) was >512 μg/mL against all strains except for Pseudomonas aeruginosa (P. aeruginosa), for which the MIC was 128 μg/mL. As a comparison, the MICs of antibiotics (kanamycin, tetracycline, and chloramphenicol) in this experiment ranged from 1 to 32 μg/mL [36]. Muramyl peptides can be used by bacteria for communication [37], participating in the coordination of the growth of the entire population and acting, along with other low molecular weight bioregulators of bacterial origin, as a quorum-sensing mediator.
The direct effect of muramyl peptides is also manifested on dormant forms of bacteria: muramyl peptide 1,6-anhydro-GMDP, added to the medium with dormant Mycobacterium smegmatis bacteria, revived dormant mycobacterial cells in the concentration range of 9–100 ng/mL [38]. The effect of muramyl peptides on the composition of the microbiocenosis of the oral cavity of healthy patients and patients after caries therapy was shown [39,40]. Oral administration of 1 mg GMDP per day for 10 days resulted in a decrease in oral fluid of Candida albicans, Clostridium difficile, and Porphyromonas gingivalis [39,40].
In experiments on J774 cell lines, GMDP inhibited the intracellular growth of M. smegmatis, M. bovis, and M. tuberculosis at concentrations of 40 μg/mL [41].
The antibacterial activity of a few dozens of muramyl peptide derivatives has been studied in experimental animal models. N-acetylmuramyl-L-alanyl-D-isoglutamine and some analogues have been found to protect mice against Klebsiella pneumoniae even when administered orally, and they also proved to be effective when administered after challenge [42,43]. Interesting data have been obtained from intermittent injections of GMDP into mice infected with Mycobacterium tuberculosis. It was found that injections of GMDP reduced the number of viable bacilli in the lungs, but increased their number in the spleen after 16 weeks, and also reduced the recurrence of infection in the lungs after chemotherapy [44].
The use of the disaccharide-containing muramyl peptide GMDP 1–4 days before the administration of a lethal dose of E. coli LPS endotoxin protects from 60 to 100% of mice from death [45], while the synthetic derivative of MDP protects against Staphylococcus aureus [46]. The muramyl dipeptide analogue provided protection against intraperitoneal Pseudomonas aeruginosa infection or intravenous Candida albicans infection in mice when administered intraperitoneally, intravenously, or subcutaneously at 80 mg/kg once a day for 4 consecutive days prior to infection. The compounds were not active when administered orally. N-acetyl-nor-muramyl-L-alanyl-D-isoglutamine potentiated the action of gentamicin and had a protective effect against Listeria monocytogenes. Post-infection protection was not observed [47]. Muramyl peptide MDP-Lys (L18) had a protective effect in C57BL/6 mice against live cultures of Salmonella enteritidis and Salmonella enterica Subspecies enterica Serovar Choleraesuis Hokkaido [48].
Muramyl peptides were able to protect animals with immunodeficiencies from infection [49]. MDP protected CBA/N mice carrying the X-linked immune deficiency mutation (xid) against the lethal bacterial infection Streptococcus pneumoniae, Salmonella typhimurium and Salmonella enteritidis [50]. Interestingly, in another study in a model of Salmonella enteritidis in CBA/N-defective mice with X-linked immunodeficiency, a single injection of either MDP or MDP-Lys(L18) did not elicit any effective protection, but repeated injections prior to bacterial challenge of MDP-Lys(L18) (100 µg per mouse per day for 3 consecutive days) protected immunodeficient mice. Multiple injections of MDP once a day for several days in a row significantly increased bactericidal activity in the abdominal cavity and spleen of mice [51]. Muramyl peptides in experimental studies on animals not only demonstrated activity against various bacterial infections, but they also had a protective effect against viruses, fungi, and protozoa [52,53,54].
4. Muramyl Peptides Activation of Various Cell Populations
To date, several drugs based on muramyl peptides have been registered, and clinical trials of vaccines containing muramyl peptides are underway [4]. These clinical trials were preceded by numerous studies on the effect of muramyl peptides on various cell populations, which established their ability to activate anti-infective and anti-tumor defenses. With the discovery in 2003 by two independent groups of scientists of the sensors of muramyl peptides—NOD1 and NOD2 proteins and their predominant localization in monocytic cells, the preferential effect of muramyl peptides on the cells of the immune system became clear [55,56]. NOD1 and NOD2 are intracellular receptors of innate immunity; they provide an adequate response to a pathogen in case of its invasion and tolerance to commensal microflora. NOD1 recognizes muramyl peptides containing γ-d-glutamyl-meso-diaminopimelic acid (iE-DAP), a peptdoglycan fragment of Gram-negative bacteria [57]. NOD2 recognizes muramyl peptides containing L-Ala-D-isoGln and its derivatives [55,56]. Mutations in NOD1 and NOD2 are associated with inflammatory [58,59,60], oncological [61], and neurodegenerative diseases [62,63]. NOD1 and NOD2 receptors in monocytes initiate an adequate response and clearance from the pathogen. Derivatives of monocytes are macrophages, dendritic cells, osteoclasts, microglia.
Muramyl peptides exhibit pronounced activity against mouse and human macrophages in vitro: their phagocytic and microbicidal ability increases due to an increase in the synthesis of cyclic adenosine monophosphate, superoxide radicals, collagenase, prostaglandins and an increase in the specific activity of beta-glucosaminidase, which is a component of the macrophage lysosome, and lactate dehydrogenase (Figure 2) [42,64]. Recorded changes were observed in peritoneal macrophages, in macrophages isolated from the bone marrow of animals, and also in macrophages isolated from the milk of healthy women [64].
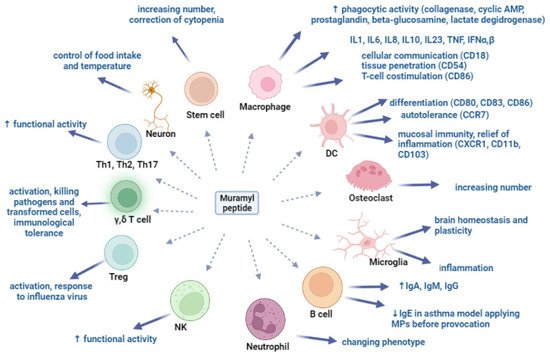
Figure 2. Muramyl peptides activation of various cell populations.
Under the action of muramyl peptides, macrophages synthesize cytokines IL-1, IL-6, IL-8, IL-23, IFN α,β [65,66,67], and regulate the expression of membrane markers, including HLA-DR and adhesion molecules CD18, CD54, CD86 [67,68,69]. CD18-integrin is involved in the implementation of intercellular communication; CD54-Inter-Cellular Adhesion Molecule 1 (ICAM-1,) is required for tissue penetration; and CD86 is a membrane protein of the immunoglobulin family that acts as a co-stimulatory signal in antigen-dependent activation of T-lymphocytes. Thus, muramyl peptides stimulate macrophages to eliminate the pathogen, and to synthesize cytokines and mediators of innate immunity, as well as membrane-associated activation markers, providing the possibility of migration to the site of inflammation and interaction of immune system cells necessary for the implementation of adaptive immunity. Simultaneous stimulation of macrophages with MDP and TLR4 and TLR9 receptor agonists contributed to a significant increase in the synthesis of TNF, IL-1, IL-6, and IFN-α and IFN-β [45,70,71,72,73]. The synergy of the action of muramyl peptides with LPS and IFN on macrophages was the basis for the introduction of MPs as components of antibacterial and antiviral vaccines [74,75]. Interesting results were obtained when MPs were injected into the crushed sciatic nerve of rats [76]. It turned out that the intrafascicular injection of MDP activates macrophages that enter the damaged nerve and support the lengthening of the regenerating axon. The introduction of MDP accelerated the process of axon length increase by 15.5% after 5 days and by 18.3% after 3 weeks [76].
The role of muramyl peptides in the differentiation of dendritic cells (DCs) was determined. Muramyl dipeptide MDP-Lys (L18) increased the expression of CD80, CD83, CD86, and CD40, but not HLA-DR, and stimulated the production of tumor necrosis factor-alpha (TNFα), IL-6, IL-8, IL-10 and IL-12 (p40) human DCs. In addition, DCs treated with MDP-Lys showed an increased antigen-presenting function compared to untreated DCs [77]. Similar results were also obtained with GMDP [69]. Dendritic cells isolated from the blood of healthy donors who used the drug based on GMDP for 10 days changed their phenotype: the expression of differentiation markers CD80, CD83, and CCR7, which is responsible for DC recruitment to secondary lymphoid organs, increased. Constitutively expressed CCR7 provides autotolerance, as well as tolerance to food and inhalation antigens [78,79]. The expression levels of the XCR1, CD11b, and CD103 genes also increased. The XCR1 receptor plays a leading role in maintaining the homeostasis of mucosal immunity and the formation of memory CD8+T cells [80,81]. In addition, the expression of XCR1 on DC is required for the maturation of regulatory T cells, for the relief of inflammation, the maintenance of autotolerance, and for a cytotoxic immune response [82,83]. The discovered ability of GMDP to increase the expression of XCR1 and CD103 demonstrates the ability of GMDP to control excessive inflammation in mucosal tissues.
MDP induced osteoclast formation, showing synergy with LPS, and did not affect osteoclastogenesis in mice treated with the parathyroid hormone [84,85]. Macrophages of bone tissue osteoclasts resorb bone tissue and, together with osteoblasts, regulate bone remodeling.
Macrophages of the central nervous system (microglia) maintain homeostasis and brain plasticity. In an experimental model, activation of the NOD1 receptor by muramyl peptides enhanced the inflammatory response caused by microglia and exacerbated brain damage after intracerebral hemorrhage in mice [86].
An increase in antibody titer under the influence of MP, confirmed in numerous experimental and clinical studies, can serve as evidence of their activating effect on B-lymphocytes.
The first studies of muramyl peptides on lymphocytes were carried out using laboratory animals [3,87,88]. MDP at a concentration of 0.1 to 1 mg/kg provoked lymphocytosis, and at 10 mg/kg lymphocytopenia and an increase in the number of young stab neutrophils and monocytes. MPs by themselves did not induce antibody synthesis [89,90], but exhibited the properties of adjuvants when administered together with an antigen [75,88,91]. The adjuvant has no antigenic determinants and no antibodies are produced against it, but it can enhance the stimulatory response to compounds that have little effect on their own [92]. MPs exhibit adjuvant properties and potentiate the action of drugs and vaccines [4,93].
The ability of MPs to increase the titers of immunoglobulins was the basis for studying the mechanism of their action and introducing them as components of vaccines [3,87,89,94], since effective animal vaccines alum and Freund’s adjuvant have not been used in humans. MDP was the minimal structure of peptidoglycan included in Freund’s complete adjuvant. The study of numerous MDP derivatives showed that muramic acid and the presence of L-Ala and D-iGln are critical for the manifestation of biological activity [95]. Later, it was shown that the disaccharide-containing MP, GMDP, which is formed, in particular, under the action of lysozyme, increases the titer of antibodies to bovalbumin M much more efficiently than MDP [87]. The developed method for the synthesis of GMDP using a disaccharide obtained from bacteria and a compound with dipeptide turned out to be less expensive than previously proposed, which served as the basis for the development of a drug based on it. It should be noted that the adjuvant activity of MDP was largely expressed against ovalbumin and, to a much lesser extent, against sheep erythrocytes [96]. It is very important that muramyl peptides increase the immune response to low doses of antigen when other adjuvants (FCA, FIA) are not effective [97]. At the same time, MDP stimulated the production of immunoglobulins of the IgG1 subclass. MPs can also increase IgG2a titers [98].
The influence of MPs on the levels of IgA in the oral cavity of humans and animals [39,40,99], in the blood serum of humans [100] and animals [99,101], and in extracts of animal feces [101] was registered. It is interesting that 2-fold intranasal administration of MDP mice as an adjuvant with the joint administration of the recombinant urease protein (rUr) of Helicobacter pylori did not affect the increase in the titer of IgA and IgG in the blood serum; a composition of several adjuvants turned out to be effective [101]. Conversely, in extracts of animal feces, IgA was detected after the introduction of rUr with MDP and was absent in the composition of adjuvants [101].
MPs reduce the IgE titer in individuals with allergic diseases in the case of taking MPs at the stage of remission, as well as in animals with the experimental model of asthma with the preliminary administration of MPs before allergen sensitization, while the severity of the inflammatory process also decreased [40,98].
When mice were immunized with the Huntavirus vaccine, muramyl peptides B30-MDP and MDP-Lys(L18) showed an adjuvant effect and stimulated elevated levels of IgG1 and IgM, while no increase in IgG2 and IgG3 was observed [102]. Muramyl peptides increased antibody titers in mice immunized with a phage suspension [103].
Thus, MPs affect the synthesis of immunoglobulins. The intensity of the impact depends on many factors. The structure of MPs, their introduction into liposomes, route of administration, duration, dose, and properties of the antigen are obvious factors. However, it is additionally necessary to take into account other factors that can have a decisive effect, such as the duration of the early administration of the MPs to the antigen, in which not only the activating effect of the MPs is recorded, but also the suppressive one [40,104]. In addition, the adjuvant properties of MPs depend on the composition of the composition [105] and may depend on the presence of IL-2 and IL-4 [106].
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms10081526
This entry is offline, you can click here to edit this entry!
