Abstract
Nutritional therapy has been conventionally recommended for people with prediabetes as a method to delay or halt progression to type 2 diabetes. However, recommended nutritional strategies evolve over time. Hence, we performed a scoping review on recently reported nutritional interventions for individuals with prediabetes. Ovid MEDLINE, PubMed, Embase, Scopus, CINAHL and PsycINFO databases were searched to identify relevant research articles published within the past 10 years. Ninety-five articles involving a total of 11,211 participants were included in this review. Nutritional strategies were broadly classified into four groups: low calorie diet, low glycemic index diet, specific foods, and a combination of diet and exercise. The most frequently assessed outcomes were plasma glucose, serum insulin, serum lipid profile, body mass index and body weight. More than 50% of reported interventions resulted in significant improvements in these parameters. Nutritional interventions have demonstrated feasibility and practicality as an effective option for prediabetes management. However, the intervention variability demonstrates the challenges of a ‘one-size-fits-all’ approach. Investigations in genetically diverse populations and objective assessment of progression rate to diabetes are necessary to better comprehend the impact of these nutritional strategies in prediabetes.
- prediabetes
- diabetes mellitus
- type 2
- hyperglycemia
- glucose intolerance
- diet therapy
1. Definition
The risk of developing type 2 diabetes mellitus (T2DM) increases with existence of one of two precursor states—impaired fasting glucose (IFG) and impaired glucose tolerance (IGT). IFG is defined as a fasting plasma glucose (FPG) concentration of ≥6.1 but <7.0 mmol/L, whereas IGT is defined as an FPG concentration of <7.0 mmol/L and a 2-h post-load plasma glucose concentration of ≥7.8 but <11.1 mmol/L following an oral glucose challenge . A glycated hemoglobin A1c (HbA1c) level of 5.7–6.4% has been included in the existing American Diabetes Association (ADA)’s criteria for high diabetes risk since 2010 .Prediabetes is broadly defined as blood glucose levels above normal but below that of diabetes [3,4]. Prediabetes is typically an umbrella term encompassing IGT, IFG as well as elevated HbA1c levels—all of which are considered substantial risk factors for progression to overt diabetes.
2. Nutritional Strategies
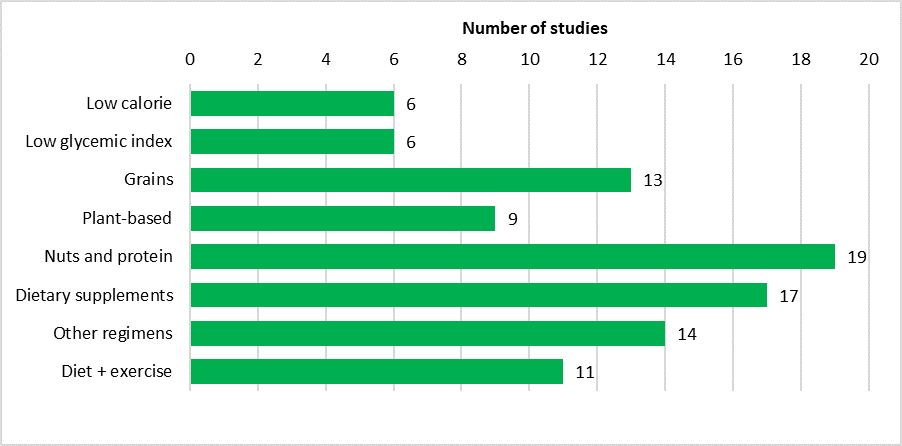
Nutritional interventions can be broadly divided into four groups: low calorie diet, low glycemic index diet, specific foods, as well as combined diet and physical activity interventions (Figure 3).
Figure 3. Bar graph showing the number of included studies classified according to interventions.
2.1. Low Calorie Diet
Six studies [62,67,71,99,102,109] reported low calorie interventions in people with prediabetes, achieved either through restrictions on all food classes or manipulation of a specific food group. Four studies reported low-calorie dietary interventions that were achieved through restrictions on the daily intake of all food classes, with intervention durations lasting from eight weeks to 12 months [62,67,102,109]. Daily energy allowances ranged from 415–1700 kcal, distributed with macronutrient compositions of 41–65% carbohydrates, 12–43.7% protein and 13–30% fat. The studies reported reductions in fasting blood glucose levels, which corresponded to a decrease in insulin resistance in three of the studies [62,102,109]. Measures of anthropometry changed considerably after intervention, with decreases in body weight [67,102,109] and waist circumference [62,67,102]. Interestingly, Christensen et al. compared a low energy diet (810 kcal/day) with a very low energy diet (415–554 kcal/day) and found that the former was preferable owing to lower lean tissue loss and fewer side effects reported [67].
A hypocaloric diet was combined with manipulation in fat intake in two studies [71,99]. These interventions resulted in weight reduction and lower insulin resistance, even in interventions as short as seven weeks [71,99].
2.2. Low Glycemic Index Diet
The studies included here are those in which a low glycemic index (GI) diet was specified by the authors or was indicated to be taken by participants. Three studies utilized nonspecific low GI diets as the intervention diet. Hari et al. [74] and Solomon et al.
Of the three studies with the nonspecific low GI diet as intervention, two established that insulin sensitivity and peripheral insulin resistance were improved and decreased respectively, whereas nonsignificant differences in insulin sensitivity between low and high GI diet were found in the study by Hari et al. [74,98,101]. Pancreatic β-cell function was successfully preserved by having a low GI diet, ensuring effective glucose metabolism [101]. This was further supported by Solomon et al. who found that postprandial glucose levels improved in a low GI diet while pancreatic β-cell function was impaired in a high GI diet [98]. All three studies demonstrated significant weight loss in low GI diets [74,98,101].
A low GI diet achieved by a combination of dairy, chicken, nuts and whole grains was contrasted against a red meat and refined grain diet in a crossover RCT by Kim et al. [105]. The former proved to be superior to the latter by demonstrating decreased postprandial glucose, insulin and triglyceride responses.
Resistant starch (RS), a low GI carbohydrate, was investigated by two RCTs. In the Korean study by Kwak et al., rice containing RS derived from corn starch, with 6.51g of resistant starch per serving, was served once daily for four weeks [21]. This intervention yielded significant overall improvements to glucose and insulin outcomes compared to the RS-free placebo. However, when a RS-rich diet was compared to a diet rich in fiber in the 12-month study by Dodevska et al., the fiber-rich diet prevailed in achieving better postprandial glycemic control [61]. This RS-rich diet—consumed by subjects under free-living conditions—consisted of plant foods proven to be good RS sources, such as cooked potatoes and beans. Nevertheless, Dodevska et al. demonstrated that body mass index (BMI) and lipid profiles were augmented by the RS diet [61], while Kwak et al. showed that oxidative stress was reduced, leading to better endothelial function .
2.3. Other Dietary Regimens
A monounsaturated fatty acid (MUFA)-enriched diet shown to be less proinflammatory compared to a medium-chain saturated fatty acid (SFA) diet [66]. Surprisingly, diets high in MUFA conferred additional benefits of diminishing hepatic fat and improving insulin sensitivity, as a trial by Errazuriz et al. [72] demonstrated. Although this study found no short-term advantage for high-fiber diets, others have shown clear improvements in body weight, blood glucose levels and plasma triglycerides [24,65]. Low advanced glycation end products (L-dAGEs) showed satisfactory results in terms of lipid profile, high-sensitivity C-reactive protein (hs-CRP) level and intima-media thickness, thereby decreasing cardiovascular risk factors [51,66].
A low-energy breakfast [107] and four types of carbohydrates [41] were each investigated in one study and provided positive outcomes. Improvements in glucose metabolism and insulin parameters were observed after consuming a low-energy breakfast [107]. Amongst the four types of carbohydrates (glucose, trehalose, sucrose, isomaltose) examined in the study by van Can et al., sucrose demonstrated the most potential in improving glucose levels, insulin parameters and postprandial inhibition of fat oxidation [41].
Food type and proportion may play important roles in determining health outcomes, as suggested by a nonrandomized study by Tippens et al., where consumption of grains, dairy, meat and fat were reduced [90]. This resulted in improved serum lipid profiles and glucose-related parameters including HbA1c, insulin and hs-CRP.
Carnevale et al. [50] examined the effects of extra virgin olive oil and found significant reductions in glucose and triglyceride levels and dipeptidyl-peptidase 4 (DPP4) activity, as well as increases in insulin and glucagon-like peptide-1 (GLP-1). When vinegar was consumed just before meals, it enhanced insulin sensitivity, insulin resistance, glucose metabolism, lipid profile as well as muscle blood flow [63].
3. Conclusion
After evaluating the studies in this review, we present a few recommendations for future research. First, there was no study regarding the effects of a solely low-fat diet that fit our inclusion criteria. Although numerous studies explored these practices within the general diabetes theme [8], only a small number of low-carbohydrate diets as well as meal patterns were investigated in the prediabetic population. Hence, this represents an important knowledge gap that future researchers are encouraged to fill by designing studies incorporating those diets as interventions, with special attention given to prediabetes.
Genetic variations and ethnic nuances need to be considered to yield evidence for nutritional interventions that are valid, reliable and generalizable. Certain regions of the world that are culturally rich and genetically diverse such as Africa and some parts of South East Asia were underrepresented in the study pool. Therefore, researchers should endeavor to involve subjects from a variety of backgrounds in future trials. We acknowledge that low-income countries may face various situational challenges effectively prohibiting research and development, but these should be systematically overcome as prediabetes is increasingly recognized as an evolving global issue [114]. A multinational, crowdfunded study involving the collaboration of multiple developing countries with limited means to implement independent research can be considered.
This entry is adapted from the peer-reviewed paper 10.3390/nu12102990