Mesenchymal stem cells (MSCs) play a critical role in response to stress such as infection. They initiate the removal of cell debris, exert major immunoregulatory activities, control pathogens, and lead to a remodeling/scarring phase. Interestingly, many viruses and particularly those associated to chronic infection and inflammation may hijack and polarize MSC’s immune regulatory activities to their own advantages. Virus will remain in the MSC perivascular niche while being protected from immune attack. In the context of immunodepression (e.g. organ transplantation) the hidden viruses may rebound and causing tissue injuries.
- innate immunity
- immune-regulation
- neural crest
- COVID-19
- chikungunya
1. MSC Viral Infection and Host Responses
| Cells | Viruses | Related Outcomes |
|---|---|---|
| Bone marrow-MSC | HIV | Inability to support Hematopoietic Stem Cells expansion and implication in HIV-related cytopenia [1]; HIV-related reactivation [2][3][4] |
| HCMV | Transmit to neighboring cells after reactivation [5][6] | |
| Modifies the physiological interaction between bone marrow (BM)-derived MSCs (BM-MSC) and hematopoietic stem cell (HSC) | ||
| Impairment of osteoblast regeneration, cartilage regeneration, hematopoiesis and properties of immune progenitor cells [6] | ||
| HHV | Lower proliferation rates and altered phenotypes related to malignant transformation [5][7][8] | |
| Influenza A H5N1 | Risk of transmission during bone marrow transplantation [9][10] | |
| RSV | Alteration of immunoregulatory functions [11] | |
| ZIKV | Impaired osteoblast differentiation and possible implication in development of bone pathologies [12] | |
| HBV | HBV-associated myocarditis and other HBV-related extrahepatic diseases [13] | |
| Lung resident MSC/pericytes | SARS-CoV-2 | Pericyte apoptosis and loss in COVID-19 patients [14][15] |
| SIV/HIV | Development of HIV-related pulmonary complications [12][16] | |
| Hepatic Stellate Cell (HepSC, Ito cells) | HIV | HepSC activation and chemotaxis through HIV gp120 envelope protein [17][18] |
| HCV | HCV proteins as well as RNA released by hepatocytes are activating HepSC [19][20]. Constant activation leading to liver fibrosis [21][22][23][24] | |
| HBV | Release of IL-17 by infected cells which stimulate liver fibrosis by activation of HepSC [25][26]. | |
| Mesangial Cell (Kidney) | HIV | HIV-associated glomerulosclerosis due to increased proliferation and matrix synthesis [27][28] |
| HCMV | Glomerulosclerosis [29][30][31][32] | |
| HCV | Glomerulonephritis [33] | |
| ZIKV | Viral reservoir (persistent viruria) [34] | |
| SARS-CoV-2 | Stromal (MSC-like cells) are infected and may contribute to kidney fibrosis in a model of spheroid cultures and to be correlated with kidney fibrosis in COVID19 patients [35]. | |
| Brain Pericytes (BP) | HIV | Decreased BPs coverage of blood brain barrier (BBB) associated with higher permeability [36][37] |
| IL-6 and PDGF-BB secretions concur in HIV-induced CNS damage and BBB disruption [36][38] | ||
| HCMV | Contribute to HCMV dissemination [39] | |
| CXCL8, CXCL11, CCL5, TNF-α, IL-1β and IL-6 secretion causing neuroinflammation [40] | ||
| JEV | IL-6 secretion leads to ZO-1 degradation and BBB impairment [41] | |
| Prostaglandin E2 (PGE2) and RANTES secretion by BPs recruit leukocytes to the site of infection. Associated with BBB impairment, this provoke leukocyte infiltration and major neuroinflammation [42] | ||
| Herpes Simplex Virus (HSV) | BBB impairment associated with leukocytes recruitment leading to major neuroinflammation [43] | |
| ZIKV | Brain abnormalities and BBB defect [44][45] | |
| Osteoblasts | HIV (gp120) | TNF-α and impaired Wnt/β-Catenin signaling promote bone demineralization and reduced bone mass leading to osteopenia and osteoprosis [46] |
| RRV | Imbalance in RANKL/OPG ratio in favor of osteoclastogenic activities and bone loss [47][48] | |
| Chikungunya Virus (CHIKV) | Proinflammatory (IL-6) and pro-osteoclastic (RANKL) effects in infected cells [49] | |
| HCV | Associated with bone density hardening and osteosclerosis [50] | |
| Increased risk of osteoporosis [51] | ||
| Increased risk of fracture [52] | ||
| Impairment of RANKL/OPG ratio [50] | ||
| ZIKV | Impaired osteoblasts function triggering an imbalance in bone homeostasis and inducing bone-related disorders [12] | |
| MeV | Higher expression of several osteogenic markers and osteogenic differentiation [53] | |
| Otosclerosis [54] | ||
| Paget’s disease [55] | ||
| Schwann Cell (SC) | HIV | Dorsal root ganglion neurotoxicity, including axon and myelin injury [56] |
| HSV/VZV | The principal mechanism evoked for HSV-induced Guillain-Barré Syndrome (GBS) is a molecular mimicry of viral proteins [57] | |
| HCMV | Probable molecular mimicry generating autoantibodies against moesin expressed by SCs [58][59][60][61][62] | |
| ZIKV | Possible direct viral pathogenic effect or a cell-mediated inflammation in pathogenesis of ZIKV-associated GBS [63] | |
2. MSCs a Gatekeper and/or Reservoir of Viruses
2.1. Bone Marrow-Derived MSC (BM-MSC)
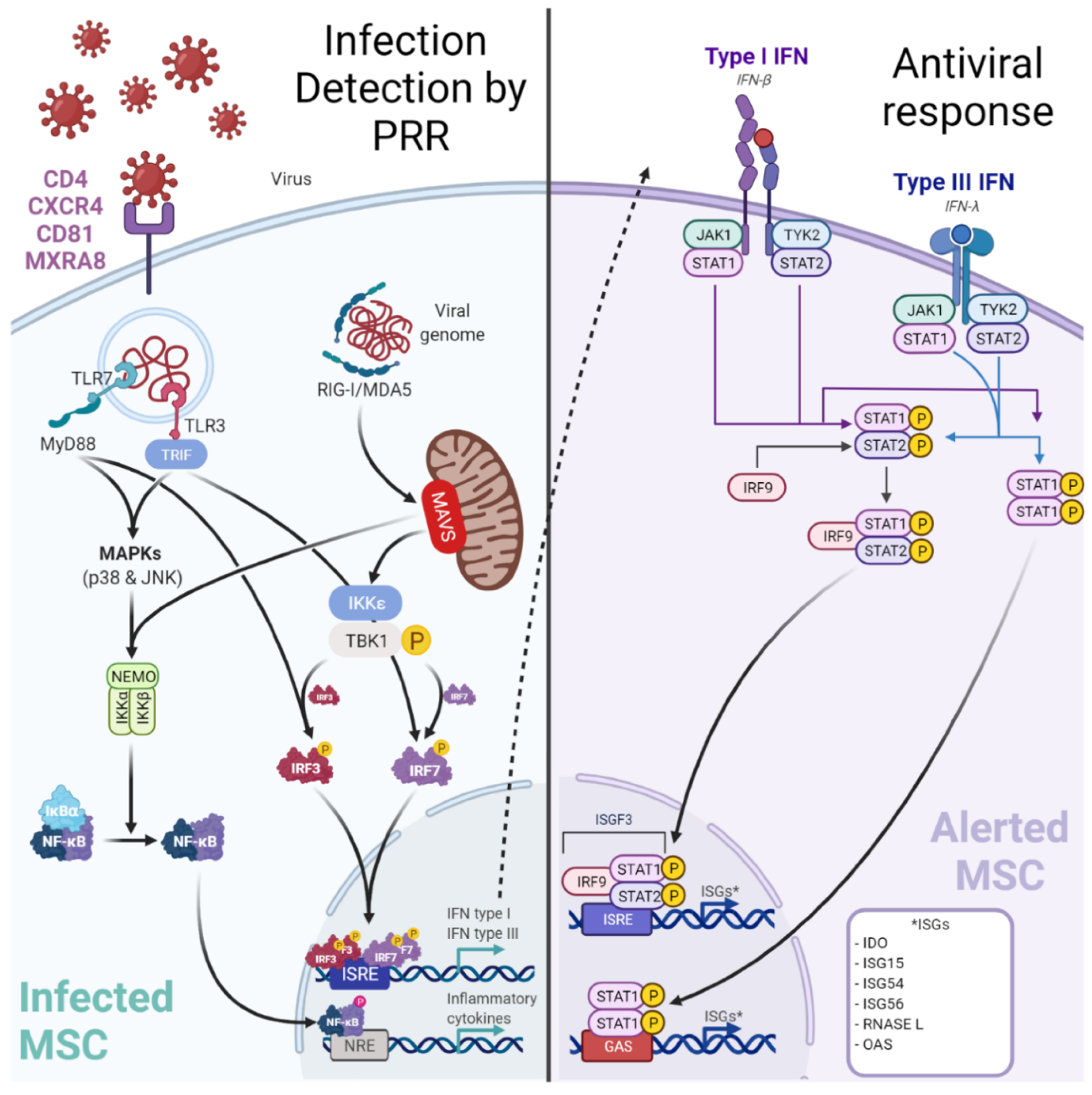
2.2. Lung Resident-MSC and Viruses
2.3. Adipose Stem Cells and Viruses
2.4. MSC of the Liver: Hepatic Stellate Cell/Ito Cell and Viruses
2.5. MSC of the Kidney: Mesangial Cell and Viruses
2.6. Brain MSC: Brain Pericytes and Viruses
3. Osteoblasts and Viral Infections
4. Schwann Cell of Peripheral Nerves and Viral Infection
This entry is adapted from the peer-reviewed paper 10.3390/ijms23148038
References
- Yuan, Y.; Zhao, S.; Wang, X.; Teng, Z.; Li, D.; Zeng, Y. HIV-1 P55-Gag Protein Induces Senescence of Human Bone Marrow Mesenchymal Stem Cells and Reduces Their Capacity to Support Expansion of Hematopoietic Stem Cells in Vitro. Cell Biol. Int. 2017, 41, 969–981.
- Chandra, P.K.; Gerlach, S.L.; Wu, C.; Khurana, N.; Swientoniewski, L.T.; Abdel-Mageed, A.B.; Li, J.; Braun, S.E.; Mondal, D. Mesenchymal Stem Cells Are Attracted to Latent HIV-1-Infected Cells and Enable Virus Reactivation via a Non-Canonical PI3K-NFκB Signaling Pathway. Sci. Rep. 2018, 8, 14702.
- Cotter, E.J.; Chew, N.; Powderly, W.G.; Doran, P.P. HIV Type 1 Alters Mesenchymal Stem Cell Differentiation Potential and Cell Phenotype Ex Vivo. AIDS Res. Hum. Retrovir. 2010, 27, 187–199.
- Beaupere, C.; Garcia, M.; Larghero, J.; Fève, B.; Capeau, J.; Lagathu, C. The HIV Proteins Tat and Nef Promote Human Bone Marrow Mesenchymal Stem Cell Senescence and Alter Osteoblastic Differentiation. Aging Cell 2015, 14, 534–546.
- Sundin, M.; Örvell, C.; Rasmusson, I.; Sundberg, B.; Ringdén, O.; Le Blanc, K. Mesenchymal Stem Cells Are Susceptible to Human Herpesviruses, but Viral DNA Cannot Be Detected in the Healthy Seropositive Individual. Bone Marrow Transplant. 2006, 37, 1051–1059.
- Smirnov, S.V.; Harbacheuski, R.; Lewis-Antes, A.; Zhu, H.; Rameshwar, P.; Kotenko, S.V. Bone-Marrow-Derived Mesenchymal Stem Cells as a Target for Cytomegalovirus Infection: Implications for Hematopoiesis, Self-Renewal and Differentiation Potential. Virology 2007, 360, 6–16.
- Lee, M.-S.; Yuan, H.; Jeon, H.; Zhu, Y.; Yoo, S.; Shi, S.; Krueger, B.; Renne, R.; Lu, C.; Jung, J.U.; et al. Human Mesenchymal Stem Cells of Diverse Origins Support Persistent Infection with Kaposi’s Sarcoma-Associated Herpesvirus and Manifest Distinct Angiogenic, Invasive, and Transforming Phenotypes. mBio 2016, 7, e02109.
- Pessina, A.; Bonomi, A.; Coccè, V.; Bernardo, M.E.; Cometa, A.M.; Ferrari, M.; Sisto, F.; Cavicchini, L.; Locatelli, F. Assessment of Human Herpesvirus-6 Infection in Mesenchymal Stromal Cells Ex Vivo Expanded for Clinical Use. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2009, 11, 491–496.
- Khatri, M.; Saif, Y.M. Influenza Virus Infects Bone Marrow Mesenchymal Stromal Cells in Vitro: Implications for Bone Marrow Transplantation. Cell Transplant. 2013, 22, 461–468.
- Thanunchai, M.; Kanrai, P.; Wiboon-Ut, S.; Puthavathana, P.; Hongeng, S.; Thitithanyanont, A. Tropism of Avian Influenza A (H5N1) Virus to Mesenchymal Stem Cells and CD34+ Hematopoietic Stem Cells. PLoS ONE 2013, 8, e81805.
- Cheung, M.B.; Sampayo-Escobar, V.; Green, R.; Moore, M.L.; Mohapatra, S.; Mohapatra, S.S. Respiratory Syncytial Virus-Infected Mesenchymal Stem Cells Regulate Immunity via Interferon Beta and Indoleamine-2,3-Dioxygenase. PLoS ONE 2016, 11, e0163709.
- Mumtaz, N.; Koedam, M.; van den Doel, P.B.; van Leeuwen, J.P.T.M.; Koopmans, M.P.G.; van der Eerden, B.C.J.; Rockx, B. Zika Virus Infection Perturbs Osteoblast Function. Sci. Rep. 2018, 8, 16975.
- Rong, Q.; Zhang, L.; Su, E.; Li, J.; Li, J.; Liu, Z.; Huang, Z.; Ma, W.; Cao, K.; Huang, J. Bone Marrow-Derived Mesenchymal Stem Cells Are Capable of Mediating Hepatitis B Virus Infection in Injured Tissues. J. Viral Hepat. 2008, 15, 607–614.
- Cardot-Leccia, N.; Hubiche, T.; Dellamonica, J.; Burel-Vandenbos, F.; Passeron, T. Pericyte Alteration Sheds Light on Micro-Vasculopathy in COVID-19 Infection. Intensive Care Med. 2020, 46, 1777–1778.
- Rendeiro, A.F.; Ravichandran, H.; Bram, Y.; Chandar, V.; Kim, J.; Meydan, C.; Park, J.; Foox, J.; Hether, T.; Warren, S.; et al. The Spatial Landscape of Lung Pathology during COVID-19 Progression. Nature 2021, 593, 564–569.
- Stephenson, S.E.; Wilson, C.L.; Bond, N.G.; Kaur, A.; Alvarez, X.; Midkiff, C.C.; Schnapp, L.M. Pericytes as Novel Targets for HIV/SIV Infection in the Lung. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 319, L848–L853.
- Zhang, L.; Bansal, M.B. Role of Kupffer Cells in Driving Hepatic Inflammation and Fibrosis in HIV Infection. Front. Immunol. 2020, 11, 1086.
- Tuyama, A.C.; Hong, F.; Saiman, Y.; Wang, C.; Ozkok, D.; Mosoian, A.; Chen, P.; Chen, B.K.; Klotman, M.E.; Bansal, M.B. Human Immunodeficiency Virus (HIV)-1 Infects Human Hepatic Stellate Cells and Promotes Collagen I and Monocyte Chemoattractant Protein-1 Expression: Implications for the Pathogenesis of HIV/Hepatitis C Virus-Induced Liver Fibrosis. Hepatology 2010, 52, 612–622.
- Schulze-Krebs, A.; Preimel, D.; Popov, Y.; Bartenschlager, R.; Lohmann, V.; Pinzani, M.; Schuppan, D. Hepatitis C Virus-Replicating Hepatocytes Induce Fibrogenic Activation of Hepatic Stellate Cells. Gastroenterology 2005, 129, 246–258.
- Cheng, J.-C.; Tseng, C.-P.; Liao, M.-H.; Peng, C.-Y.; Yu, J.-S.; Chuang, P.-H.; Huang, J.-T.; Chen, J.J.W. Activation of Hepatic Stellate Cells by the Ubiquitin C-Terminal Hydrolase 1 Protein Secreted from Hepatitis C Virus-Infected Hepatocytes. Sci. Rep. 2017, 7, 4448.
- He, L.; Yuan, H.; Liang, J.; Hong, J.; Qu, C. Expression of Hepatic Stellate Cell Activation-Related Genes in HBV-, HCV-, and Nonalcoholic Fatty Liver Disease-Associated Fibrosis. PLoS ONE 2020, 15, e0233702.
- Salloum, S.; Holmes, J.A.; Jindal, R.; Bale, S.S.; Brisac, C.; Alatrakchi, N.; Lidofsky, A.; Kruger, A.J.; Fusco, D.N.; Luther, J.; et al. Exposure to Human Immunodeficiency Virus/Hepatitis C Virus in Hepatic and Stellate Cell Lines Reveals Cooperative Profibrotic Transcriptional Activation between Viruses and Cell Types: Salloum et Al. Hepatology 2016, 64, 1951–1968.
- Mazzocca, A.; Sciammetta, S.C.; Carloni, V.; Cosmi, L.; Annunziato, F.; Harada, T.; Abrignani, S.; Pinzani, M. Binding of Hepatitis C Virus Envelope Protein E2 to CD81 Up-Regulates Matrix Metalloproteinase-2 in Human Hepatic Stellate Cells. J. Biol. Chem. 2005, 280, 11329–11339.
- Bataller, R.; Paik, Y.; Lindquist, J.N.; Lemasters, J.J.; Brenner, D.A. Hepatitis C Virus Core and Nonstructural Proteins Induce Fibrogenic Effects in Hepatic Stellate Cells. Gastroenterology 2004, 126, 529–540.
- Wang, Q.; Zhou, J.; Zhang, B.; Tian, Z.; Tang, J.; Zheng, Y.; Huang, Z.; Tian, Y.; Jia, Z.; Tang, Y.; et al. Hepatitis B Virus Induces IL-23 Production in Antigen Presenting Cells and Causes Liver Damage via the IL-23/IL-17 Axis. PLoS Pathog. 2013, 9, e1003410.
- Tan, Z.; Qian, X.; Jiang, R.; Liu, Q.; Wang, Y.; Chen, C.; Wang, X.; Ryffel, B.; Sun, B. IL-17A Plays a Critical Role in the Pathogenesis of Liver Fibrosis through Hepatic Stellate Cell Activation. J. Immunol. 2013, 191, 1835–1844.
- Green, D.F.; Resnick, L.; Bourgoignie, J.J. HIV Infects Glomerular Endothelial and Mesangial but Not Epithelial Cells in Vitro. Kidney Int. 1992, 41, 956–960.
- Tokizawa, S.; Shimizu, N.; Hui-Yu, L.; Deyu, F.; Haraguchi, Y.; Oite, T.; Hoshino, H. Infection of Mesangial Cells with HIV and SIV: Identification of GPR1 as a Coreceptor. Kidney Int. 2000, 58, 607–617.
- Alcendor, D.J. Human Vascular Pericytes and Cytomegalovirus Pathobiology. Int. J. Mol. Sci. 2019, 20, 1456.
- Popik, W.; Correa, H.; Khatua, A.; Aronoff, D.M.; Alcendor, D.J. Mesangial Cells, Specialized Renal Pericytes and Cytomegalovirus Infectivity: Implications for HCMV Pathology in the Glomerular Vascular Unit and Post-Transplant Renal Disease. J. Transl. Sci. 2018, 5.
- Ustinov, J.A.; Loginov, R.J.; Mattila, P.M.; Nieminen, V.K.; Suni, J.I.; Häyry, P.J.; Lautenschlager, I.T. Cytomegalovirus Infection of Human Kidney Cells in Vitro. Kidney Int. 1991, 40, 954–960.
- Heieren, M.H.; van der Woude, F.J.; Balfour, H.H. Cytomegalovirus Replicates Efficiently in Human Kidney Mesangial Cells. Proc. Natl. Acad. Sci. USA 1988, 85, 1642–1646.
- Sansonno, D.; Gesualdo, L.; Manno, C.; Schena, F.P.; Dammacco, F. Hepatitis C Virus-Related Proteins in Kidney Tissue from Hepatitis C Virus-Infected Patients with Cryoglobulinemic Membranoproliferative Glomerulonephritis. Hepatology 1997, 25, 1237–1244.
- Alcendor, D.J. Zika Virus Infection of the Human Glomerular Cells: Implications for Viral Reservoirs and Renal Pathogenesis. J. Infect. Dis. 2017, 216, 162–171.
- Jansen, J.; Reimer, K.C.; Nagai, J.S.; Varghese, F.S.; Overheul, G.J.; de Beer, M.; Roverts, R.; Daviran, D.; Fermin, L.A.S.; Willemsen, B.; et al. SARS-CoV-2 Infects the Human Kidney and Drives Fibrosis in Kidney Organoids. Cell Stem Cell 2022, 29, 217–231.
- Niu, F.; Yao, H.; Zhang, W.; Sutliff, R.L.; Buch, S. Tat 101-Mediated Enhancement of Brain Pericyte Migration Involves Platelet-Derived Growth Factor Subunit B Homodimer: Implications for Human Immunodeficiency Virus-Associated Neurocognitive Disorders. J. Neurosci. 2014, 34, 11812–11825.
- Nakagawa, S.; Castro, V.; Toborek, M. Infection of Human Pericytes by HIV-1 Disrupts the Integrity of the Blood–Brain Barrier. J. Cell. Mol. Med. 2012, 16, 2950–2957.
- Persidsky, Y.; Hill, J.; Zhang, M.; Dykstra, H.; Winfield, M.; Reichenbach, N.L.; Potula, R.; Mukherjee, A.; Ramirez, S.H.; Rom, S. Dysfunction of Brain Pericytes in Chronic Neuroinflammation. J. Cereb. Blood Flow Metab. 2016, 36, 794–807.
- Cheeran, M.C.-J.; Lokensgard, J.R.; Schleiss, M.R. Neuropathogenesis of Congenital Cytomegalovirus Infection: Disease Mechanisms and Prospects for Intervention. Clin. Microbiol. Rev. 2009, 22, 99–126, Table of Contents.
- Alcendor, D.J.; Charest, A.M.; Zhu, W.Q.; Vigil, H.E.; Knobel, S.M. Infection and Upregulation of Proinflammatory Cytokines in Human Brain Vascular Pericytes by Human Cytomegalovirus. J. Neuroinflamm. 2012, 9, 95.
- Chen, C.-J.; Ou, Y.-C.; Li, J.-R.; Chang, C.-Y.; Pan, H.-C.; Lai, C.-Y.; Liao, S.-L.; Raung, S.-L.; Chang, C.-J. Infection of Pericytes In Vitro by Japanese Encephalitis Virus Disrupts the Integrity of the Endothelial Barrier. J. Virol. 2014, 88, 1150–1161.
- Chang, C.-Y.; Li, J.-R.; Ou, Y.-C.; Lin, S.-Y.; Wang, Y.-Y.; Chen, W.-Y.; Hu, Y.-H.; Lai, C.-Y.; Chang, C.-J.; Chen, C.-J. Interplay of Inflammatory Gene Expression in Pericytes Following Japanese Encephalitis Virus Infection. Brain Behav. Immun. 2017, 66, 230–243.
- Liu, H.; Qiu, K.; He, Q.; Lei, Q.; Lu, W. Mechanisms of Blood-Brain Barrier Disruption in Herpes Simplex Encephalitis. J. Neuroimmune Pharm. 2019, 14, 157–172.
- Kim, J.; Alejandro, B.; Hetman, M.; Hattab, E.M.; Joiner, J.; Schroten, H.; Ishikawa, H.; Chung, D.-H. Zika Virus Infects Pericytes in the Choroid Plexus and Enters the Central Nervous System through the Blood-Cerebrospinal Fluid Barrier. PLoS Pathog. 2020, 16, e1008204.
- Krauer, F.; Riesen, M.; Reveiz, L.; Oladapo, O.T.; Martínez-Vega, R.; Porgo, T.V.; Haefliger, A.; Broutet, N.J.; Low, N.; Group, W.Z.C.W. Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain–Barré Syndrome: Systematic Review. PLoS Med. 2017, 14, e1002203.
- Butler, J.S.; Dunning, E.C.; Murray, D.W.; Doran, P.P.; O’Byrne, J.M. HIV-1 Protein Induced Modulation of Primary Human Osteoblast Differentiation and Function via a Wnt/β-Catenin-Dependent Mechanism. J. Orthop. Res. 2013, 31, 218–226.
- Chen, W.; Foo, S.-S.; Li, R.W.; Smith, P.N.; Mahalingam, S. Osteoblasts from Osteoarthritis Patients Show Enhanced Susceptibility to Ross River Virus Infection Associated with Delayed Type I Interferon Responses. Virol. J. 2014, 11, 189.
- Chen, W.; Foo, S.-S.; Rulli, N.E.; Taylor, A.; Sheng, K.-C.; Herrero, L.J.; Herring, B.L.; Lidbury, B.A.; Li, R.W.; Walsh, N.C.; et al. Arthritogenic Alphaviral Infection Perturbs Osteoblast Function and Triggers Pathologic Bone Loss. Proc. Natl. Acad. Sci. USA 2014, 111, 6040–6045.
- Noret, M.; Herrero, L.; Rulli, N.; Rolph, M.; Smith, P.N.; Li, R.W.; Roques, P.; Gras, G.; Mahalingam, S. Interleukin 6, RANKL, and Osteoprotegerin Expression by Chikungunya Virus–Infected Human Osteoblasts. J. Infect. Dis. 2012, 206, 455–457.
- Manganelli, P.; Giuliani, N.; Fietta, P.; Mancini, C.; Lazzaretti, M.; Pollini, A.; Quaini, F.; Pedrazzoni, M. OPG/RANKL System Imbalance in a Case of Hepatitis C-Associated Osteosclerosis: The Pathogenetic Key? Clin. Rheumatol. 2005, 24, 296–300.
- Kluger, R.; Mühlberger, H.; Hoffmann, O.; Berger, C.E.; Engel, A.; Pavlova, B.G. Osteoprogenitor Cells and Osteoblasts Are Targets for Hepatitis C Virus. Clin. Orthop. Relat. Res. 2005, 433, 251–257.
- Hansen, A.-B.E.; Omland, L.H.; Krarup, H.; Obel, N. Fracture Risk in Hepatitis C Virus Infected Persons: Results from the DANVIR Cohort Study. J. Hepatol. 2014, 61, 15–21.
- Ayala-Peña, V.; Santillán, G.; Scolaro, L. Experimental in Vitro Infection of Rat Osteoblasts with Measles Virus Stimulates Osteogenic Differentiation. Biochem. Biophys. Res. Commun. 2014, 451, 609–614.
- Potocka-Bakłażec, M.; Sakowicz-Burkiewicz, M.; Kuczkowski, J.; Pawełczyk, T.; Stankiewicz, C.; Sierszeń, W.; Jankowski, Z.; Buczny, J. Expression of TNF-α, OPG, IL-1β and the Presence of the Measles Virus RNA in the Stapes of the Patients with Otosclerosis. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 1907–1912.
- Teramachi, J.; Nagata, Y.; Mohammad, K.; Inagaki, Y.; Ohata, Y.; Guise, T.; Michou, L.; Brown, J.P.; Windle, J.J.; Kurihara, N.; et al. Measles Virus Nucleocapsid Protein Increases Osteoblast Differentiation in Paget’s Disease. J. Clin. Investig. 2016, 126, 1012–1022.
- Keswani, S.C.; Polley, M.; Pardo, C.A.; Griffin, J.W.; McArthur, J.C.; Hoke, A. Schwann Cell Chemokine Receptors Mediate HIV-1 Gp120 Toxicity to Sensory Neurons. Ann. Neurol. 2003, 54, 287–296.
- Dilena, R.; Strazzer, S.; Esposito, S.; Paglialonga, F.; Tadini, L.; Barbieri, S.; Giannini, A. Locked-in–like Fulminant Infantile Guillain–Barré Syndrome Associated with Herpes Simplex Virus 1 Infection. Muscle Nerve 2016, 53, 140–143.
- Behar, R.; Wiley, C.; McCutchan, J.A. Cytomegalovirus Polyradiculoneuropathy in Acquired Immune Deficiency Syndrome. Neurology 1987, 37, 557.
- Orlikowski, D.; Porcher, R.; Sivadon-Tardy, V.; Quincampoix, J.-C.; Raphael, J.-C.; Durand, M.-C.; Sharshar, T.; Roussi, J.; Caudie, C.; Annane, D.; et al. Guillain-Barre Syndrome Following Primary Cytomegalovirus Infection: A Prospective Cohort Study. Clin. Infect. Dis. 2011, 52, 837–844.
- Mohan, A.; Smith-Rohrberg, D.; Sethu, M.; Sharma, S.K. Cytomegalovirus Polyradiculopathy: A Rare Neurological Manifestation of Acquired Immunodeficiency Syndrome. Neurol. India 2008, 56, 493–494.
- Sawai, S.; Satoh, M.; Mori, M.; Misawa, S.; Sogawa, K.; Kazami, T.; Ishibashi, M.; Beppu, M.; Shibuya, K.; Ishige, T.; et al. Moesin Is a Possible Target Molecule for Cytomegalovirus-Related Guillain-Barre Syndrome. Neurology 2014, 83, 113–117.
- Miyaji, K.; Devaux, J.; Yuki, N.; Sawai, S.; Mori, M.; Kuwabara, S.; Miyaji, K.; Yuki, N.; Sawai, S.; Mori, M.; et al. Moesin Is a Possible Target Molecule for Cytomegalovirus-Related Guillain-Barre Syndrome. Neurology 2014, 83, 2314–2315.
- Cumberworth, S.L.; Barrie, J.A.; Cunningham, M.E.; de Figueiredo, D.P.G.; Schultz, V.; Wilder-Smith, A.J.; Brennan, B.; Pena, L.J.; Freitas de Oliveira França, R.; Linington, C.; et al. Zika Virus Tropism and Interactions in Myelinating Neural Cell Cultures: CNS Cells and Myelin Are Preferentially Affected. Acta Neuropathol. Commun. 2017, 5, 50.
- Caplan, A.I. New MSC: MSCs as Pericytes Are Sentinels and Gatekeepers. J. Orthop. Res. 2017, 35, 1151–1159.
- Le Blanc, K.; Davies, L.C. Mesenchymal Stromal Cells and the Innate Immune Response. Immunol. Lett. 2015, 168, 140–146.
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal Stromal Cells: Sensors and Switchers of Inflammation. Cell Stem Cell 2013, 13, 392–402.
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A New Mesenchymal Stem Cell (MSC) Paradigm: Polarization into a Pro-Inflammatory MSC1 or an Immunosuppressive MSC2 Phenotype. PLoS ONE 2010, 5, e10088.
- Tomchuck, S.L.; Zwezdaryk, K.J.; Coffelt, S.B.; Waterman, R.S.; Danka, E.S.; Scandurro, A.B. Toll-like Receptors on Human Mesenchymal Stem Cells Drive Their Migration and Immunomodulating Responses. Stem Cells 2008, 26, 99–107.
- Friedenstein, A.J.; Chailakhyan, R.K.; Latsinik, N.V.; Panasyuk, A.F.; Keiliss-Borok, I.V. Stromal Cells Responsible for Transferring the Microenvironment of the Hemopoietic Tissues. Cloning in Vitro and Retransplantation in Vivo. Transplantation 1974, 17, 331–340.
- Isern, J.; Garcia-Garcia, A.; Martin, A.M.; Arranz, L.; Martin-Perez, D.; Torroja, C.; Sanchez-Csabo, F.; Mendez-Ferrer, S. The Neural Crest Is a Source of Mesenchymal Stem Cells with Specialized Hematopoietic Stem-Cell-Niche Function. eLife 2014, 3, e03696.
- Mendez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; MacArthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and Haematopoietic Stem Cells Form a Unique Bone Marrow Niche. Nature 2010, 466, 829–834.
- Schneider, R.K.; Mullally, A.; Dugourd, A.; Peisker, F.; Hoogenboezem, R.; Van Strien, P.M.H.; Bindels, E.M.; Heckl, D.; Büsche, G.; Fleck, D.; et al. Gli1+ Mesenchymal Stromal Cells Are a Key Driver of Bone Marrow Fibrosis and an Important Cellular Therapeutic Target. Cell Stem Cell 2017, 20, 785–800.
- Sacchetti, B.; Funari, A.; Michienzi, S.; Di Cesare, S.; Piersanti, S.; Saggio, I.; Tagliafico, E.; Ferrari, S.; Robey, P.G.; Riminucci, M.; et al. Self-Renewing Osteoprogenitors in Bone Marrow Sinusoids Can Organize a Hematopoietic Microenvironment. Cell 2007, 131, 324–336.
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.R.; Soria, B.; Capilla-Gonzalez, V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 43.
- Collino, F.; Bruno, S.; Incarnato, D.; Dettori, D.; Neri, F.; Provero, P.; Pomatto, M.; Oliviero, S.; Tetta, C.; Quesenberry, P.J.; et al. AKI Recovery Induced by Mesenchymal Stromal Cell-Derived Extracellular Vesicles Carrying MicroRNAs. J. Am. Soc. Nephrol. 2015, 26, 2349–2360.
- Kallmeyer, K.; Ryder, M.A.; Pepper, M.S. Mesenchymal Stromal Cells: A Possible Reservoir for HIV-1? Stem Cell Rev. Rep. 2022, 18, 1253–1280.
- Soland, M.A.; Keyes, L.R.; Bayne, R.; Moon, J.; Porada, C.D.; Jeor, S.S.; Almeida-Porada, G. Perivascular Stromal Cells as a Potential Reservoir of Human Cytomegalovirus. Am. J. Transplant. 2014, 14, 820–830.
- Rollín, R.; Álvarez-Lafuente, R.; Marco, F.; Jover, J.A.; Hernández-García, C.; Rodríguez-Navas, C.; López-Durán, L.; Fernández-Gutiérrez, B. Human Parvovirus B19, Varicella Zoster Virus, and Human Herpesvirus-6 in Mesenchymal Stem Cells of Patients with Osteoarthritis: Analysis with Quantitative Real-Time Polymerase Chain Reaction. Osteoarthr. Cartil. 2007, 15, 475–478.
- Le Blanc, K.; Mougiakakos, D. Multipotent Mesenchymal Stromal Cells and the Innate Immune System. Nat. Rev. Immunol. 2012, 12, 383–396.
- Raicevic, G.; Najar, M.; Busser, H.; Crompot, E.; Bron, D.; Toungouz, M.; Lagneaux, L. Comparison and Immunobiological Characterization of Retinoic Acid Inducible Gene-I-like Receptor Expression in Mesenchymal Stromal Cells. Sci. Rep. 2017, 7, 2896.
- Mastri, M.; Shah, Z.; McLaughlin, T.; Greene, C.J.; Baum, L.; Suzuki, G.; Lee, T. Activation of Toll-like Receptor 3 Amplifies Mesenchymal Stem Cell Trophic Factors and Enhances Therapeutic Potency. Am. J. Physiol.-Cell Physiol. 2012, 303, C1021–C1033.
- Raicevic, G.; Rouas, R.; Najar, M.; Stordeur, P.; Id Boufker, H.; Bron, D.; Martiat, P.; Goldman, M.; Nevessignsky, M.T.; Lagneaux, L. Inflammation Modifies the Pattern and the Function of Toll-like Receptors Expressed by Human Mesenchymal Stromal Cells. Hum. Immunol. 2010, 71, 235–244.
- Meisel, R.; Brockers, S.; Heseler, K.; Degistirici, Ö.; Bülle, H.; Woite, C.; Stuhlsatz, S.; Schwippert, W.; Jäger, M.; Sorg, R.; et al. Human but Not Murine Multipotent Mesenchymal Stromal Cells Exhibit Broad-Spectrum Antimicrobial Effector Function Mediated by Indoleamine 2,3-Dioxygenase. Leukemia 2011, 25, 648–654.
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754.
- Sabatini, F.; Petecchia, L.; Tavian, M.; de Villeroché, V.J.; Rossi, G.A.; Brouty-Boyé, D. Human Bronchial Fibroblasts Exhibit a Mesenchymal Stem Cell Phenotype and Multilineage Differentiating Potentialities. Lab. Investig. 2005, 85, 962–971.
- Sinclair, K.; Yerkovich, S.T.; Chambers, D.C. Mesenchymal Stem Cells and the Lung. Respirology 2013, 18, 397–411.
- Humphreys, B.D.; Lin, S.-L.; Kobayashi, A.; Hudson, T.E.; Nowlin, B.T.; Bonventre, J.V.; Valerius, M.T.; McMahon, A.P.; Duffield, J.S. Fate Tracing Reveals the Pericyte and Not Epithelial Origin of Myofibroblasts in Kidney Fibrosis. Am. J. Pathol. 2010, 176, 85–97.
- Kramann, R.; Schneider, R.K.; DiRocco, D.P.; Machado, F.; Fleig, S.; Bondzie, P.A.; Henderson, J.M.; Ebert, B.L.; Humphreys, B.D. Perivascular Gli1+ Progenitors Are Key Contributors to Injury-Induced Organ Fibrosis. Cell Stem Cell 2015, 16, 51–66.
- Avanzini, M.A.; Mura, M.; Percivalle, E.; Bastaroli, F.; Croce, S.; Valsecchi, C.; Lenta, E.; Nykjaer, G.; Cassaniti, I.; Bagnarino, J.; et al. Human Mesenchymal Stromal Cells Do Not Express ACE2 and TMPRSS2 and Are Not Permissive to SARS-CoV-2 Infection. Stem Cells Transl. Med. 2021, 10, 636–642.
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. MBoC 2002, 13, 4279–4295.
- Uzbas, F.; May, I.D.; Parisi, A.M.; Thompson, S.K.; Kaya, A.; Perkins, A.D.; Memili, E. Molecular Physiognomies and Applications of Adipose-Derived Stem Cells. Stem Cell Rev. Rep. 2015, 11, 298–308.
- Bajek, A.; Gurtowska, N.; Olkowska, J.; Kazmierski, L.; Maj, M.; Drewa, T. Adipose-Derived Stem Cells as a Tool in Cell-Based Therapies. Arch. Immunol. Ther. Exp. 2016, 64, 443–454.
- Fang, Y.; Zhang, Y.; Zhou, J.; Cao, K. Adipose-Derived Mesenchymal Stem Cell Exosomes: A Novel Pathway for Tissues Repair. Cell Tissue Bank 2019, 20, 153–161.
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-Derived Stem Cells Differentiate into a Schwann Cell Phenotype and Promote Neurite Outgrowth in Vitro. Exp. Neurol. 2007, 207, 267–274.
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in Inflammation and Metabolic Disease. Nat. Rev. Immunol. 2011, 11, 85–97.
- Vernochet, C.; Azoulay, S.; Duval, D.; Guedj, R.; Cottrez, F.; Vidal, H.; Ailhaud, G.; Dani, C. Human Immunodeficiency Virus Protease Inhibitors Accumulate into Cultured Human Adipocytes and Alter Expression of Adipocytokines. J. Biol. Chem. 2005, 280, 2238–2243.
- Wang, Y.; Wang, F.; Zhao, H.; Zhang, X.; Chen, H.; Zhang, K. Human Adipose-Derived Mesenchymal Stem Cells Are Resistant to HBV Infection during Differentiation into Hepatocytes in Vitro. Int. J. Mol. Sci. 2014, 15, 6096–6110.
- Choi, J.E.; Hur, W.; Kim, J.-H.; Li, T.Z.; Lee, E.B.; Lee, S.W.; Kang, W.; Shin, E.-C.; Wakita, T.; Yoon, S.K. MicroRNA-27a Modulates HCV Infection in Differentiated Hepatocyte-Like Cells from Adipose Tissue-Derived Mesenchymal Stem Cells. PLoS ONE 2014, 9, e91958.
- Geerts, A. History, Heterogeneity, Developmental Biology, and Functions of Quiescent Hepatic Stellate Cells. Semin. Liver Dis. 2001, 21, 311–336.
- Kupffer, C. Ueber Sternzellen der Leber: Briefliche Mittheilung an Prof. Waldeyer. Arch. Mikrosk. Anat. 1876, 12, 353–358.
- Cassiman, D.; Libbrecht, L.; Desmet, V.; Denef, C.; Roskams, T. Hepatic Stellate Cell/Myofibroblast Subpopulations in Fibrotic Human and Rat Livers. J. Hepatol. 2002, 36, 200–209.
- Neubauer, K.; Knittel, T.; Aurisch, S.; Fellmer, P.; Ramadori, G. Glial Fibrillary Acidic Protein—A Cell Type Specific Marker for Ito Cells in Vivo and in Vitro. J. Hepatol. 1996, 24, 719–730.
- Ito, T.; Nemoto, M. Über Die Kupfferschen Sternzellen Und Die “Fettspeicherungszellen” (“Fat Storing Cells”) in Der Blutkapillarenwand Der Menschlichen Leber. Okajimas Folia Anat. Jpn. 1952, 24, 243–258.
- Asada, N.; Takase, M.; Nakamura, J.; Oguchi, A.; Asada, M.; Suzuki, N.; Yamarnura, K.; Nagoshi, N.; Shibata, S.; Rao, T.N.; et al. Dysfunction of Fibroblasts of Extrarenal Origin Underlies Renal Fibrosis and Renal Anemia in Mice. J. Clin. Investig. 2011, 121, 3981–3990.
- Miyata, E.; Masuya, M.; Yoshida, S.; Nakamura, S.; Kato, K.; Sugimoto, Y.; Shibasaki, T.; Yamamura, K.; Ohishi, K.; Nishii, K.; et al. Hematopoietic Origin of Hepatic Stellate Cells in the Adult Liver. Blood 2008, 111, 2427–2435.
- Friedman, S.L. Hepatic Stellate Cells: Protean, Multifunctional, and Enigmatic Cells of the Liver. Physiol. Rev. 2008, 88, 125–172.
- Wake, K. Perisinusoidal Stellate Cells (Fat-Storing Cells, Interstitial Cells, Lipocytes), Their Related Structure in and around the Liver Sinusoids, and Vitamin A-Storing Cells in Extrahepatic Organs. In International Review of Cytology; Elsevier: Amsterdam, The Netherlands, 1980; Volume 66, pp. 303–353. ISBN 978-0-12-364466-4.
- Zhang, C.-Y.; Yuan, W.-G.; He, P.; Lei, J.-H.; Wang, C.-X. Liver Fibrosis and Hepatic Stellate Cells: Etiology, Pathological Hallmarks and Therapeutic Targets. World J. Gastroenterol. 2016, 22, 10512.
- Yan, C.; Zhou, L.; Han, Y.-P. Contribution of Hepatic Stellate Cells and Matrix Metalloproteinase 9 in Acute Liver Failure: HSCs and MMP 9 in ALF. Liver Int. 2008, 28, 959–971.
- Gupta, G.; Khadem, F.; Uzonna, J.E. Role of Hepatic Stellate Cell (HSC)-Derived Cytokines in Hepatic Inflammation and Immunity. Cytokine 2019, 124, 154542.
- Wang, B.; Trippler, M.; Pei, R.; Lu, M.; Broering, R.; Gerken, G.; Schlaak, J.F. Toll-like Receptor Activated Human and Murine Hepatic Stellate Cells Are Potent Regulators of Hepatitis C Virus Replication. J. Hepatol. 2009, 51, 1037–1045.
- Da Silva Meirelles, L.; Caplan, A.I.; Nardi, N.B. In Search of the In Vivo Identity of Mesenchymal Stem Cells. Stem Cells 2008, 26, 2287–2299.
- Lin, S.-L.; Kisseleva, T.; Brenner, D.A.; Duffield, J.S. Pericytes and Perivascular Fibroblasts Are the Primary Source of Collagen-Producing Cells in Obstructive Fibrosis of the Kidney. Am. J. Pathol. 2008, 173, 1617–1627.
- Duffield, J.S. Cellular and Molecular Mechanisms in Kidney Fibrosis. J. Clin. Investig. 2014, 124, 2299–2306.
- Blüm, P.; Pircher, J.; Merkle, M.; Czermak, T.; Ribeiro, A.; Mannell, H.; Krötz, F.; Hennrich, A.; Spannagl, M.; Köppel, S.; et al. Arterial Thrombosis in the Context of HCV-Associated Vascular Disease Can Be Prevented by Protein C. Cell. Mol. Immunol. 2017, 14, 986–996.
- Kobayashi, N.; Bagheri, N.; Nedrud, J.G.; Strieter, R.M.; Tomino, Y.; Lamm, M.E.; Emancipator, S.N. Differential Effects of Sendai Virus Infection on Mediator Synthesis by Mesangial Cells from Two Mouse Strains. Kidney Int. 2003, 64, 1675–1684.
- Pasch, A.; Frey, F.J. Coxsackie B Viruses and the Kidney—A Neglected Topic. Nephrol. Dial. Transplant. 2006, 21, 1184–1187.
- Popik, W.; Khatua, A.K.; Fabre, N.F.; Hildreth, J.E.K.; Alcendor, D.J. BK Virus Replication in the Glomerular Vascular Unit: Implications for BK Virus Associated Nephropathy. Viruses 2019, 11, 583.
- Zhai, S.; Hu, L.; Zhong, L.; Guo, Y.; Dong, L.; Jia, R.; Wang, Z. Respiratory Syncytial Virus Aggravates Renal Injury through Cytokines and Direct Renal Injury. Front. Cell. Infect. Microbiol. 2016, 6, 112.
- Mattana, J.; Abramovici, M.; Singhal, P.C. Effects of Human Immunodeficiency Virus Sera and Macrophage Supernatants on Mesangial Cell Proliferation and Matrix Synthesis. Am. J. Pathol. 1993, 143, 814–822.
- Kotton, C.N.; Fishman, J.A. Viral Infection in the Renal Transplant Recipient. J. Am. Soc. Nephrol. 2005, 16, 1758–1774.
- Baumert, T.F.; Berg, T.; Lim, J.K.; Nelson, D.R. Status of Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection and Remaining Challenges. Gastroenterology 2019, 156, 431–445.
- Flür, K.; Allam, R.; Zecher, D.; Kulkarni, O.P.; Lichtnekert, J.; Schwarz, M.; Beutler, B.; Vielhauer, V.; Anders, H.-J. Viral RNA Induces Type I Interferon-Dependent Cytokine Release and Cell Death in Mesangial Cells via Melanoma-Differentiation-Associated Gene-5: Implications for Viral Infection-Associated Glomerulonephritis. Am. J. Pathol. 2009, 175, 2014–2022.
- Imaizumi, T.; Tanaka, H.; Matsumiya, T.; Yoshida, H.; Tanji, K.; Tsuruga, K.; Oki, E.; Aizawa-Yashiro, T.; Ito, E.; Satoh, K. Retinoic Acid-Inducible Gene-I Is Induced by Double-Stranded RNA and Regulates the Expression of CC Chemokine Ligand (CCL) 5 in Human Mesangial Cells. Nephrol. Dial. Transplant. 2010, 25, 3534–3539.
- Allam, R.; Lichtnekert, J.; Moll, A.G.; Taubitz, A.; Vielhauer, V.; Anders, H.-J. Viral RNA and DNA Trigger Common Antiviral Responses in Mesangial Cells. J. Am. Soc. Nephrol. 2009, 20, 1986–1996.
- Patole, P.S.; Gröne, H.-J.; Segerer, S.; Ciubar, R.; Belemezova, E.; Henger, A.; Kretzler, M.; Schlöndorff, D.; Anders, H.-J. Viral Double-Stranded RNA Aggravates Lupus Nephritis through Toll-Like Receptor 3 on Glomerular Mesangial Cells and Antigen-Presenting Cells. J. Am. Soc. Nephrol. 2005, 16, 1326–1338.
- Appaix, F. Brain Mesenchymal Stem Cells: The Other Stem Cells of the Brain? World J. Stem Cells 2014, 6, 134–143.
- Armulik, A.; Genove, G.; Betsholtz, C. Pericytes: Developmental, Physiological, and Pathological Perspectives, Problems, and Promises. Dev. Cell 2011, 21, 193–215.
- Dupin, E.; Sommer, L. Neural Crest Progenitors and Stem Cells: From Early Development to Adulthood. Dev. Biol. 2012, 366, 83–95.
- Nyúl-Tóth, Á.; Kozma, M.; Nagyőszi, P.; Nagy, K.; Fazakas, C.; Haskó, J.; Molnár, K.; Farkas, A.E.; Végh, A.G.; Váró, G.; et al. Expression of Pattern Recognition Receptors and Activation of the Non-Canonical Inflammasome Pathway in Brain Pericytes. Brain Behav. Immun. 2017, 64, 220–231.
- Kovac, A.; Erickson, M.A.; Banks, W.A. Brain Microvascular Pericytes Are Immunoactive in Culture: Cytokine, Chemokine, Nitric Oxide, and LRP-1 Expression in Response to Lipopolysaccharide. J. Neuroinflamm. 2011, 8, 139.
- Duan, L.; Zhang, X.-D.; Miao, W.-Y.; Sun, Y.-J.; Xiong, G.; Wu, Q.; Li, G.; Yang, P.; Yu, H.; Li, H.; et al. PDGFRβ Cells Rapidly Relay Inflammatory Signal from the Circulatory System to Neurons via Chemokine CCL2. Neuron 2018, 100, 183–200.e8.
- Cho, H.J.; Kuo, A.M.-S.; Bertrand, L.; Toborek, M. HIV Alters Gap Junction-Mediated Intercellular Communication in Human Brain Pericytes. Front. Mol. Neurosci. 2017, 10, 410.
- Bertrand, L.; Cho, H.J.; Toborek, M. Blood–Brain Barrier Pericytes as a Target for HIV-1 Infection. Brain 2019, 142, 502–511.
- Piekna-Przybylska, D.; Nagumotu, K.; Reid, D.M.; Maggirwar, S.B. HIV-1 Infection Renders Brain Vascular Pericytes Susceptible to the Extracellular Glutamate. J. Neurovirol. 2019, 25, 114–126.
- Gaceb, A.; Özen, I.; Padel, T.; Barbariga, M.; Paul, G. Pericytes Secrete Pro-Regenerative Molecules in Response to Platelet-Derived Growth Factor-BB. J. Cereb. Blood Flow Metab. 2018, 38, 45–57.
- Blecharz-Lang, K.G.; Wagner, J.; Fries, A.; Nieminen-Kelhä, M.; Rösner, J.; Schneider, U.C.; Vajkoczy, P. Interleukin 6-Mediated Endothelial Barrier Disturbances Can Be Attenuated by Blockade of the IL6 Receptor Expressed in Brain Microvascular Endothelial Cells. Transl. Stroke Res. 2018, 9, 631–642.
- Saint-Pastou Terrier, C.; Gasque, P. Bone Responses in Health and Infectious Diseases: A Focus on Osteoblasts. J. Infect. 2017, 75, 281–292.
- Zhang, Q.; Fu, L.; Liang, Y.; Guo, Z.; Wang, L.; Ma, C.; Wang, H. Exosomes Originating from MSCs Stimulated with TGF-β and IFN-γ Promote Treg Differentiation. J. Cell. Physiol. 2018, 233, 6832–6840.
- Chen, W.; Foo, S.-S.; Taylor, A.; Lulla, A.; Merits, A.; Hueston, L.; Forwood, M.R.; Walsh, N.C.; Sims, N.A.; Herrero, L.J.; et al. Bindarit, an Inhibitor of Monocyte Chemotactic Protein Synthesis, Protects against Bone Loss Induced by Chikungunya Virus Infection. J. Virol. 2015, 89, 581–593.
- Chen, C.-H.; Lin, C.-L.; Kao, C.-H. Relation Between Hepatitis C Virus Exposure and Risk of Osteoporosis: A Nationwide Population-Based Study. Medicine 2015, 94, e2086.
- Gibellini, D.; Crignis, E.D.; Ponti, C.; Cimatti, L.; Borderi, M.; Tschon, M.; Giardino, R.; Re, M.C. HIV-1 Triggers Apoptosis in Primary Osteoblasts and HOBIT Cells through TNFα Activation. J. Med. Virol. 2008, 80, 1507–1514.
- Bar-Shavit, Z. Taking a Toll on the Bones: Regulation of Bone Metabolism by Innate Immune Regulators. Autoimmunity 2008, 41, 195–203.
- Nakamura, K.; Deyama, Y.; Yoshimura, Y.; Suzuki, K.; Morita, M. Toll-like Receptor 3 Ligand-Induced Antiviral Response in Mouse Osteoblastic Cells. Int. J. Mol. Med. 2007, 19, 771–775.
- Nakamura, K.; Deyama, Y.; Yoshimura, Y.; Suzuki, K.; Morita, M. Synthetic Double-Stranded RNA Induces Retinoic Acid-Inducible Gene-I in Mouse Osteoblastic Cells. Mol. Med. Rep. 2008, 1, 833–836.
- Larousserie, F.; Bsiri, L.; Dumaine, V.; Dietrich, C.; Audebourg, A.; Radenen-Bussière, B.; Anract, P.; Vacher-Lavenu, M.-C.; Devergne, O. Frontline Science: Human Bone Cells as a Source of IL-27 under Inflammatory Conditions: Role of TLRs and Cytokines. J. Leukoc. Biol. 2017, 101, 1289–1300.
- Matsuse, D.; Kitada, M.; Kohama, M.; Nishikawa, K.; Makinoshima, H.; Wakao, S.; Fujiyoshi, Y.; Heike, T.; Nakahata, T.; Akutsu, H.; et al. Human Umbilical Cord-Derived Mesenchymal Stromal Cells Differentiate Into Functional Schwann Cells That Sustain Peripheral Nerve Regeneration. J. Neuropathol. Exp. Neurol. 2010, 69, 973–985.
- Sun, X.; Zhu, Y.; Yin, H.; Guo, Z.; Xu, F.; Xiao, B.; Jiang, W.; Guo, W.; Meng, H.; Lu, S.; et al. Differentiation of Adipose-Derived Stem Cells into Schwann Cell-like Cells through Intermittent Induction: Potential Advantage of Cellular Transient Memory Function. Stem Cell Res. Ther. 2018, 9, 133.
- Assouline, J.G.; Levin, M.J.; Straus, E.; Ostrovet, J.M. Varicella-Zoster Virus Infection of Human Astrocytes, Schwann Ceils, and Neurons. Virology 1990, 179, 834–844.
- Shimeld, C.; Efstathiou, S.; Hill, T. Tracking the Spread of a LacZ-Tagged Herpes Simplex Virus Type 1 between the Eye and the Nervous System of the Mouse: Comparison of Primary and Recurrent Infection. J. Virol. 2001, 75, 5252–5262.
- Volpi, V.G.; Pagani, I.; Ghezzi, S.; Iannacone, M.; D’Antonio, M.; Vicenzi, E. Zika Virus Replication in Dorsal Root Ganglia Explants from Interferon Receptor1 Knockout Mice Causes Myelin Degeneration. Sci. Rep. 2018, 8, 10166.
- Dhiman, G.; Abraham, R.; Griffin, D.E. Human Schwann Cells Are Susceptible to Infection with Zika and Yellow Fever Viruses, but Not Dengue Virus. Sci. Rep. 2019, 9, 9951.
- Muñoz, L.; Barreras, P.; Pardo, C. Zika Virus–Associated Neurological Disease in the Adult: Guillain–Barré Syndrome, Encephalitis, and Myelitis. Semin. Reprod. Med. 2016, 34, 273–279.
- Lebeau, G.; Frumence, E.; Turpin, J.; Begue, F.; Hoarau, J.-J.; Gadea, G.; Krejbich-Trotot, P.; Desprès, P.; Viranaicken, W. Zika E Glycan Loop Region and Guillain–Barré Syndrome-Related Proteins: A Possible Molecular Mimicry to Be Taken in Account for Vaccine Development. Vaccines 2021, 9, 283.
- Goethals, S.; Ydens, E.; Timmerman, V.; Janssens, S. Toll-like Receptor Expression in the Peripheral Nerve. Glia 2010, 58, 1701–1709.
