The role of extracellular matrix in breast cancer progression has recently been realized. Here, the effect of various ECM properties including fiber structure, stiffness and biochemical composition are reviewed, with a special emphasis on the extracellular vesicles.
- breast cancer
- tumor microenvironment
- extracellular matrix
- extracellular vesicles
- stiffness
- biochemical composition
- cytokines
- fiber structure
- matrix metalloproteinases
- exosomes
1. Introduction
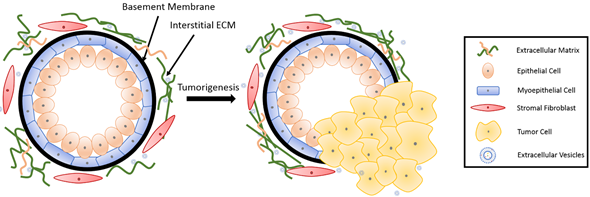
The basic mammary structure consists of luminal epithelial cells lining a central lumen, surrounded by a layer of myoepithelial cells, the stroma, and the basement membrane that separates the epithelium from the stroma (Figure 1). The basement membrane and the stroma make up an important part of the ECM in the mammary gland. The basement membrane is a thin layer of matrix mainly composed of type IV collagen, laminin and entactin[1]. Stromal ECM, which mainly contains type I collagen, fibronectin, laminins, and glycoproteins, serves as a structural scaffold that maintains breast tissue integrity and sustainability[2]. However, the role of ECM is way more significant than simply providing structural support. It plays multiple roles in regulating cell behavior in the breast tissue, such as survival, proliferation, differentiation[3], invasion[4][5], as well as immune responses[6]. Furthermore, the ECM mediates stromal–epithelial communication and serves as a guide that regulates breast development[7].
In recent decades, a growing body of research has revealed an important role of the ECM in breast cancer progression and metastasis[8][9]. During tumorigenesis, the structure and composition of the ECM is also significantly altered, further contributing to cancer progression[7]. In this section, we will discuss the influence of both the biophysical properties and biochemical composition of ECM on breast cancer progression and metastasis, as well as drug resistance. Moreover, we will discuss alterations taking place in the ECM during breast cancer development.
Figure 1. Breast tissue undergoing tumorigenesis. The basement membrane is a thin layer of pericellular matrix separating the epithelium and the stroma. Following tumorigenesis, a microenvironment is created, supporting tumor progression. Tumor cells surpass the basement membrane, which becomes more permissive in the tumor microenvironment (TME), invade the stroma, and eventually metastasize to distant sites through vasculature.
2. Role of Mechanical and Biophysical Properties of ECM in Breast Cancer
The ECM provides mechanical support to cells and guides them through mechanical stimuli. The cells adjust their behavior and remodel their microenvironment as a result of these forces[10]. These mechanical cues, including ECM density and stiffness, alter mechanotransduction signaling[11][12] and thus the protein[13] and miRNA[14] expression in cells. These alterations influence cell behavior including cell morphogenesis[15], stem cell differentiation[16][17][18], and cancer-associated fibroblast (CAF) activation[19][20], thereby potentiating and stimulating aggressive behaviors in malignant epithelial cells. For example, in a meta-analysis study, the risk of breast cancer demonstrated a compelling increase of up to 4–5-fold in women with 75% mammographic density compared to those with less than 10% density[21].
Stiffness is a well-known regulator of breast cancer cell behavior. ECM stiffness leads to profound changes in cancer cell growth, metastatic potential, as well as chemotherapeutic responses[22]. Collagen crosslinking also increases the ECM stiffness, and thus promotes tumor metastasis[23]. The collagen fibril bending stiffness of 3D collagen matrices was demonstrated to direct the spreading and clustering of breast cancer cells[24]. A previous study by Yue et al.[12] revealed that the influence of breast cancer cells on stromal cells was stiffness-dependent; breast cancer cells reduced the degree of adipogenesis only on stiff substrates. A more recent study showed that scaffold stiffness exerts its impact on breast tumor cell invasion through EGFR-linked Mena upregulation and matrix remodeling[25], altering matrix organization. Additionally, tissue stiffness regulates integrin-linked kinase (ILK) expression to control stem-like breast cancer cells under hypoxic conditions[26]. Tissue mechanical properties also modulate miR-18a expression to reduce PTEN and HOXA9 levels, and subsequently regulate cancer invasive progression[14]. In the same vein, stromal miR-200s-regulated ECM stiffness contributes to breast cancer metastasis through CAF activation[20].
The organization of the ECM is another factor influencing breast cancer cell behavior. Interestingly, collagen alignment was reported to promote migration in invasive breast cancer cells more than in non-invasive cells[27][28]. The stromal tissue is rich in type I collagen, and the collagen network in the stroma serves as a physical barrier against cancer cell invasion[29]. On the other hand, normal epithelial cells grown on type I collagen bind to type I collagen and go through epithelial-to-mesenchymal transition (EMT)[30]. Type I collagen leads to increased secretion of matrix metalloproteinases (MMPs) which facilitate ECM degradation, and induces invasive behavior[31]. Upon ECM fragmentation by MMPs, ECM bound growth factors are released[32] and a path is opened for the cancer cells to migrate through.
3. Role of Biochemical Composition of ECM in Breast Cancer
Aside from the mechanical and biophysical properties, the biochemical composition of the ECM also has a significant impact on breast tumor progression, metastasis, and response to treatments. Growing evidence indicates that many ECM proteins serve a major functional role in breast cancer progression, metastatic niche construction, and metastatic growth promotion. A study from Staren group[33] proved that ECM proteins, such as vitronectin and fibronectin, can enhance the metastatic potential of breast cancer cells by regulating cell adhesion and migration with integrin subunits. Many ECM proteins, including collagen, osteonectin, and hyaluronic acid, are involved in breast cancer development. Type I collagen poses a versatile role in breast cancer development. Fibronectin expression level in breast cancer cells is significantly associated with a higher probability of metastasis[34]. Upregulation of fibronectin also promotes formation of the pre-metastatic niche[8]. Proteoglycans various pathological processes, including cancer progression and metastasis[35]. Proteoglycan expression is altered in the breast TME during tumor development, and such an alteration affects cancer cell growth, adhesion, signaling, migration and angiogenesis[36]. A higher expression of proteoglycan in breast cancer cells is often correlated with increased tumor risk[37], grade[38] and size[37], directing the cells toward metastasis[39].
Cytokines and growth factors are becoming a significant part of breast-cancer-related studies. Many cytokines are considered as prognostic markers in breast cancer. These cytokines also impact breast cancer progression. Table 1 summarizes the principal cytokines involved in the prevention or progression of breast cancer. Transforming growth factor b (TGF-b), one of the most significant and widely studied cytokines in cancer research, is pro-tumorigenic and involved in breast cancer cell proliferation[40]. Tumor necrosis factor α (TNF-α) enhances the dendritic cell (DC) antitumor effect, inhibits growth and promotes apoptosis of breast cancer cells[41]. Fibroblast growth factor acidic (acidic FGF) is involved in the estrogen-independent and antiestrogen-resistant growth of MCF7 breast cancer cells[33]. Many interleukins (IL) are involved in the cellular immunity and communication of stromal cells with breast cancer cells[42]. IL-1α is known to promote metastasis[43], as it contributes to the induction of pro-metastatic genes in breast cancer[44]. IL-6 induces T cell and B cell differentiation, stimulates cytotoxic T cells and assists in killer cell activation to promote antitumor activity[45], demonstrating its therapeutic potential.
Table 1. Common cytokines involved in breast cancer.
|
Cytokine Type |
Role in Breast Cancer |
Ref. |
|
IL-1 family |
Promote angiogenesis, tumor proliferation and local tumor invasion |
|
|
IL-4 |
Inhibit breast cancer cell growth |
|
|
IL-6 |
Promote tumor cell proliferation, induce T- and B-cell activation |
[50] |
|
IL-8 |
Promote tumor growth and metastasis |
|
|
IL-10 |
Inhibit tumor growth, induce drug resistance |
|
|
IL-12 |
Inhibit breast cancer cell proliferation and invasion |
|
|
IL-18 |
Inhibit metastasis |
[57] |
|
IL-33 |
Promote breast cancer cell proliferation |
[58] |
|
Type I Interferon (a,b) |
Inhibit tumor proliferation and invasion |
[59] |
|
Interferon g p |
Promote breast cancer proliferation and invasion |
[60] |
|
TGF-b |
Promote breast cancer cell proliferation |
[40] |
|
gp130 |
Promote breast cancer cell proliferation and invasion |
[61] |
|
TNF-a |
Promote breast cancer metastasis |
[62] |
|
Vascular endothelial growth factor (VEGF) |
Promote breast cancer metastasis |
[63] |
|
MMP-2 |
Stimulate breast cancer metastasis |
[64] |
|
Acidic FGF |
Inhibit breast cancer proliferation |
[33] |
|
Platelet-derived growth factor (PDGF)-BB |
Promote breast cancer cell invasion |
[65] |
|
Leukemia inhibitory factor (LIF) |
Promote breast cancer cell proliferation and invasion |
[66] |
|
Cystatin C |
Inhibit breast cancer cell proliferation |
[67] |
|
Resistin |
Facilitate breast cancer progression and promote breast cancer metastasis |
Under normal conditions, breast tissue maintains homeostasis. As the ECM starts to change and becomes suitable for cancer development, disruption of homeostasis follows. While tumor cells create their own microenvironment by remodeling the ECM, the TME also impacts cancer cell behavior, leading to a more aggressive phenotype. In a pathological microenvironment, collagen fibers tend to become relatively straight, forming a more organized alignment[28]. ECM protein components could be degraded or modified by cancer-associated enzymes. MMPs are an important category of enzymes involved in ECM degradation and remodeling, and play a role in tumor cell invasiveness. MMP1, 2, 7–11, 13, 14, and 16 are constitutively expressed in tumor cell lines but not in normal breast epithelial cells[25]. MMP expression alters the rigidity, porosity, and many other characteristics of the ECM, facilitating cell migration and invasion. Breast cancer cells can activate the surrounding stromal cells to create CAFs or cancer-associated adipocytes (CAAs), which remodel the ECM and promote tumor invasiveness[70]. Breast cancer cells also modify the dynamics of stromal fibronectin and collagen interactions, with the help of MMPs[25]. The sequestered pro-angiogenic factors are released as the ECM remodels, further facilitating downstream breast cancer invasion.
Nucleic acid cargo is another influential component of ECM. Lots of effort has been put into identifying the nucleic acid profiles of the breast TME. MicroRNAs (miRNAs) are a family of small-size, non-coding RNA molecules that function as post-transcriptional gene regulators, playing roles in cancer proliferation and invasion[71]. In breast tissue, miRNAs regulate the expression of cytokines and growth factors[72] that can affect ECM composition and pave the way for pathogenesis. miRNAs are often dysregulated in breast cancer[73]. Researchers found out that a single-nucleotide polymorphism (SNP) with miR-196a2 is associated with a decreased risk of cancer[74], while an SNP in miR-146a has been reported to be linked to earlier onset of breast cancer[75]. In a study with more than 1000 patients, the upregulation of miR-103/107 was shown to be associated with metastasis and poor outcome of breast cancer patients[76]. The downregulation of miR-210 was reported to be inversely correlated with cancer aggressiveness and metastatic capability[77]. In a study by Song group[78] with 32 patients, miR-21 was shown to target MMP3 expression to regulate breast cancer invasion. Stromal miR-200s might also regulate CAF activation and ECM remodeling to promote breast cancer cell invasion[12].
4. The ECM as a Physical Barrier for Breast Cancer Treatment
The biophysical and biochemical properties of the breast significantly impact the treatment outcome of the patient. ECM components affect the penetration of immune cells, antibodies and drugs into tumor sites[79]. The dense and stiff collagen network may also serve as a physical barrier against drug penetration[80]. Hence, collagenase treatment can significantly enhance drug penetration for collagen-rich tumors[81]. Glycoseaminoglycans, such as hyaluronic acid and chondroitin sulfate, may also limit drug penetration to the tumor site.
In the meanwhile, interactions between cancer cells and the ECM can drastically affect the sensitivity of cells to apoptosis and their response to chemotherapeutic drugs. ECM proteins mediate drug resistance in breast cancer in multiple therapies. Stromal-derived MMPs are involved in tamoxifen resistance. Loss of function experiments showed that MMPs facilitated the release of heparin-bound EGF, which further regulated cell behavior, resulting in the paracrine induction of 4-OH-tamoxifen resistance through EGFR and PI3K/AKT pathways[82][83]. In HER2-positive breast cancer, ECM/integrin signaling promoted drug resistance to combination therapy aiming at HER2 and PI3K inhibition[84]. Doxorubicin was shown to be more effective against MDA-MB-231 cells when ECM-cell signaling was disrupted by inhibiting β1-integrin[85].
miRNAs are also involved in the modulation of chemotherapy responses. The dysregulation of miRNAs also affects the success of therapeutic interventions. miR-19, miR-21 and miR-203 expression in the breast results in resistance to chemotherapy[73]. Moreover, the expression of miR-34 and miR-155 suppresses radiotherapy sensitivity[73]. miRNA-34a has been reported to be associated with docetaxel resistance in human breast cancer cells[86].
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering7040124
References
- Ki M. Mak; Rena Mei; Basement Membrane Type IV Collagen and Laminin: An Overview of Their Biology and Value as Fibrosis Biomarkers of Liver Disease. The Anatomical Record 2017, 300, 1371-1390, 10.1002/ar.23567.
- Matthew W. Conklin; Patricia J. Keely; Why the stroma matters in breast cancer. Cell Adhesion & Migration 2012, 6, 249-260, 10.4161/cam.20567.
- Daniel Timothy Sweet; Zhongming Chen; David M. Wiley; Victoria L. Bautch; Ellie Tzima; The adaptor protein Shc integrates growth factor and ECM signaling during postnatal angiogenesis. Blood 2012, 119, 1946-1955, 10.1182/blood-2011-10-384560.
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219.
- Bahcecioglu, G.; Yue, X.; Howe, E.; Guldner, I.; Stack, M.S.; Nakshatri, H.; Zhang, S.; Zorlutuna, P. Aged Breast Extracellular Matrix Drives Mammary Epithelial Cells to an Invasive and Cancer-Like Phenotype. BioRxiv 2020.
- Lydia Sorokin; The impact of the extracellular matrix on inflammation. Nature Reviews Immunology 2010, 10, 712-723, 10.1038/nri2852.
- Jacob Insua-Rodríguez; Thordur Oskarsson; The extracellular matrix in breast cancer. Advanced Drug Delivery Reviews 2016, 97, 41-55, 10.1016/j.addr.2015.12.017.
- Oskarsson, T. Extracellular matrix components in breast cancer progression and metastasis. Breast 2013, 22 (Suppl. 2), S66–S72.
- Bahcecioglu, G.; Basara, G.; Ellis, B.W.; Ren, X.; Zorlutuna, P. Breast cancer models: Engineering the tumor microenvironment. Acta Biomater. 2020, 106, 1–21.
- Gokhan Bahcecioglu; Xiaoshan Yue; Erin Howe; Ian Guldner; M. Sharon Stack; Harikrishna Nakshatri; Siyuan Zhang; Pinar Zorlutuna; Aged Breast Extracellular Matrix Drives Mammary Epithelial Cells to an Invasive and Cancer-Like Phenotype. null 2020, null, null, 10.1101/2020.09.30.320960.
- Casey, J.; Yue, X.; Nguyen, T.D.; Acun, A.; Zellmer, V.R.; Zhang, S.; Zorlutuna, P. 3D hydrogel-based microwell arrays as a tumor microenvironment model to study breast cancer growth. Biomed. Mater. 2017, 12, 025009.
- Yue, X.; Nguyen, T.D.; Zellmer, V.; Zhang, S.; Zorlutuna, P. Stromal cell-laden 3D hydrogel microwell arrays as tumor microenvironment model for studying stiffness dependent stromal cell-cancer interactions. Biomaterials 2018, 170, 37–48.
- Akiko Mammoto; Kip M. Connor; Tadanori Mammoto; Chong Wing Yung; Dongeun Huh; Christopher M. Aderman; Gustavo Mostoslavsky; Kimberly A. Smith; Donald E. Ingber; A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 2009, 457, 1103-1108, 10.1038/nature07765.
- Janna K Mouw; Yoshihiro Yui; Laura Damiano; Russell O Bainer; Johnathon N Lakins; Irene Acerbi; Guanqing Ou; Amanda C Wijekoon; Kandice R Levental; Penney M Gilbert; et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nature Medicine 2014, 20, 360-367, 10.1038/nm.3497.
- Matthew J. Paszek; Nastaran Zahir; Kandice R. Johnson; Johnathon N. Lakins; Gabriela I. Rozenberg; Amit Gefen; Cynthia A. Reinhart-King; Susan S. Margulies; Micah Dembo; David Boettiger; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241-254, 10.1016/j.ccr.2005.08.010.
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689.
- Chandler, E.M.; Seo, B.R.; Califano, J.P.; Andresen Eguiluz, R.C.; Lee, J.S.; Yoon, C.J.; Tims, D.T.; Wang, J.X.; Cheng, L.; Mohanan, S.; et al. Implanted adipose progenitor cells as physicochemical regulators of breast cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 9786–9791.
- Pang, M.F.; Siedlik, M.J.; Han, S.; Stallings-Mann, M.; Radisky, D.C.; Nelson, C.M. Tissue stiffness and hypoxia modulate the integrin-linked kinase ilk to control breast cancer stem-like cells. Cancer Res. 2016, 76, 5277–5287.
- Kirsi Rilla; Anne-Mari Mustonen; Uma Thanigai Arasu; Kai Härkönen; Johanna Matilainen; Petteri Nieminen; Extracellular vesicles are integral and functional components of the extracellular matrix. Matrix Biology 2019, 75–76, 201-219, 10.1016/j.matbio.2017.10.003.
- X Tang; Y Hou; G Yang; X Wang; S Tang; Y-E Du; L Yang; T Yu; H Zhang; M Zhou; et al. Stromal miR-200s contribute to breast cancer cell invasion through CAF activation and ECM remodeling. Cell Death & Differentiation 2015, 23, 132-145, 10.1038/cdd.2015.78.
- Norman Boyd; Qing Li; Olga Melnichouk; Ella Huszti; Lisa J. Martin; Anoma Gunasekara; Gord Mawdsley; Martin J. Yaffe; Salomon Minkin; Evidence That Breast Tissue Stiffness Is Associated with Risk of Breast Cancer. PLoS ONE 2014, 9, e100937, 10.1371/journal.pone.0100937.
- Scott H. Medina; Brian Bush; Maggie Cam; Emily Sevcik; Frank W. DelRio; Kaustav Nandy; Joel P. Schneider; Identification of a mechanogenetic link between substrate stiffness and chemotherapeutic response in breast cancer. Biomaterials 2019, 202, 1-11, 10.1016/j.biomaterials.2019.02.018.
- Marta Cavo; Marco Fato; Leonardo Peñuela; Francesco Beltrame; Roberto Raiteri; Silvia Scaglione; Microenvironment complexity and matrix stiffness regulate breast cancer cell activity in a 3D in vitro model. Scientific Reports 2016, 6, 1-13, 10.1038/srep35367.
- Jiranuwat Sapudom; Liv Kalbitzer; Xiancheng Wu; Steve Martin; Klaus Kroy; Tilo Pompe; Fibril bending stiffness of 3D collagen matrices instructs spreading and clustering of invasive and non-invasive breast cancer cells. Biomaterials 2019, 193, 47-57, 10.1016/j.biomaterials.2018.12.010.
- Anthony J. Berger; Carine M. Renner; Isaac Hale; Xinhai Yang; Suzanne M. Ponik; Paul S. Weisman; Kristyn S. Masters; Pamela K. Kreeger; Scaffold stiffness influences breast cancer cell invasion via EGFR-linked Mena upregulation and matrix remodeling. Matrix Biology 2020, null, 80-93, 10.1016/j.matbio.2019.07.006.
- Gregg L. Semenza; The hypoxic tumor microenvironment: A driving force for breast cancer progression. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms 2016, 1863, 382-391, 10.1016/j.bbamcr.2015.05.036.
- Campbell, J.J.; Husmann, A.; Hume, R.D.; Watson, C.J.; Cameron, R.E. Development of three-dimensional collagen scaffolds with controlled architecture for cell migration studies using breast cancer cell lines. Biomaterials 2017, 114, 34–43.
- Schedin, P.; Keely, P.J. Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–22
- Eliza L.S. Fong; Daniel A. Harrington; Mary C. Farach-Carson; Hanry Yu; Heralding a new paradigm in 3D tumor modeling. Biomaterials 2016, 108, 197-213, 10.1016/j.biomaterials.2016.08.052.
- Damian Medici; Ali Nawshad; Type I collagen promotes epithelial–mesenchymal transition through ILK-dependent activation of NF-κB and LEF-1. Matrix Biology 2010, 29, 161-165, 10.1016/j.matbio.2009.12.003.
- Matthew W. Conklin; Ronald E. Gangnon; Brian L. Sprague; Lisa Van Germert; John M. Hampton; Kevin W. Eliceiri; Jeremy S. Bredfeldt; Yuming Liu; Nuntida Surachaicharn; Polly A. Newcomb; et al. Collagen Alignment as a Predictor of Recurrence after Ductal CarcinomaIn Situ. Cancer Epidemiology Biomarkers & Prevention 2017, 27, 138-145, 10.1158/1055-9965.epi-17-0720.
- Myriam Polette; Philippe Birembaut; Matrix Metalloproteinases in Breast Cancer. The Breast Journal 1996, 2, 209-220, 10.1111/j.1524-4741.1996.tb00097.x.
- L Zhang; S Kharbanda; J Hanfelt; F G Kern; Both autocrine and paracrine effects of transfected acidic fibroblast growth factor are involved in the estrogen-independent and antiestrogen-resistant growth of MCF-7 breast cancer cells.. Cancer Research 1998, 58, 352–361, .
- Belen Fernandez-Garcia; Noemí Eiró; Laura Marín; Salomé González-Reyes; Luis O González; Maria Luz Lamelas; Francisco J. Vizoso; Expression and prognostic significance of fibronectin and matrix metalloproteases in breast cancer metastasis. Histopathology 2013, 64, 512-522, 10.1111/his.12300.
- Achilleas D. Theocharis; Spyros S. Skandalis; Thomas Neill; Hinke A. B. Multhaupt; Mario Hubo; Helena Frey; Sandeep Gopal; Angélica Gomes; Nikos Afratis; Hooi Ching Lim; et al. Insights into the key roles of proteoglycans in breast cancer biology and translational medicine. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2015, 1855, 276-300, 10.1016/j.bbcan.2015.03.006.
- J. E. Ferguson; A. M. Schor; Anthony Howell; M. W. J. Ferguson; Changes in the extracellular matrix of the normal human breast during the menstrual cycle. Cell and Tissue Research 1992, 268, 167-177, 10.1007/bf00338066.
- Carmela Ricciardelli; John H Brooks; Supaporn Suwiwat; Andrew J. Sakko; Keiko Mayne; Wendy A Raymond; Ram Seshadri; Richard G. LeBaron; David J Horsfall; Regulation of stromal versican expression by breast cancer cells and importance to relapse-free survival in patients with node-negative primary breast cancer.. Clinical Cancer Research 2002, 8, 1054–1060, .
- Fusun Baba; Kathryn Swartz; Regina Van Buren; Jens Eickhoff; Yong Zhang; William Wolberg; A Friedl; Syndecan-1 and syndecan-4 are overexpressed in an estrogen receptor-negative, highly proliferative breast carcinoma subtype. Breast Cancer Research and Treatment 2006, 98, 91-98, 10.1007/s10549-005-9135-2.
- Thomas R. Cawthorn; Juan C. Moreno; Moyez Dharsee; Danh Tran-Thanh; Suzanne Ackloo; Pei Hong Zhu; Girish Sardana; Jian Chen; Peter Kupchak; Lindsay M. Jacks; et al. Proteomic Analyses Reveal High Expression of Decorin and Endoplasmin (HSP90B1) Are Associated with Breast Cancer Metastasis and Decreased Survival. PLoS ONE 2012, 7, e30992, 10.1371/journal.pone.0030992.
- Jeffrey Donovan; Joyce Slingerland; Transforming growth factor-β and breast cancer: Cell cycle arrest by transforming growth factor-β and its disruption in cancer. Breast Cancer Research 2000, 2, 116-124, 10.1186/bcr43.
- K A Candido; K Shimizu; J C McLaughlin; R Kunkel; J A Fuller; Bruce G. Redman; E K Thomas; B J Nickoloff; J J Mulé; Local administration of dendritic cells inhibits established breast tumor growth: implications for apoptosis-inducing agents.. Cancer Research 2001, 61, 228–236, .
- Andrea Nicolini; A. Carpi; G. Rossi; Cytokines in breast cancer. Cytokine & Growth Factor Reviews 2006, 17, 325-337, 10.1016/j.cytogfr.2006.07.002.
- Ron N. Apte; Shahar Dotan; Moshe Elkabets; Malka R. White; Eli Reich; Yaron Carmi; Xiaping Song; Tatyana Dvozkin; Yakov Krelin; Elena Voronov; et al. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer and Metastasis Reviews 2006, 25, 387-408, 10.1007/s10555-006-9004-4.
- Shinichi Nozaki; George W. Sledge; Harikrishna Nakshatri; Cancer Cell-Derived Interleukin 1α Contributes to Autocrine and Paracrine Induction of Pro-metastatic Genes in Breast Cancer. Biochemical and Biophysical Research Communications 2000, 275, 60-62, 10.1006/bbrc.2000.3241.
- Mohit Trikha; Robert Corringham; Bernard Klein; Jean-François Rossi; Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clinical Cancer Research 2003, 9, 4653-4665, .
- Pantschenko, A.G.; Pushkar, I.; Anderson, K.H.; Wang, Y.; Miller, L.J.; Kurtzman, S.H.; Barrows, G.; Kreutzer, D.L. The interleukin-1 family of cytokines and receptors in human breast cancer: Implications for tumor progression. Int. J. Oncol. 2003, 23, 269–284.
- Singer, C.F.; Kronsteiner, N.; Hudelist, G.; Marton, E.; Walter, I.; Kubista, M.; Czerwenka, K.; Schreiber, M.; Seifert, M.; Kubista, E. Interleukin 1 System and Sex Steroid Receptor Expression in Human Breast Cancer: Interleukin 1α Protein Secretion Is Correlated with Malignant Phenotype. Clin. Cancer Res. 2003, 9, 4877–4883.
- Gooch, J.L.; Lee, A.V.; Yee, D. Interleukin 4 inhibits growth and induces apoptosis in human breast cancer cells. Cancer Res. 1998, 58, 4199–4205.
- Nagai, S.; Toi, M. Interleukin-4 and breast cancer. Breast Cancer 2000, 7, 181–186.
- Peter C. Heinrich; Iris Behrmann; Gerhard Müller-Newen; Fred Schaper; Lutz Graeve; Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway1. Biochemical Journal 1998, 334, 297-314, 10.1042/bj3340297.
- Singh, B.; Berry, J.A.; Vincent, L.E.; Lucci, A. Involvement of IL-8 in COX-2-Mediated Bone Metastases from Breast Cancer. J. Surg. Res. 2006, 134, 44–51.
- David, J.; Dominguez, C.; Hamilton, D.; Palena, C. The IL-8/IL-8R Axis: A Double Agent in Tumor Immune Resistance. Vaccines 2016, 4, 22.
- Kundu, N.; Beaty, T.L.; Jackson, M.J.; Fulton, A.M. Antimetastatic and antitumor activities of interleukin 10 in a murine model of breast cancer. J. Natl. Cancer Inst. 1996, 88, 536–541.
- Mocellin, S.; Panelli, M.C.; Wang, E.; Nagorsen, D.; Marincola, F.M. The dual role of IL-10. Trends Immunol. 2003, 24, 36–43.
- Yang, C.; He, L.; He, P.; Liu, Y.; Wang, W.; He, Y.; Du, Y.; Gao, F. Increased drug resistance in breast cancer by tumor-associated macrophages through IL-10/STAT3/bcl-2 signaling pathway. Med. Oncol. 2015, 32, 352.
- Sergio Dias; Robert Boyd; Frances Balkwill; IL-12 regulates VEGF and MMPs in a murine breast cancer model. International Journal of Cancer 1998, 78, 361-365, 10.1002/(sici)1097-0215(19981029)78:3<361::aid-ijc17>3.0.co;2-9.
- A Nakata; T Tsujimura; Ayako Sugihara; H Okamura; T Iwasaki; K Shinkai; N Iwata; E Kakishita; H Akedo; N Terada; et al. Inhibition by interleukin 18 of osteolytic bone metastasis by human breast cancer cells.. Anticancer Research 2000, 19, 4131–4138, .
- Xuejian Liu; Leilei Zhu; Xin Lu; Hairong Bian; Xia Wu; Wenchuan Yang; Qingliang Qin; IL-33/ST2 pathway contributes to metastasis of human colorectal cancer. Biochemical and Biophysical Research Communications 2014, 453, 486-492, 10.1016/j.bbrc.2014.09.106.
- Aldo A. Rossini; Dale L. Greiner; John P. Mordes; Induction of immunologic tolerance for transplantation.. Physiological Reviews 1999, 79, 99-141, 10.1152/physrev.1999.79.1.99.
- Anjana Saha; Ashish Dhir; Anand Ranjan; Vibhuti Gupta; Narendra Bairwa; Rameshwar N.K. Bamezai; Functional IFNG polymorphism in intron 1 in association with an increased risk to promote sporadic breast cancer. Immunogenetics 2005, 57, 165-171, 10.1007/s00251-005-0783-5.
- Katri S. Selander; Li Li; Latania Watson; Melinda Merrell; Heike Dahmen; Peter C. Heinrich; Gerhard Müller-Newen; Kevin W. Harris; Inhibition of gp130 Signaling in Breast Cancer Blocks Constitutive Activation of Stat3 and Inhibitsin VivoMalignancy. Cancer Research 2004, 64, 6924-6933, 10.1158/0008-5472.can-03-2516.
- Sangmin Kim; Jae Hyuck Choi; Jong Bin Kim; Seok Jin Nam; Jung-Hyun Yang; Jung-Han Kim; Jeong Eon Lee; Berberine Suppresses TNF-α-induced MMP-9 and Cell Invasion through Inhibition of AP-1 Activity in MDA-MB-231 Human Breast Cancer Cells. Molecules 2008, 13, 2975-2985, 10.3390/molecules13122975.
- Mihaela Skobe; Thomas Hawighorst; David G. Jackson; Remko Prevo; Lauren Janes; Paula Velasco; Lucia Riccardi; Kari Alitalo; Kevin Claffey; Michael Detmar; et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nature Medicine 2001, 7, 192-198, 10.1038/84643.
- U M Muñoz-Nájar; K M Neurath; F Vumbaca; K P Claffey; Hypoxia stimulates breast carcinoma cell invasion through MT1-MMP and MMP-2 activation. Oncogene 2005, 25, 2379-2392, 10.1038/sj.onc.1209273.
- Yi-Chu Yu; Pei-Ming Yang; Qiu-Yu Chuah; Yao-Huei Huang; Chih-Wen Peng; Yi-Jang Lee; Shu-Jun Chiu; Radiation-induced senescence in securin-deficient cancer cells promotes cell invasion involving the IL-6/STAT3 and PDGF-BB/PDGFR pathways. Scientific Reports 2013, 3, 1–11, 10.1038/srep01675.
- Xiaoyan Li; QiFeng Yang; Haiyang Yu; Lihua Wu; Yuhan Zhao; Cen Zhang; Xuetian Yue; Zhen Liu; Hao Wu; Bruce G. Haffty; et al. LIF promotes tumorigenesis and metastasis of breast cancer through the AKT-mTOR pathway. Oncotarget 2014, 5, 788-801, 10.18632/oncotarget.1772.
- Janja Završnik; Miha Butinar; Mojca Trstenjak Prebanda; Aleksander Krajnc; Robert Vidmar; Marko Fonović; Anders Grubb; Vito Turk; Boris Turk; Olga Vasiljeva; et al. Cystatin C deficiency suppresses tumor growth in a breast cancer model through decreased proliferation of tumor cells. Oncotarget 2017, 8, 73793-73809, 10.18632/oncotarget.17379.
- Wang, C.H.; Wang, P.J.; Hsieh, Y.C.; Lo, S.; Lee, Y.C.; Chen, Y.C.; Tsai, C.H.; Chiu, W.C.; Hu, S.C.S.; Lu, C.W.; et al. Resistin facilitates breast cancer progression via TLR4- mediated induction of mesenchymal phenotypes and stemness properties. Oncogene 2018, 37, 589–600.
- Lee, J.O.; Kim, N.; Lee, H.J.; Lee, Y.W.; Kim, S.J.; Park, S.H.; Kim, H.S. Resistin, a fat-derived secretory factor, promotes metastasis of MDA-MB-231 human breast cancer cells through ERM activation. Sci. Rep. 2016, 6, 1–11.
- Karin Wang; Fei Wu; Bo Ri Seo; Claudia Fischbach; Weisi Chen; Lauren Hsu; Delphine Gourdon; Breast cancer cells alter the dynamics of stromal fibronectin-collagen interactions. Matrix Biology 2017, 60–61, 86-95, 10.1016/j.matbio.2016.08.001.
- Jonathan Chou; Payam Shahi; Zena Werb; microRNA-mediated regulation of the tumor microenvironment. Cell Cycle 2013, 12, 3262-3271, 10.4161/cc.26087.
- Zina Jeyapalan Rutnam; Thomas N. Wight; Burton B Yang; miRNAs regulate expression and function of extracellular matrix molecules.. Matrix Biology 2012, 32, 74-85, 10.1016/j.matbio.2012.11.003.
- Laoighse Mulrane; Sharon F. McGee; W M Gallagher; Darran P. O'connor; miRNA Dysregulation in Breast Cancer. Cancer Research 2013, 73, 6554-6562, 10.1158/0008-5472.can-13-1841.
- Aaron E. Hoffman; Tongzhang Zheng; Chunhui Yi; Derek Leaderer; Joanne Weidhaas; Frank Slack; Yawei Zhang; Trupti Paranjape; Yong Zhu; microRNA miR-196a-2 and Breast Cancer: A Genetic and Epigenetic Association Study and Functional Analysis. Cancer Research 2009, 69, 5970-5977, 10.1158/0008-5472.can-09-0236.
- Jie Shen; Christine B. Ambrosone; Richard A. DiCioccio; Kunle Odunsi; Shashikant Lele; Hua Zhao; A functional polymorphism in the miR-146a gene and age of familial breast/ovarian cancer diagnosis. Carcinogenesis 2008, 29, 1963-1966, 10.1093/carcin/bgn172.
- Graziano Martello; Antonio Rosato; Francesco Ferrari; Andrea Manfrin; Michelangelo Cordenonsi; Sirio Dupont; Elena Enzo; Vincenza Guzzardo; Maria Rondina; Thomas Spruce; et al. A MicroRNA Targeting Dicer for Metastasis Control. Cell 2010, 141, 1195-1207, 10.1016/j.cell.2010.05.017.
- John A. Foekens; Anieta M. Sieuwerts; Marcel Smid; Maxime P. Look; Vanja De Weerd; Antonius W. M. Boersma; Jan G. M. Klijn; Erik A. C. Wiemer; John W.M. Martens; Four miRNAs associated with aggressiveness of lymph node-negative, estrogen receptor-positive human breast cancer. Proceedings of the National Academy of Sciences 2008, 105, 13021-13026, 10.1073/pnas.0803304105.
- Bao Song; Chuanxi Wang; Jie Liu; Xingwu Wang; Liyan Lv; Ling Wei; Li Xie; Yan Zheng; Xianrang Song; MicroRNA-21 regulates breast cancer invasion partly by targeting tissue inhibitor of metalloproteinase 3 expression. Journal of Experimental & Clinical Cancer Research 2010, 29, 29-29, 10.1186/1756-9966-29-29.
- Andrew W. Holle; Jennifer L. Young; Joachim P. Spatz; In vitro cancer cell–ECM interactions inform in vivo cancer treatment. Advanced Drug Delivery Reviews 2016, 97, 270-279, 10.1016/j.addr.2015.10.007.
- Darci T. Butcher; Tamara Alliston; Valerie M. Weaver; A tense situation: forcing tumour progression. Nature Reviews Cancer 2009, 9, 108-122, 10.1038/nrc2544.
- P A Netti; D A Berk; M A Swartz; A J Grodzinsky; R K Jain; Role of extracellular matrix assembly in interstitial transport in solid tumors.. Cancer Research 2000, 60, 2497–2503, .
- Pontiggia, O.; Sampayo, R.; Raffo, D.; Motter, A.; Xu, R.; Bissell, M.J.; De Kier Joffé, E.B.; Simian, M. The tumor microenvironment modulates tamoxifen resistance in breast cancer: A role for soluble stromal factors and fibronectin through β1 integrin. Breast Cancer Res. Treat. 2012, 133, 459–471.
- Block, K.I.; Gyllenhaal, C.; Lowe, L.; Amedei, A.; Ruhul Amin, A.R.M.; Amin, A.; Aquilano, K.; Arbiser, J.; Arreola, A.; Arzumanyan, A.; et al. Designing a broad-spectrum integrative approach for cancer prevention and treatment. Semin. Cancer Biol. 2015, 35, S276–S304.
- Ariella B. Hanker; Mónica Valeria Estrada; Giampaolo Bianchini; Preston D. Moore; Junfei Zhao; Feixiong Cheng; James P. Koch; Luca Gianni; Darren R. Tyson; Violeta Sánchez; et al. Extracellular Matrix/Integrin Signaling Promotes Resistance to Combined Inhibition of HER2 and PI3K in HER2+ Breast Cancer. Cancer Research 2017, 77, 3280-3292, 10.1158/0008-5472.can-16-2808.
- Britta Weigelt; Alvin T. Lo; Catherine C. Park; Joe W. Gray; Mina J. Bissell; HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment. Breast Cancer Research and Treatment 2009, 122, 35-43, 10.1007/s10549-009-0502-2.
- L. Kastl; I. Brown; A. C. Schofield; miRNA-34a is associated with docetaxel resistance in human breast cancer cells. Breast Cancer Research and Treatment 2011, 131, 445-454, 10.1007/s10549-011-1424-3.