Fullerenes are all-carbon molecules discovered in 1985. They are spherical or ellipsoidal in shape, with a hollow cage structure. Three discoverers of fullerene C60 won the Nobel Prize in chemistry in 1996. Fullerene C60, the representative fullerene, is ~0.7 nm in diameter. In the past 30 years, with the continuous development of fullerene preparation technology, fullerenes have presented unprecedented opportunities in the fields of biomedicine, catalysis, superconduction, and photovoltaics.
Unlike traditional small molecule drugs, fullerene is an all-carbon nanomolecule with a spherical cage structure. Fullerene exhibits high levels of antiviral activity, inhibiting virus replication in vitro and in vivo. In this review, we systematically summarize the latest research regarding the different types of fullerenes investigated in antiviral studies. We discuss the unique structural advantage of fullerenes, present diverse modification strategies based on the addition of various functional groups, assess the effect of structural differences on antiviral activity, and describe the possible antiviral mechanism. Finally, we discuss the prospective development of fullerenes as antiviral drugs.
- fullerene
- antivirus
- nanodrug
1. Introduction
Currently, more than 90% of infectious diseases in humans are caused by viruses. The most well-known include the influenza virus, human immunodeficiency virus (HIV), and Ebola virus, which have caused serious damage [1–5]. Although several anti-HIV and anti-Ebola drugs, such as saquinavir, ritonavir, T20, lopinavir, ribavirin, tenofovir, and remdesivir, have been developed, their efficacy is not satisfactory. Severe acute respiratory syndrome (SARS), which broke out in China in 2003, is a respiratory infection caused by a coronavirus. So far, there is no specific medicine for SARS. The novel coronavirus, SARS-CoV-2, now circulating worldwide, is more infectious than SARS or HIV. For patients infected with SARS-CoV-2, there are no specific antiviral drugs.
Fullerenes and their derivatives exert significant inhibitory effects against HIV [1], herpes simplex virus (HSV) [2], influenza virus [6], Ebola virus [7], cytomegalovirus (CMV) [8], and other viruses in vitro and in vivo (Figure 1). Fullerenes and their derivatives, as a class of new, broad-spectrum antiviral drugs, have attracted increasing attention as a potential treatment for SARS-CoV-2.
In the past, hundreds of fullerene derivatives have been synthesized and used to inhibit viruses in vitro. Most of these derivatives are water soluble. These fullerene de-rivatives can be classified as the following six types: (1) amino acid, peptide, and primary amine derivatives; (2) piperazine and pyrrolidine derivatives; (3) carboxyl derivatives; (4) hydroxyl derivatives; (5) glycofullerene derivatives; and (6) fullerene complexes. Numerous antiviral studies have been conducted to evaluate fullerene C60 and its derivatives; this review assesses the latest research on the ability of fullerene C60 and its derivatives to inhibit virus replication.
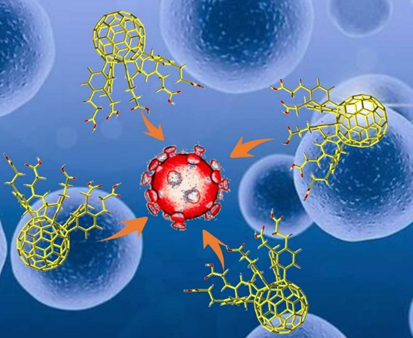 Figure 1. Possible interaction between fullerene molecules and coronavirus, in which fullerene molecules inhibit virus replication.
Figure 1. Possible interaction between fullerene molecules and coronavirus, in which fullerene molecules inhibit virus replication.
2. Synthesis and Antiviral Properties of Amino Acid, Peptide, and Primary Amine Fullerene Derivatives
In 1993, Friedman et al. [1] reported a landmark study based on model building and experimental evidence. Theoretical calculations revealed that the hydrophobic cavity of HIVP accommodates C60 molecules; the spherical C60 molecules fit perfectly within the active site, facilitating strong interactions between HIVP and fullerene. However, because C60 spheres are insoluble in polar solvents, it is critical to dissolve C60 in a medium suitable for biological testing. By modifying fullerene with strong polar groups, Sijbesma et al. [9] synthesized the water-soluble fullerene derivative bis (phenylenaminosuccinic acid)-C60 (1) (Figure 2). In vitro studies revealed that 1 inhibited acute and chronic HIV-1 infection in human peripheral blood mononuclear (PBM) cells, with a half-effective concentration (EC50) of 7.0 µM, while showing no cytotoxicity to uninfected PBM cells. This work is of great significance because fullerene derivatives as virus inhibitors are unprecedented in the field of antiviral research.
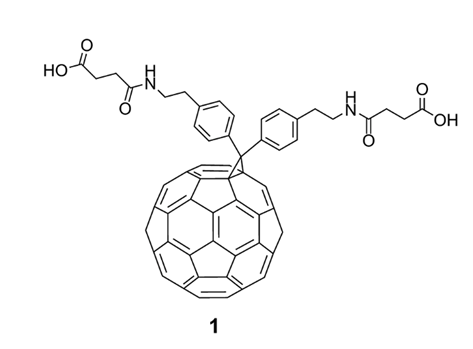 Figure 2. The first example of a water-soluble fullerene derivative (1) used as a virus inhibitor.
Figure 2. The first example of a water-soluble fullerene derivative (1) used as a virus inhibitor.
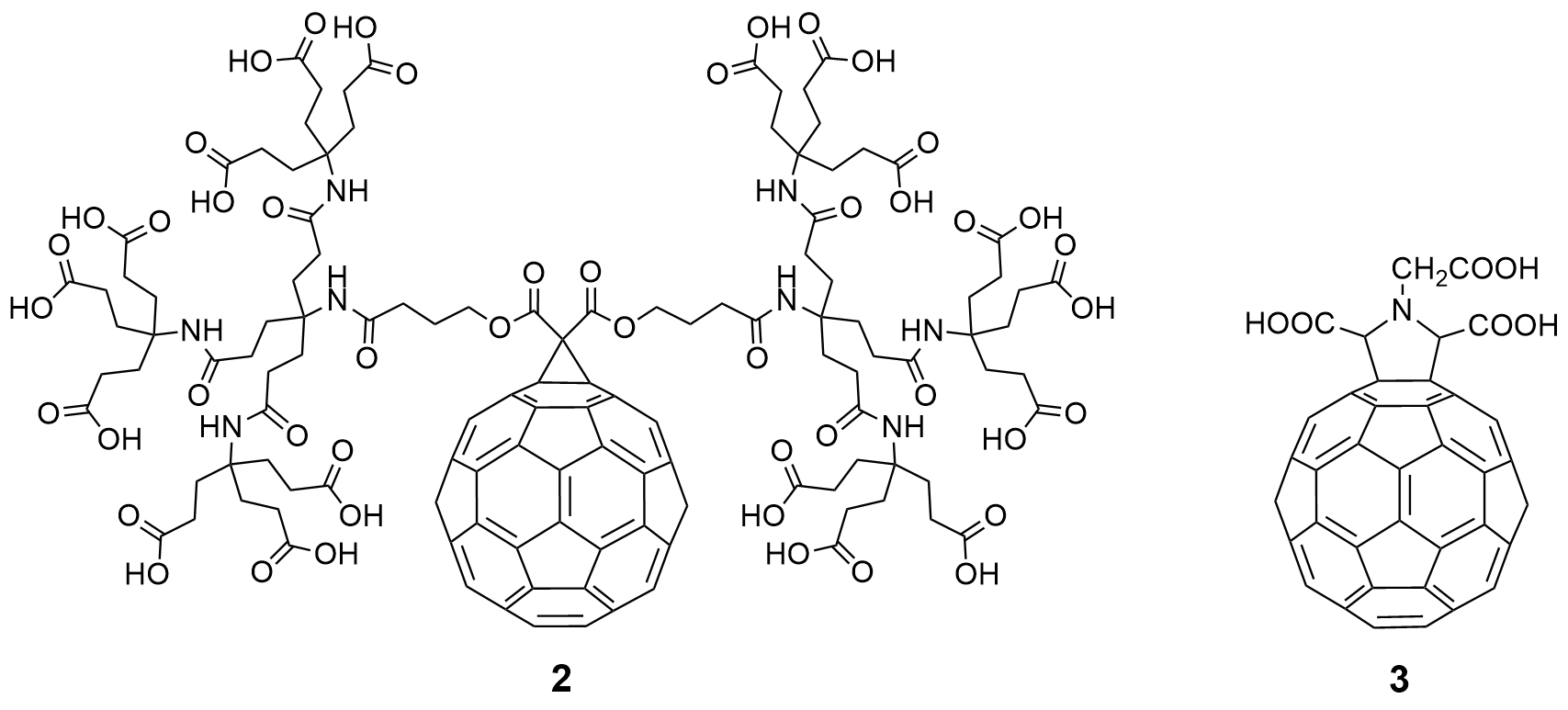
In the same time period, many research groups synthesized various fullerene derivatives with different functional groups and demonstrated effective virus inhibition by introducing appropriate carboxylic acid and amino acid groups at specific positions on fullerenes. Among them, the fullerene dendritic amino acid derivative 2, prepared by Brettreich and Hirsch [10] (Figure 3), is highly water soluble and has an EC50 of 0.22 µM in human lymphocytes acutely infected with HIV-1 [11]. This compound also shows no toxicity up to 100 μM in PBM, Vero, and CEM cells. The fullerene amino acid derivative 3 (Figure 3), synthesized by Mashino et al. [2], strongly inhibits HIV reverse transcriptase (HIV-RT), with an EC50 of 0.029 μM. Additionally, Toniolo et al. [12] synthesized fullerene peptide derivatives, which also exhibit anti-HIVP activity.
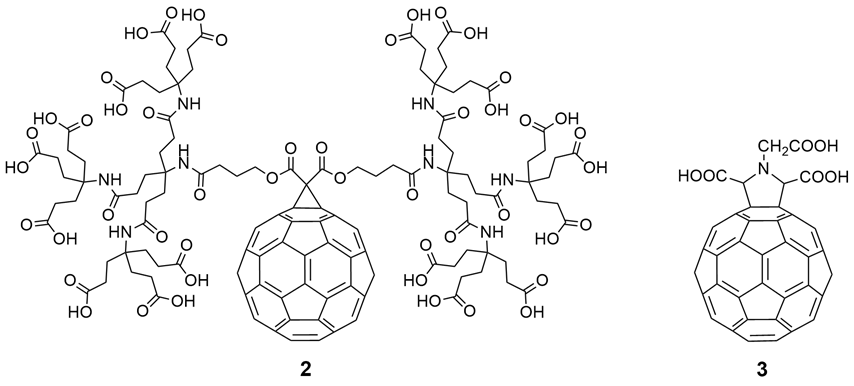 Figure 3. Fullerene amino acid derivatives (2) and (3) as potential inhibitors of HIV-1 cell replication.
Figure 3. Fullerene amino acid derivatives (2) and (3) as potential inhibitors of HIV-1 cell replication.
3. Synthesis and Antiviral Studies of Fullerene Piperazine and Pyrrolidine Derivatives
As shown in Figure 4, the reaction of C60Cl6 with N-methylpiperazine (4) can efficiently generate a fullerene-piperazine derivative (5) in the absence of any base [5]. By adding six times the amount of compound 4 to a C60Cl6 toluene solution, 5 is precipitated immediately, with more than a 95% yield. In vivo cell experiments have indicated that 5 exhibits high acute toxicity when administered via intraperitoneal injection in mice, suggesting the latter is safe for biomedical applications. This example also revealed that the toxicity of the water-soluble fullerene derivatives depends largely on the organic functional groups attached to the fullerene carbon cage.
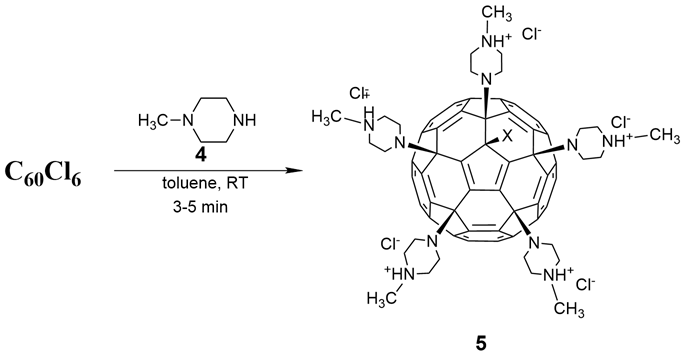 Figure 4. Synthesis route for fullerene penta-N-methyl piperazine salt (5).
Figure 4. Synthesis route for fullerene penta-N-methyl piperazine salt (5).
As mentioned, Mashino et al. (2005) reported that fullerene amino acid-type derivatives exhibited HIV-RT inhibitory activity, while cationic fullerene derivatives such as the pyrrolidinium-type derivatives showed weaker HIV-RT inhibitory activity [2]. The carboxyl groups on the pyrrolidine-type fullerene derivatives were considered crucial to HIV-RT inhibitory activity [2,13]. However, ten years later, Mashino et al. found that fullerene pyrrolidine-pyridine and pyrrolidine-pyridinium salt derivatives without any carboxyl groups [4], such as 6–18, which are functionalized with pyridine or pyridinium groups (Figure 5), exhibit strong HIV-RT inhibition. This is useful information for the future design of fullerene derivatives as HIV-RT inhibitors. In addition to HIV-RT inhibition, recently, Kobayashi et al. [14] found that compound 11 can inhibit HIV-PR, and HCV NS5B polymerase (HCV NS5B) with IC50 values in the micromolar range. Compound 18, the exo-substituent on the most potent derivative (11), exhibits no HIV-RT inhibitory activity in cell culture, indicating that HIV-RT inhibition is dependent on the fullerene skeleton. The conventional trypan blue dye exclusion test (Table 1) was used to evaluate the cytotoxicity of all fullerene pyrrolidine-pyridine and pyrrolidine-pyridinium salt derivatives (6–17) to HL60 cells. The CC50 of all derivatives except 12 (CC50 = 39.4 µM) was greater than 50 µM, which suggests that the new fullerene-pyrrolidine-pyridine or pyridinium salt derivatives effectively inhibit HIV-RT activity without damaging living cells.
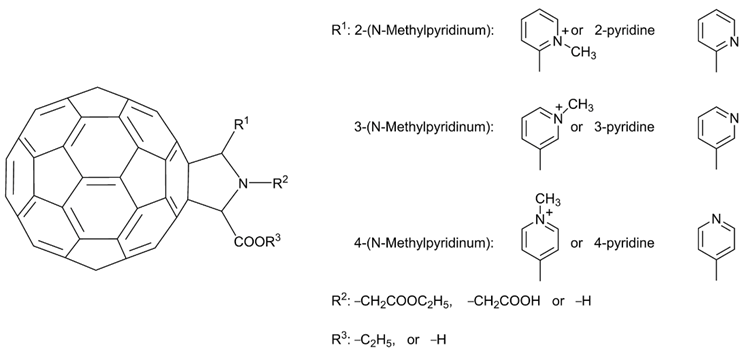 Figure 5. Structure diagram of fullerene pyrrolidine derivatives (6–18).
Figure 5. Structure diagram of fullerene pyrrolidine derivatives (6–18).
Table 1. HIV-RT inhibitory activity and cytotoxicity of fullerene pyrrolidine derivatives (6–18).
|
Compound |
R1 |
R2 |
R3 |
IC50 * |
CC50 * |
|
6 |
2-(N-Methylpyridinium) |
–CH2COOC2H5 |
–C2H5 |
0.30 |
>50 |
|
7 |
2-(N-Methylpyridinium) |
–H |
–C2H5 |
0.33 |
>50 |
|
8 |
2-Pyridine |
–CH2COOH |
–H |
0.20 |
>50 |
|
9 |
2-Pyridine |
–H |
–H |
0.46 |
>50 |
|
10 |
3-(N-Methylpyridinium) |
–CH2COOC2H5 |
–C2H5 |
0.25 |
>50 |
|
11 |
3-(N-Methylpyridinium) |
–H |
–C2H5 |
0.094 |
>50 |
|
12 |
3-Pyridine |
–CH2COOH |
–H |
0.41 |
39.4 |
|
13 |
3-Pyridine |
–H |
–H |
0.080 |
>50 |
|
14 |
4-(N-Methylpyridinium) |
–CH2COOC2H5 |
–C2H5 |
0.74 |
>50 |
|
15 |
4-(N-Methylpyridinium) |
–H |
–C2H5 |
0.37 |
>50 |
|
16 |
4-Pyridine |
–CH2COOH |
–H |
1.60 |
>50 |
|
17 |
4-Pyridine |
–H |
–H |
0.80 |
>50 |
|
18 |
|
|
|
>500 |
>50 |
|
Nevirapine |
|
|
|
3.52 |
- |
* These values (~µM) are based on the average of three test results for each test compound.
In 2016, Echegoyen and co-workers [3] reported a novel cationic N,N-dimethyl C70 fullerene-pyrrolidine iodized salt derivative (19–21), with fullerene C70 as the starting material (Figure 6), that inhibits more than 99% of HIV-1 infectivity at a low micromolar concentration. These three compounds have an EC50 of 0.41, 0.33, and 0.54 µM, respectively. An analysis of the life cycle of HIV-1 suggested that these compounds inhibit viral maturation by influencing the processing of Gag and GAG-POL. Significantly, fullerene derivatives 19–21 do not inhibit protease activity in vitro, and strongly interact with immature HIV capsid proteins in a pull-down assay. Moreover, these compounds may block infection by viruses carrying either a mutant protease that is resistant to multiple protease inhibitors, or the mutant Gag protein, which is resistant to the mature inhibitor bevirimat. Fullerenes 19–21 do not inhibit HIV-1 proteases at doses that strongly inhibit HIV-1 infection in vitro, suggesting that this mechanism is independent of the HIV-1 protease. This finding differed from previous reports that fullerene derivatives affect HIV-1 protease activity in vitro. Echegoyen et al. [15] then proposed that fullerene derivatives 19–21 act through a novel anti-HIV-1 mechanism, rather than interacting with other capsid proteins as previously reported. Unraveling the details of this mechanism will facilitate the discovery of novel anti-HIV-1 inhibitors.
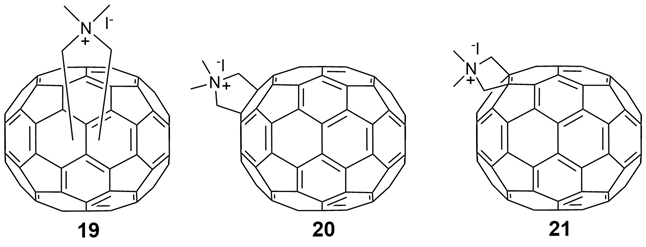 Figure 6. Chemical structures of the cationic N,N-dimethyl [70] ullerene pyrrolidine iodide derivatives (19–21).
Figure 6. Chemical structures of the cationic N,N-dimethyl [70] ullerene pyrrolidine iodide derivatives (19–21).
4. Synthesis and Antiviral Studies of Fullerene Carboxyl Derivatives
In 2007, Troshin and coworkers [16] proposed an effective method for the synthesis of water-soluble fullerene carboxylic acid derivatives. With C60Cl6 as the starting point, C60(Ar)5Cl, with ester groups linked to aryl groups, was obtained via the simple and efficient Friedel–Crafts arylation of C60Cl6 with methyl esters of phenylacetate at 100 °C. The fullerene carboxylic acid derivative (23) was prepared in almost a quantitative yield by removing the methyl group from the methyl ester under acidic conditions, as shown in Figure 7. Compound 23, with five carboxyl groups, is insoluble in water but soluble in DMSO. In order to improve the water solubility, potassium carbonate was added to neutralize the carboxylic acid group of 23 and form the corresponding ionic potassium salts, with a water solubility of up to 50–100 mg/mL at pH < 7.5. A virus-induced cytopathicity assay revealed that the fullerene carboxylic acid potassium derivative has pronounced anti-HIV-1 activity, with an IC50 of 1.20 ± 0.44 μM and a low cytotoxicity (>52 μM).
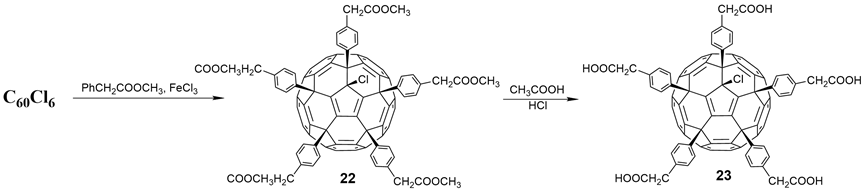 Figure 7. Schematic diagram of synthetic route of carboxylic fullerene derivative (23).
Figure 7. Schematic diagram of synthetic route of carboxylic fullerene derivative (23).
5. Synthesis and Antiviral Studies of Fullerene Hydroxyl Derivatives
Fullerenol is a polyhydroxylated fullerene C60 derivative with suitable water solubility and biocompatibility. In 2013, Eropkin et al. [17] synthesized a series of fullerenols (Figure 8). Three different groups of fullerenols, C60(OH)12–14, C60(OH)18–24, and C60(OH)30–38, can be prepared by changing the reaction conditions to control the number of hydroxyl groups attached to the fullerene skeleton. Experimental studies revealed that fullerenols containing 12~14 hydroxyl groups are insoluble in water and have no biological activity when introduced into cell culture as suspensions. The other two groups of fullerenols show broad spectrum antiviral activity in vitro against the human influenza viruses H1N1 and H3N2, avian influenza virus A (H5N1), adenovirus, human HSV, and respiratory syncytial virus. C60(OH)18–24 demonstrates better antiviral activity than C60(OH)30–38. Moreover, the three water-soluble fullerenols exhibit no toxicity in vitro to human and animal cells.
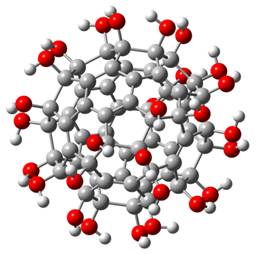 Figure 8. Structure diagram of fullerenol. Oxygen, carbon, and hydrogen atoms are marked in red, gray, and white, respectively.
Figure 8. Structure diagram of fullerenol. Oxygen, carbon, and hydrogen atoms are marked in red, gray, and white, respectively.
6. Synthesis and Antiviral Studies of Glycofullerene Derivatives
Based on an octahedral addition pattern, soluble hexakis-adduct glycofullerene derivatives can act as spherical carbohydrates, thus serving as a potential multivalent spherical ligand. In 2013, Martin and coworkers [18] designed and synthesized a class of hexakis-adduct [19] glycofullerene derivatives (24), containing 36 mannoses (Figure 9), and used them to inhibit cell infection by pseudotyped Ebola virus particles. This was the first time that glycofullerene derivatives were demonstrated to effectively inhibit cell infection. In the pseudotyped Ebola infection model, the antiviral activity of 24 was in the low nanomolar range, with an IC50 of 0.3 μM. The glycofullerene with 12 galactosyl had no inhibitory effect on virus infection, indicating that the inhibitory effect is dependent on mannose. Interestingly, only an increase in the valence of glycofullerene resulted in a loss of the antiviral effect. This phenomenon is related to the spatial crowding of sugars at the surface of glycofullerene. Martin et al. speculated that the high binding affinity occurs not only because of the extensive spatial presentation of multivalent ligands, but also the frequent interactions between the ligands and corresponding receptors. They demonstrated that a rational design of compounds with the same valency but longer spacers can significantly increase the antiviral activity, likely due to more efficient interactions with receptors. Therefore, the selection of suitable scaffolds (such as spherical fullerenes) to achieve multivalence, as well as the accessibility and flexibility of ligands, are key factors for improving antiviral activity.
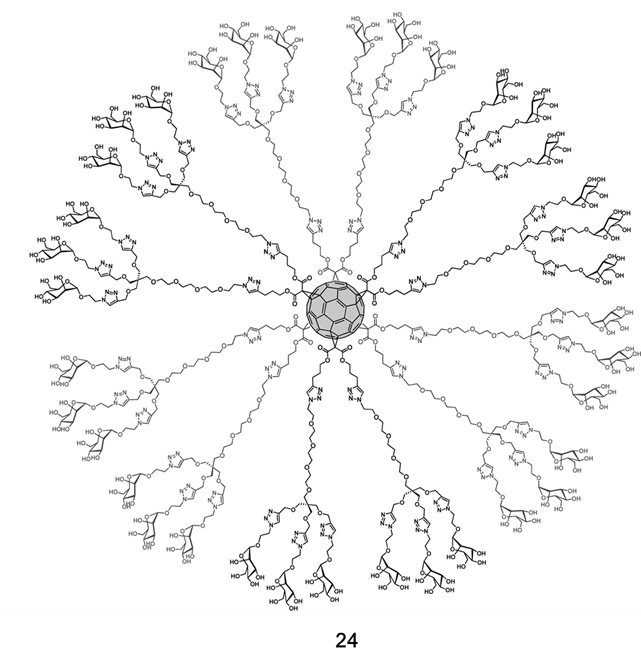 Figure 9. Structure diagram of glycofullerene (24). Adapted with permission from Ref. [18]. Copyright 2013 American Chemical Society].
Figure 9. Structure diagram of glycofullerene (24). Adapted with permission from Ref. [18]. Copyright 2013 American Chemical Society].
7. Synthesis and Antiviral Studies of Fullerene Complexes
Yang and coworkers [20] prepared a fullerene [60] liposome complex (Figure 10) and studied its anti-H1N1 activity in vivo. The fullerene liposome complex significantly reduces the average lung virus yields and lung index; prolongs the mean time to death; and decreases the mortality of mice infected with H1N1. In addition, the fullerene liposome complex has good water solubility and low toxicity, and its anti-influenza activity in vivo is much higher than that of rimantadine. Therefore, the fullerene [60] liposome complex is a promising clinical candidate drug against influenza infection.
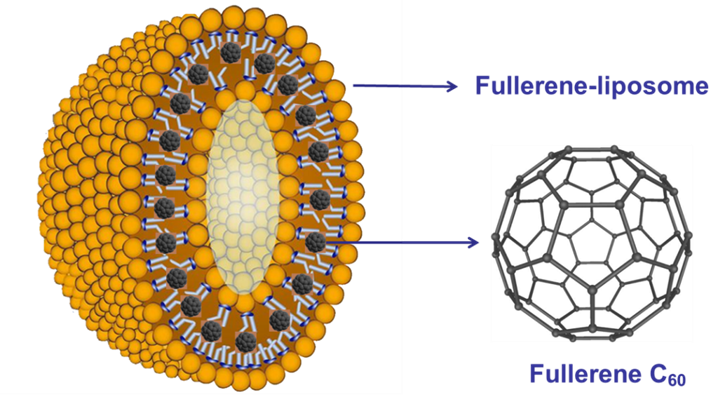 Figure 10. The structure of the fullerene liposome complex [20].
Figure 10. The structure of the fullerene liposome complex [20].
8. Conclusions
This review summarized the latest antiviral research conducted on fullerenes and their derivatives. Numerous water-soluble fullerene derivatives or fullerene complexes have shown great antiviral potential, mainly because fullerenes have three advantages. First, pristine fullerenes are hydrophobic, which is conducive to the formation of strong hydrophobic interactions with the active site surfaces of viruses. Second, hydrophilic groups with various functions (such as amino, carboxyl, amino acid, hydroxyl, pyrrolidine, and sugar groups) can be used to selectively modify the unique spherical skeleton of fullerenes via organic reactions. Third, fullerenes and their derivatives exhibit no or low cytotoxicity at relatively high concentrations. Although fullerenes are promising prospective antiviral drugs, antiviral research on fullerenes requires improvement. Most fullerene derivatives exhibit good antiviral effects in vitro, but the antiviral mechanism has not been thoroughly studied. Additionally, most of the studies on fullerenes have only focused on virus inhibition in vitro; there have been few antiviral studies in vivo, and relevant clinical studies involving fullerenes have not been conducted.
Viruses constantly threaten human health. Fullerenes have become an important molecular platform for the development of antiviral drugs. Research on fullerenes as antiviral drugs urgently needs the joint efforts of scientists working in synthesis, molecular design, biology, and medicine. Some fullerene derivatives display inhibitory activity against multiple types of viruses. Therefore, fullerene derivatives have the potential to become a class of broad-spectrum antiviral drugs effective against SARS-CoV-2, which remains a global threat. We believe that this review will encourage more researchers to synthesize fullerene derivatives and study their antiviral properties and applications.
References
- Friedman, H.; DeCamp, D.L.; Sijbesma, R.P.; Srdanov, G.; Wudl, F.; Kenyon, G.L. Inhibition of the HIV-1 protease by fullerene derivatives: Model building studies and experimental verification. J. Am. Chem. Soc. 1993, 115, 6506–6509. https://doi.org/10.1021/ja00068a005.
- Mashino, ; Shimotohno, K.; Ikegami, N.; Nishikawa, D.; Okuda, K.; Takahashi, K.; Nakamura, S.; Mochizuki, M. Human immunodeficiency virus-reverse transcriptase inhibition and hepatitis C virus RNA-dependent RNA polymerase inhibition activities of fullerene derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 1107–1109. https://doi.org/10.1016/j.bmcl.2004.12.030.
- Castro, ; Martinez, Z.S.; Seong, C.-S.; Cabrera-Espinoza, A.; Ruiz, M.; Hernandez Garcia, A.; Valdez, F.; Llano, M.; Echegoyen, L. Characterization of New Cationic N,N-Dimethyl [70]fulleropyrrolidinium Iodide Derivatives as Potent HIV-1 Maturation Inhibitors. J. Med. Chem. 2016, 59, 10963–10973. https://doi.org/10.1021/acs.jmedchem.6b00994.
- Yasuno, ; Ohe, T.; Takahashi, K.; Nakamura, S.; Mashino, T. The human immunodeficiency virus-reverse transcriptase inhibition activity of novel pyridine/pyridinium-type fullerene derivatives. Bioorg. Med. Chem. Lett. 2015, 25, 3226–3229. https://doi.org/10.1016/j.bmcl.2015.05.086.
- Kornev, B.; Khakina, E.A.; Troyanov, S.I.; Kushch, A.A.; Peregudov, A.; Vasilchenko, A.; Deryabin, D.G.; Martynenko, V.M.; Troshin, P.A. Facile preparation of amine and amino acid adducts of [60]fullerene using chlorofullerene C60Cl6 as a precursor. Chem. Commun. 2012, 48, 5461–5463. https://doi.org/10.1039/C2CC00071G.
- Tollas, ; Bereczki, I.; Borbás, A.; Batta, G.; Vanderlinden, E.; Naesens, L.; Herczegh, P. Synthesis of a cluster-forming sialylthio-d-galactose fullerene conjugate and evaluation of its interaction with influenza virus hemagglutinin and neuraminidase. Bioorg. Med. Chem. Lett. 2014, 24, 2420–2423. https://doi.org/10.1016/j.bmcl.2014.04.032.
- Muñoz, ; Sigwalt, D.; Illescas, B.M.; Luczkowiak, J.; Rodríguez-Pérez, L.; Nierengarten, I.; Holler, M.; Remy, J.-S.; Buffet, K.; Vincent, S.P.; et al. Synthesis of giant globular multivalent glycofullerenes as potent inhibitors in a model of Ebola virus infection. Nat. Chem. 2016, 8, 50–57. https://doi.org/10.1038/nchem.2387.
- Fedorova, E.; Klimova, R.R.; Tulenev, Y.A.; Chichev, E.V.; Kornev, A.B.; Troshin, P.A.; Kushch, A.A. Carboxylic Fullerene C60 Derivatives: Efficient Microbicides Against Herpes Simplex Virus and Cytomegalovirus Infections In Vitro. Mendeleev Commun. 2012, 22, 254–256. https://doi.org/10.1016/j.mencom.2012.09.009.
- Sijbesma, ; Srdanov, G.; Wudl, F.; Castoro, J.A.; Wilkins, C.; Friedman, S.H.; DeCamp, D.L.; Kenyon, G.L. Synthesis of a fullerene derivative for the inhibition of HIV enzymes. J. Am. Chem. Soc. 1993, 115, 6510–6512. https://doi.org/10.1021/ja00068a006.
- Brettreich, ; Hirsch, A. A highly water-soluble dendro [60]fullerene. Tetrahedron Lett. 1998, 39, 2731–2734. https://doi.org/10.1016/S0040-4039(98)00491-2.
- Schuster, ; Wilson, S.; Kirschner, A.; Schinazi, R.; Schlueter-Wirtz, S.; Barnett, T.; Martin, S.; Brettreich, M.; Hirsch, A. Evaluation of the anti-HIV Potency of a Water- Soluble Dendrimeric Fullerene Derivative. Proc. Electrochem. Soc. 2000
- Toniolo, ; Bianco, A.; Maggini, M.; Scorrano, G.; Prato, M.; Marastoni, M.; Tomatis, R.; Spisani, S.; Palu, G.; Blair, E.D. A Bioactive Fullerene Peptide. J. Med. Chem. 1994, 37, 4558–4562. https://doi.org/10.1021/jm00052a015.
- Nakamura, ; Mashino, T. Water-soluble Fullerene Derivatives for Drug Discovery. J. Nippon Med. Sch. 2012, 79, 248–254. https://doi.org/10.1272/jnms.79.248.
- Kobayashi, ; Yasuno, T.; Takahashi, K.; Nakamura, S.; Mashino, T.; Ohe, T. Novel pyridinium-type fullerene derivatives as multitargeting inhibitors of HIV-1 reverse transcriptase, HIV-1 protease, and HCV NS5B polymerase. Bioorg. Med. Chem. Lett. 2021, 49, 128267. https://doi.org/10.1016/j.bmcl.2021.128267.
- Martinez, S.; Castro, E.; Seong, C.-S.; Cerón, M.R.; Echegoyen, L.; Llano, M. Fullerene Derivatives Strongly Inhibit HIV-1 Replication by Affecting Virus Maturation without Impairing Protease Activity. Antimicrob. Agents Chemother. 2016, 60, 5731–5741. https://doi.org/10.1128/AAC.00341-16.
- Troshina, A.; Troshin, P.A.; Peregudov, A.S.; Kozlovskiy, V.I.; Balzarini, J.; Lyubovskaya, R.N. Chlorofullerene C60Cl6: A precursor for straightforward preparation of highly water-soluble polycarboxylic fullerene derivatives active against HIV. Org. Biomol. Chem. 2007, 5, 2783–2791. https://doi.org/10.1039/B705331B.
- Eropkin, Y.; Melenevskaya, E.Y.; Nasonova, K.V.; Bryazzhikova, T.S.; Eropkina, E.M.; Danilenko, D.M.; Kiselev, O.I. Synthesis and Biological Activity of Fullerenols with Various Contents of Hydroxyl Groups. Pharm. Chem. J. 2013, 47, 87–91. https://doi.org/10.1007/s11094-013-0901-x.
- Luczkowiak, ; Muñoz, A.; Sánchez-Navarro, M.; Ribeiro-Viana, R.; Ginieis, A.; Illescas, B.M.; Martín, N.; Delgado, R.; Rojo, J. Glycofullerenes Inhibit Viral Infection. Biomacromolecules 2013, 14, 431–437. https://doi.org/10.1021/bm3016658.
- Hirsch, ; Vostrowsky, O. C60 Hexakisadducts with an Octahedral Addition Pattern—A New Structure Motif in Organic Chemistry. Eur. J. Org. Chem. 2001, 2001, 829–848. https://doi.org/10.1002/1099-0690(200103)2001:5<829::AID-EJOC829>3.0.CO;2-V.
- Du, X. The antiviral effect of fullerene-liposome complex against influenza virus (H1N1) in vivo. Sci. Res. Essays 2012, 7, 705–711.
This entry is adapted from the peer-reviewed paper 10.3390/nano12152547