Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Health Care Sciences & Services
Cryptococcal meningoencephalitis, a disease with poor patient outcomes, remains the most prevalent invasive fungal infection worldwide, accounting for approximately 180,000 deaths each year.
- Cryptococcus neoformans
- genetic diversity
- fungal disease
- cryptococcosis
1. Introduction
Cryptococcus neoformans causes cryptococcal meningitis (CM) and is a major cause of mortality throughout the developing world, especially among individuals living with advanced HIV/AIDS [1]. Cryptococcosis is a common AIDS-defining illness and a leading cause of mortality among adults with HIV [1]. Despite the advent of antiretroviral therapy, which drastically reduced the number of HIV cases in the developed world, CM remains a major problem in resource-limited regions [2].
Due to the burden of HIV in Africa, CM is the most common cause of adult meningitis in Sub-Saharan Africa, with 70% of all cases of CM globally occurring in sub-Saharan Africa [1,2]. Survival after cryptococcosis in sub-Saharan Africa is often ≤40% [3,4]. A number of clinical adverse prognostic markers in HIV-associated CM have been identified, including high fungal burden at CM diagnosis, poor rate of cryptococcal clearance from patient cerebrospinal fluid (CSF) during antifungal treatment, and altered mental status at presentation [5]. Patient-to-patient differences in clinical phenotype likely reflect a complex interplay between host factors (level of immunosuppression, immune response phenotype [6]), pathogen virulence [7], and health system factors such as delays in diagnosis and treatment [8,9]. Furthermore, long-term natural selection of C. neoformans within individuals by human antimicrobial defenses is proposed to occur [10,11], with the resultant likelihood that virulence factors will demonstrate natural variation within and amongst lineages [5].
2. Molecular Classification of Cryptococcus spp.
Cryptococcus is a genus of basidiomycetous fungi with more than 30 species found in the environment. Within this genus, a number of species are known to cause human disease, with the species most commonly associated with human disease being Cryptococcus neoformans, Cryptococcus deneoformans and the five species that compose the Cryptococcus gattii species complex (Figure 1) [19]. Recently proposed taxonomy based on molecular and genetic studies divided the major human pathogenic Cryptococcus species from the original single Cryptococcus neoformans designation into these seven more clearly defined species. At present, these seven major human pathogenic Cryptococcus species are sub-divided into two species complexes: the Cryptococcus neoformans species complex that contains C. neoformans (serotype A; genotypes VNI, VNII, VNB) and C. deneoformans (serotype D; genotype VNIV) and the Cryptococcus gattii species complex that contains an additional five species—C. gattii, C. bacillisporus, C. deuterogattii, C. tetragattii, and C. decagattii (serotypes B and C; genotypes VGI-IV) [20,21]. The molecular taxonomy of the Cryptococcus genus is a vibrant area of research that is enhancing our understanding of specific strain characteristics, including fitness, predilection for certain environmental niches, and association with human disease outcomes. Several molecular methods are used for taxonomic analysis of the C. neoformans and C. gattii species complexes, including restriction fragment length polymorphism (RFLP), microsatellite fingerprinting, multi-locus sequence typing (MLST), and whole-genome sequencing (WGS) [22,23,24,25]. Genome sequencing has identified only minor discordance between phylogenies produced using the previously defined VN/VG clade designations or MLST-defined sequence types (STs); thus, the field largely uses VN/VG or sequence type as a standardized system to classify genotypes within the human pathogenic Cryptococcus spp.
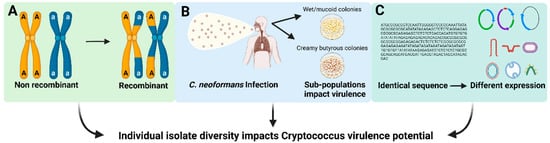
Figure 1. Factors that may contribute to individual isolate variation in Cryptococcus species. (A) Variation in pathogen genetic diversity. (B) Selection of isolate-specific genetic alterations with the host or environment. (C) Gene expression variation across isolates.
Most clinical and environmental isolates within the human pathogenic Cryptococcus spp. are haploid, although diploid and aneuploid isolates are observed [26,27,28]. Genomes range in size from 16 to 19 Mb and contain variable numbers of chromosomes. The C. neoformans, C. deneoformans, and C. gattii species complex reference strains all have 14 chromosomes along with the mitochondrial genome [29]. The human pathogenic Cryptococcus spp. have a bipolar mating system encoded by the MAT locus, with strains designated as either MATa or MATα [30,31]. The two mating types are morphologically similar in appearance and thus must be distinguished using molecular methods (e.g., PCR, sequence analysis, etc.) or by using a mating assay [28,32]. Interestingly, MATα isolates predominate and this mating type also appears to undergo monokaryotic mating more readily than MATa isolates, possibly explaining the reason for their increased prevalence [28].
3. African C. neoformans Strains Have Unique Characteristics
There are a number of possibilities for the high mortality rates observed with African cryptococcosis. The rates of cryptococcosis in Africa are consistent with HIV prevalence in this region of the world [1,33], showing a positive association between HIV and the prevalence of C. neoformans co-infection. However, along with high incidence, mortality in sub-Saharan Africa is also higher than many other regions of the world. Currently, the reason for this higher mortality is unclear. Clinical trials performed in Uganda that utilize the same standard-of-care as in the United States show lower mortality rates compared to general care, suggesting that clinical practices in sub-Saharan Africa may impact patient mortality [34,35,36]. Similarly, flucytosine, which is recommended to be used in conjunction with amphotericin B, is not available currently in much of sub-Saharan Africa [37,38]. The regimen of amphotericin B and fluconazole that is typically used in Africa was recently shown to be inferior to the amphotericin and flucytosine regiment [39,40]. Interestingly, this study found that the completely oral regimen of flucytosine and fluconazole was as effective as amphotericin B and flucytosine in African patients [40]. These data were surprising and suggest that optimal drug treatment strategies for African patients should be explored further [38].
Whether there is a genetic basis for the high cryptococcosis mortality rates in Africa is less well defined. Human genetic factors that influence cryptococcosis have been identified [19,41], but whether these genetic factors could account for the increased mortality observed in African patients has not been extensively investigated. Instead, early reports suggest that differences in C. neoformans genotypes could contribute to the high mortality observed in Africa [42]. A study performed by Litvintseva et al. on isolates from 200 HIV-seropositive patients in Botswana identified novel genotypes that differed from isolates found globally and thus were referred to as the VNB lineage. Analyses of mating types in this population indicated that 12% of these strains possess the MATa mating type, higher than among non-African isolates [43]. While it was originally proposed that this VNB lineage was geographically confined to sub-Saharan Africa, later studies identified VNB isolates in other regions of the world [43,44]. Interestingly, while the global VNI and VNII lineages appear to be highly clonal, the VNB lineage is not and led to speculation that sub-Saharan Africa could be the origin of C. neoformans [5,45].
Importantly, other common sequence types found in Africa can be globally distributed. For example, the most common sequence type in Uganda, ST93, is also frequently observed in South America, where it is also associated with high mortality rates [46]. In a study in South African pediatric patients, the most prevalent sequence type (ST8) was also global. In South Africa, ST8 was associated with male patients, the isolates exhibited high genetic diversity, and a high percentage of the strains were diploid [39,47]. In a study carried out in Yaoundé, Cameroon, 24% of HIV-infected patients with cryptococcal meningitis co-infections had infection with multiple genotypes [48]. At least two isolates with different antifungal susceptibilities were identified within a single patient sample, despite lack of antifungal treatment prior to sample collection [49]. These studies highlight the increased rates of genetic variability in Africa, even within globally distributed sequence types. In vivo microevolution has been reported and the frequency of mixed infections in African patients, whether due to microevolution or co-infection, may be higher than reported globally [50,51].
4. Disease Manifestations and Epidemiology
Successful disease initiation and progression likely rely on numerous genotypic and phenotypic factors of both the host and the fungus. More simply, a host must be susceptible and exposed to a Cryptococcus isolate that is sufficiently pathogenic before disease can occur. Most human exposure begins with inhalation of aerosolized cells, most likely spores, from the environment [52] into the lungs where the yeast cells are either cleared by the immune system or establish a latent pulmonary infection [53,54,55,56,57,58]. The timing of this initial environmental exposure to Cryptococcus may vary by geographic region and may depend on other socio-cultural factors, but by adulthood approximately 70% of people have developed antibodies to the C. neoformans species complex [59,60,61].
Infections with Cryptococcus are predominantly classified based on the site of infection. Clinically, cryptococcosis typically presents as cryptococcal meningitis (CM), although pulmonary or disseminated cryptococcosis is also frequently observed. Recently, classification based upon Cryptococcus species complex has also become more prevalent due to an outbreak of C. deuterogattii in North America and Europe, but a better understanding of the role that Cryptococcus species play in disease epidemiology is needed [62]. While latent pulmonary cryptococcosis is the most common infection due to Cryptococcus, it is predominantly asymptomatic [63]. Cryptococcus is also one of the yeasts most frequently described as members of the pulmonary mycobiome, as demonstrated in a study by Rubio-Portillo et al. [64]. Thus, initial pulmonary infection is acquired almost exclusively from the environment via inhalation of infectious, aerosolized basidiospores or desiccated yeast cells [52,65,66]. Extra-pulmonary infections are thought to be secondary to the primary pulmonary infection, even in cases where the latter is not readily evident. Disseminated cryptococcosis, particularly to the central nervous system (CNS), where it can produce meningoencephalitis and cryptococcomas during CM, is often observed in severely immunocompromised individuals. Cryptococcosis is classically seen in patients with advanced HIV and/or in individuals with CD4+ T-cell counts below 100 [67].
Susceptible hosts may experience an asymptomatic latent pulmonary infection that becomes acute pulmonary cryptococcosis (PC) during an immunosuppressive event and/or disseminate throughout the body to the CNS to ultimately cause cryptococcal meningitis [67,68] Alternatively, in a host that is susceptible upon exposure to the yeast, acute infection may manifest and disseminate without a latent stage. Current theories propose that many of the traits that promote Cryptococcus survival within its environmental niche also act as virulence factors in humans and contribute to fungal survival, disease initiation, immune evasion and dissemination of the infection from the lungs to the predominant site of disease in the brain [69].
In general, Cryptococcus preferentially localizes to the lungs and brain during infection; however, most organs have been reported as secondary sites of infection (e.g., skin, prostate) due to dissemination [70,71,72,73]. The epidemiology of human pathogenic Cryptococcus spp. has been studied since the 1980s. Disease due to the C. neoformans species complex is predominantly observed in immunocompromised individuals, but is also observed in some individuals that have no known immune deficiencies [74]. The C. gattii species complex, conversely, was historically regarded as a pathogen of apparently immunocompetent patients. However, pre-existing conditions and immunocompromised states, including subclinical immune defects, are also frequently observed in patients with C. gattii infections [75,76,77]. Thus, it is unclear whether a better understanding of the subpopulations within each species will explain the apparent patient variability, or whether the species differences in clinical presentation are primarily determined by variable host predilections [78,79,80].
As described in a recent review by Altamirano et al. [46], the various Cryptococcus spp. also display different disease epidemiology and clinical manifestations. C. neoformans is the most common species to cause infections globally, accounting for 95% of infections overall, and 99% of infections in individuals with advanced HIV [81]. While the majority of C. neoformans infections occur in patients with an immunocompromising condition, infections by the C. gattii species complex predominantly occur in immunocompetent individuals [46]. CM is a common disease manifestation in patients with C. neoformans species complex infections, with over 80% of patients displaying meningitis symptoms [82]. In contrast, CM is less common in C. gattii species complex infections, with patients typically presenting with pneumonia [79,83]. While rare globally, C. deneoformans infections are more frequently observed in Europe, often in the context of hybrids with C. neoformans, and in approximately 14% of infections where skin lesions are observed [84].
While less frequent, a few other species of Cryptococcus have been documented to cause disease in severely immunocompromised individuals. Cryptococcus laurentii is associated with fungemia, lung abscesses, and meningitis [85]. Cryptococcus albidus is another very rare species associated with ocular infections and meningitis [86,87]. Cryptococcus uniguttulatus is associated with ventriculitis and was first isolated from a human nail [88]. Given that the vast majority of human infections are caused by C. neoformans, we will predominantly focus our discussion on this species.
5. Host–Pathogen Interactions during Cryptococcus Infection
Many fungal species kill mammalian tissue culture cells upon in vitro co-culture [89,90]. Surprisingly, Cryptococcus exhibits minimal toxicity to mammalian cells in culture, leading to the suggestion that growth within mammalian cells may be beneficial for fungal cell survival or dissemination [91,92]. IFNγ-producing CD4+ T-cells are required for the activation of myeloid cells, especially macrophages, to enable fungal killing and clearance. However, macrophages may also act as a reservoir of the fungal yeast cells, shielding them from host immune detection and thus promoting latent infection or persistent chronic inflammation [93]. In a previous study, macrophage cell lines with phagocytosed C. neoformans were capable of growth and cell division, with the fungal cells able to transmit to the daughter macrophage [94]. These in vitro studies lead to the hypothesis that some C. neoformans isolates do not produce high levels of cytotoxic factors, promoting their survival within the phagocyte.
The outcome of the C. neoformans–macrophage interaction is a critical determinant for the fate of the pathogen and host during infection. The ability of C. neoformans to replicate inside macrophages correlates with infection susceptibility in animal models [95]. Similarly, the capacity of C. neoformans isolates to replicate in macrophages is correlated with worse human clinical outcomes [17,96]. These data suggest that factors and interventions that modulate macrophage function, especially in patients where adaptive immunity from T-cell function is impaired, could reduce disease, whereas the capacity of the pathogen to efficiently replicate intracellularly could be associated with progression of infection. Reduced macrophage activation impairs the antifungal capacity of these cells, which in turn facilitates intracellular Cryptococcus growth [97,98]. Finally, damage to mitochondria, reduced phagosome maturation, and induction of programmed cell death pathways in host cells during Cryptococcus infection directly aid in fungal cell survival in vivo and in vitro [99].
Consistent with these observations, Cryptococcus infections are not associated with large amounts of tissue necrosis, in contrast to infections caused by other fungal pathogens such as Aspergillus spp. or Mucorales. Instead, cryptococcosis infections tend to have features consistent with a chronic infection, with host death frequently resulting from physical compression of tissue, such as meningoencephalitis, or the presence of fungal masses, called cryptococcomas, in the brain that are associated with minimal to no inflammation [97]. The cryptococcomas have a distinctive appearance in magnetic resonance imaging that are sometimes referred to as “soap bubbles” and are composed of gelatinous pseudo cysts containing Crytococcus cells with large amounts of capsule polysaccharide. The cryptococcomas displace or destroy brain tissue to create the space for the fungal mass. Combined with the observation that C. neoformans replicates inside host cells, the cryptococcomas may be the result of progressive lysis of host cells during the host–pathogen interaction [97]. Alternatively, the cryptococcomas may compress surrounding brain tissue [100]. Defects in the resorption of the cerebrospinal fluid (CSF), thought to be due to its increased viscosity due to the presence of the capsule polysaccharide being released into CSF, are also known to cause overwhelming brain edema [101].
These observations lead to the conclusion that C. neoformans infections are associated with minimal host damage, but several reports indicate that C. neoformans is able to cause direct damage to host cells and tissues, with the damage attributed to both the fungus and the host immune response. For example, Immune Reconstitution Inflammatory Syndrome (IRIS), an exaggerated inflammatory response causing a subset of persons with recent CM to deteriorate with improving immune function, often occurs in the absence of culturable fungus and is due to inappropriate immune system activation in response to residual Cryptococcus antigen [102,103,104,105,106,107].
The blood–brain barrier (BBB) is a highly selective semipermeable border of endothelial cells that prevents solutes in the circulating blood from non-selectively crossing into the extracellular fluid of the central nervous system [108]. For Cryptococcus to invade the central nervous system, it must cross the BBB. Studies have provided evidence that C. neoformans crosses the BBB using at least three mechanisms—active transcytosis, passive transcytosis, and within host cells using a Trojan Horse-like mechanism. During active transcytosis, the Cryptococcus cells induce uptake by the BBB endothelial cells, crossing this cell layer without damaging the BBB integrity [109,110]. Alternatively, in passive transcytosis, the fungal cells are trapped in the brain capillaries because of their size, resulting in a lesion that ruptures the capillary and disrupts the BBB integrity [110,111,112]. In Trojan Horse BBB penetration, the Cryptococcus cells are first phagocytosed by monocytes or macrophages, and then are thought to transit across the BBB within the phagocyte [113,114,115]. It is currently unclear whether a fourth mechanism for BBB penetration exists that requires an interaction with host phagocytes, but this interaction does not occur at the BBB [115,116].
Thus, disease is likely a complex balancing act between fungal virulence potential and host susceptibility [97]. Several mechanisms of host–pathogen interaction come into play to cause maximum damage during infection. These host–pathogen interactions have been previously reported to occur at molecular, cellular, tissue and organism levels. Damage at the molecular level in C. neoformans infections has been shown to be the result of secretion of various enzymes, such as proteases, nuclease, urease, and phospholipase, that result in degradation of host molecules, such as antibodies, and/or modification of cell membranes [97]. At the cellular level, host damage involves modification of host cellular compartments, fungal cell shape, organelles and accumulation of fungal materials in the cell leading to cellular damage. At the tissue level, disruption of host intracellular organization and accumulation of fungal cells leading to the creation of fungal masses has been reported. Finally, at the organismal level, damage is due to fungal growth and dissemination and the host immune response that often leads to intracranial hypertension ending in death [97]. A damage–response framework for Cryptococcus pathogenesis has also been proposed where disease occurs at one extreme due to lack of appropriate immune response and at the other extreme when aberrant or excessive host immune responses directly cause host damage and exacerbate disease [117].
This entry is adapted from the peer-reviewed paper 10.3390/jof8070734
This entry is offline, you can click here to edit this entry!
