Gastrointestinal cancer refers to malignancy of the accessory organs of digestion, and it includes colorectal cancer (CRC) and pancreatic cancer (PC). Worldwide, CRC is the second most common cancer among women and the third most common among men. PC has a poor prognosis and high mortality, with 5-year relative survival of approximately 11.5%. Conventional chemotherapy treatments for these cancers are limited due to severe side effects and the development of drug resistance. Therefore, there is an urgent need to develop new and safe drugs for effective treatment of PC and CRC. Historically, natural sources—plants in particular—have played a dominant role in traditional medicine used to treat a wide spectrum of diseases. In recent decades, marine natural products (MNPs) have shown great potential as drugs, but drug leads for treating various types of cancer, including CRC and PC, are scarce. To date, marine-based drugs have been used against leukemia, metastatic breast cancer, soft tissue sarcoma, and ovarian cancer.
- marine natural products
- colon cancer
- pancreatic cancer
1. Introduction
Gastrointestinal cancer refers to malignant of accessory organs of digestion, and it includes colorectal cancer (CRC) and pancreatic cancer (PC). In 2020, 1.9 million new CRC cases and 0.9 million CRC deaths were estimated worldwide [1]. The CRC incidence is higher in developed countries; however, the number of cases is increasing in non-developed countries every year [2]. PC is a malignant tumor that usually occurs as a pancreatic adenocarcinoma. It has a poor prognosis and high mortality, with an estimated 5-year relative survival of 11.5% [3].
2. Marine Natural Products (MNPs) with the Potential to Treat Cancer
3. Mechanisms of Action of MNPs in CRC and PC
3.1. Induction of Apoptosis through Caspase Activation
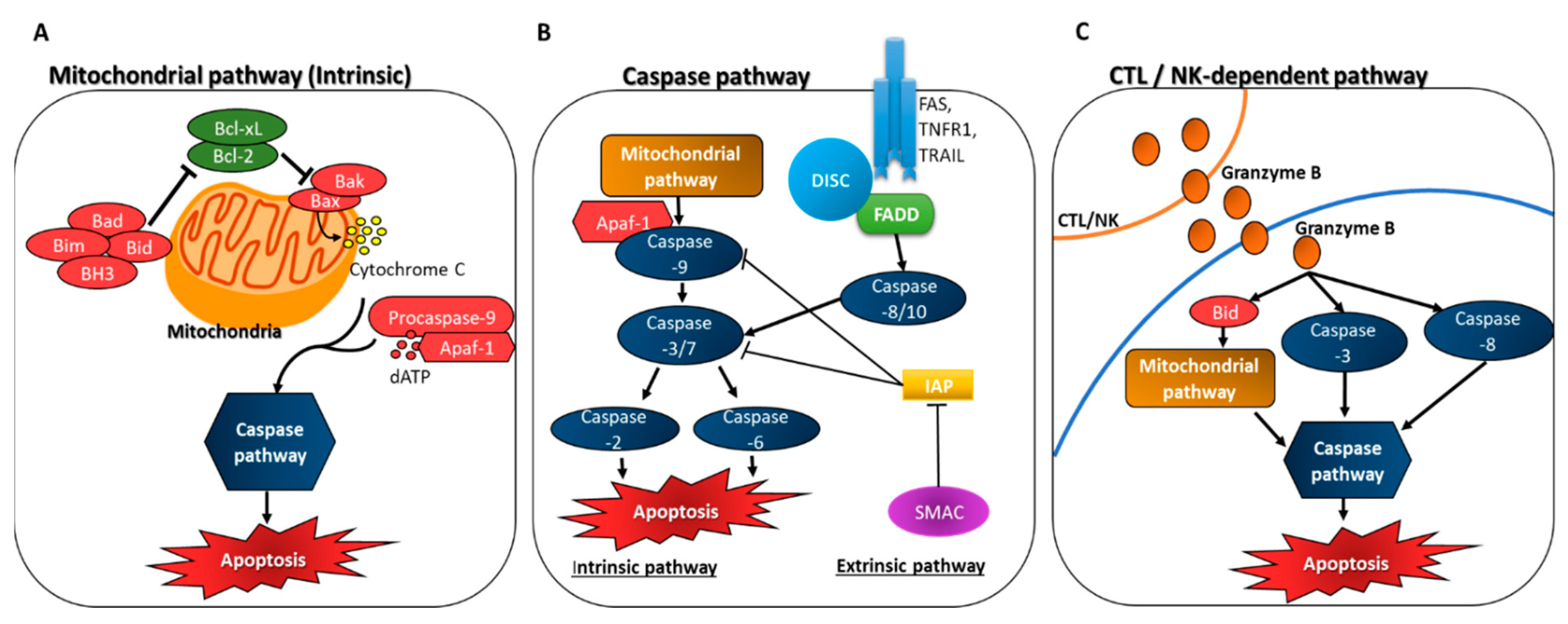
3.2. Inhibition of Anti-Apoptotic Factors
3.3. Interaction of MNPs with Tubulin to Cause Anti-Mitotic Activity
3.4. Suppression of Cell Cycle Progression
3.5. The Role of NFκB and p53 in Apoptosis
3.6. Increased Intracellular ROS Accumulation and Induction of Apoptosis
4. Conclusions
MNPs have great potential as new compounds that can assist in the prevention and treatment of cancer, but extensive exploration is needed. Over the past 50 years, many MNPs with beneficial effects on the prevention and treatment of various types of cancer have been reported. For example, cytarabine, eribulin mesylate, brentuximab vedotin, and trabectidine are marine-based drugs used against leukemia, metastatic breast cancer, soft tissue sarcoma, and ovarian cancer [51][52]. MNPs compounds have different activities including the inhibition of the transformation of normal cells into tumor cells, halting tumor cell growth and microtumors development, and inducing apoptosis. A higher consumption of sea food is suggested as a promising strategy to prevent cancer [53][54]. Many marine edible organisms contain lipids enriched by polyunsaturated fatty acids (PUFAs), such as ω-3 fatty acids that have been shown in many experimental studies to suppress most forms of tumor development, including breast, colon, prostate, liver, and pancreatic tumors [55][56][57].
This entry is adapted from the peer-reviewed paper 10.3390/ijms23148048
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249.
- Xi, Y.; Xu, P. Global Colorectal Cancer Burden in 2020 and Projections to 2040. Transl. Oncol. 2021, 14, 101174.
- NIH National Cancer Institute. Pancreatic Cancer-Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 13 March 2022).
- Cancer.Net. Colorectal Cancer: Types of Treatment. Available online: https://www.cancer.net/cancer-types/colorectal-cancer/types-treatment (accessed on 13 March 2022).
- Pancreatic Cancer: Types of Treatment. Available online: https://www.cancer.net/cancer-types/pancreatic-cancer/types-treatment (accessed on 26 April 2022).
- Aslam, M.S.; Naveed, S.; Ahmed, A.; Abbas, Z.; Gull, I.; Athar, M.A. Side Effects of Chemotherapy in Cancer Patients and Evaluation of Patients Opinion about Starvation Based Differential Chemotherapy. J. Cancer Ther. 2014, 2014, 817–822.
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2018, 35, 8–53.
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2016, 33, 382–431.
- Wu, L.; Ye, K.; Jiang, S.; Zhou, G. Marine Power on Cancer: Drugs, Lead Compounds, and Mechanisms. Mar. Drugs 2021, 19, 488.
- Parate, S.; Kumar, V.; Lee, G.; Rampogu, S.; Hong, J.C.; Lee, K.W. Marine-Derived Natural Products as ATP-Competitive MTOR Kinase Inhibitors for Cancer Therapeutics. Pharmaceuticals 2021, 14, 282.
- Mbaoji, F.N.; Nweze, J.A.; Yang, L.; Huang, Y.; Huang, S.; Onwuka, A.M.; Peter, I.E.; Mbaoji, C.C.; Jiang, M.; Zhang, Y.; et al. Novel Marine Secondary Metabolites Worthy of Development as Anticancer Agents: A Review. Molecules 2021, 26, 5769.
- Guzmán, E.A.; Pitts, T.P.; Diaz, M.C.; Wright, A.E. The Marine Natural Product Scalarin Inhibits the Receptor for Advanced Glycation End Products (RAGE) and Autophagy in the PANC-1 and MIA PaCa-2 Pancreatic Cancer Cell Lines. Investig. New Drugs 2019, 37, 262–270.
- Wang, E.; Sorolla, M.A.; Krishnan, P.D.G.; Sorolla, A. From Seabed to Bedside: A Review on Promising Marine Anticancer Compounds. Biomolecules 2020, 10, 248.
- Dyshlovoy, S.A.; Honecker, F. Marine Compounds and Cancer: Updates 2020. Mar. Drugs 2020, 18, 643.
- Wu, A.C.; Jelielek, K.K.; Le, H.Q.; Butt, M.; Newman, D.J.; Glaser, K.B.; Pierce, M.L.; Mayer, A.M. The 2021 Marine Pharmacology and Pharmaceuticals Pipeline. FASEB J. 2022, 36, L7586.
- Li, T.; Wang, N.; Zhang, T.; Zhang, B.; Sajeevan, T.P.; Joseph, V.; Armstrong, L.; He, S.; Yan, X.; Benjamin Naman, C. A Systematic Review of Recently Reported Marine Derived Natural Product Kinase Inhibitors. Mar. Drugs 2019, 17, 493.
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.E.F.; Moustafa, M.S.; El-Wahed, A.A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491.
- Sarabia, F.; Han, N.; Li, J.; Li, X. Natural Marine Products: Anti-Colorectal Cancer In Vitro and In Vivo. Mar. Drugs 2022, 20, 349.
- Denicourt, C.; Dowdy, S.F. Targeting Apoptotic Pathways in Cancer Cells. Science 2004, 305, 1411–1413.
- Creagh, E.M.; Conroy, H.; Martin, S.J. Caspase-Activation Pathways in Apoptosis and Immunity. Immunol. Rev. 2003, 193, 10–21.
- Slee, E.A.; Harte, M.T.; Kluck, R.M.; Wolf, B.B.; Casiano, C.A.; Newmeyer, D.D.; Wang, H.-G.; Reed, J.C.; Nicholson, D.W.; Alnemri, E.S.; et al. Ordering the Cytochrome c–Initiated Caspase Cascade: Hierarchical Activation of Caspases-2,-3,-6,-7,-8, and-10 in a Caspase-9–Dependent Manner. J. Cell Biol. 1999, 144, 281–292.
- Adrain, C.; Martin, S. The Mitochondrial Apoptosome: A Killer Unleashed by the Cytochrome Seas. Trends Biochem. Sci. 2001, 26, 390–397.
- Lee, H.; Chung, K.; Hwang, I.; Gwak, J.; Park, S.; Ju, B.; Yun, E.; Kim, D.; Chung, Y.; Na, M.; et al. Activation of P53 with Ilimaquinone and Ethylsmenoquinone, Marine Sponge Metabolites, Induces Apoptosis and Autophagy in Colon Cancer Cells. Mar. Drugs 2015, 13, 543–557.
- LaCasse, E.C.; Baird, S.; Korneluk, R.G.; MacKenzie, A.E. The Inhibitors of Apoptosis (IAPs) and Their Emerging Role in Cancer. Oncogene 1998, 17, 3247–3259.
- Deveraux, Q.L.; Reed, J.C. IAP Family Proteins—Suppressors of Apoptosis. Genes Dev. 1999, 13, 239–252.
- Green, D.R. Apoptotic Pathways: Paper Wraps Stone Blunts Scissors. Cell 2000, 102, 1–4.
- Kim, E.J.; Park, S.Y.; Lee, J.Y.; Park, J.H.Y. Fucoidan Present in Brown Algae Induces Apoptosis of Human Colon Cancer Cells. BMC Gastroenterol. 2010, 10, 96.
- Zhang, W.; Zhu, Y.; Yu, H.; Liu, X.; Jiao, B.; Lu, X. Libertellenone H, a Natural Pimarane Diterpenoid, Inhibits Thioredoxin System and Induces ROS-Mediated Apoptosis in Human Pancreatic Cancer Cells. Molecules 2021, 26, 315.
- Lu, J.; Holmgren, A. The Thioredoxin Antioxidant System. Free Radic. Biol. Med. 2014, 66, 75–87.
- Meuillet, E.J.; Mahadevan, D.; Berggren, M.; Coon, A.; Powis, G. Thioredoxin-1 Binds to the C2 Domain of PTEN Inhibiting PTEN’s Lipid Phosphatase Activity and Membrane Binding: A Mechanism for the Functional Loss of PTEN’s Tumor Suppressor Activity. Arch. Biochem. Biophys. 2004, 429, 123–133.
- Saitoh, M.; Nishitoh, H.; Fujii, M.; Takeda, K.; Tobiume, K.; Sawada, Y.; Kawabata, M.; Miyazono, K.; Ichijo, H. Mammalian Thioredoxin Is a Direct Inhibitor of Apoptosis Signal-Regulating Kinase (ASK) 1. EMBO J. 1998, 17, 2596–2606.
- Nogales, E. Structural Insights into Microtubule Function. Annu. Rev. Biochem. 2000, 69, 277–302.
- Valiron, O.; Caudron, N.; Job, D. Microtubule Dynamics. Cell. Mol. Life Sci. CMLS 2001, 58, 2069–2084.
- Checchi, P.M.; Nettles, J.H.; Zhou, J.; Snyder, J.P.; Joshi, H.C. Microtubule-Interacting Drugs for Cancer Treatment. Rends Pharmacol. Sci. 2003, 24, 361–365.
- Guzman, E.A.; Xu, Q.; Pitts, T.P.; Mitsuhashi, K.O.; Baker, C.; Linley, P.A.; Oestreicher, J.; Tendyke, K.; Winder, P.L.; Suh, E.M.; et al. Leiodermatolide, a Novel Marine Natural Product, Has Potent Cytotoxic and Antimitotic Activity against Cancer Cells, Appears to Affect Microtubule Dynamics, and Exhibits. Int. J. Cancer 2016, 139, 2116–2126.
- Martínez-Díez, M.; Guillén-Navarro, M.; Pera, B.; Bouchet, B.; Martínez-Leal, J.; Barasoain, I.; Cuevas, C.; Andreu, J.; García-Fernández, L.; Díaz, J.; et al. PM060184, a New Tubulin Binding Agent with Potent Antitumor Activity Including P-Glycoprotein over-Expressing Tumors. Biochem. Pharmacol. 2014, 88, 291–302.
- Lim, S.; Kaldis, P. Cdks, Cyclins and CKIs: Roles beyond Cell Cycle Regulation. Development 2013, 140, 3079–3093.
- Wang, W.; Abbruzzese, J.L.; Evans, D.B.; Larry, L.; Cleary, K.R.; Chiao, P.J. The Nuclear Factor-ΚB RelA Transcription Factor Is Constitutively Activated in Human Pancreatic Adenocarcinoma Cells. Clin. Cancer Res. 1999, 5, 119–127.
- Nakanishi, C.; Toi, M. Nuclear Factor-ΚB Inhibitors as Sensitizers to Anticancer Drugs. Nat. Rev. Cancer 2005, 5, 297–309.
- Arlt, A.; Vorndamm, J.; Breitenbroich, M.; FoÈlsch, U.; Kalthoff, H.; Schmidt, W.E.; SchaÈfer, H. Inhibition of NF-ΚB Sensitizes Human Pancreatic Carcinoma Cells to Apoptosis Induced by Etoposide (VP16) or Doxorubicin. Oncogene 2001, 20, 859–868.
- Guo, J.; Verma, U.N.; Gaynor, R.B.; Frenkel, E.P.; Becerra, C.R. Enhanced Chemosensitivity to Irinotecan by RNA Interference-Mediated down-Regulation of the Nuclear Factor-ΚB P65 Subunit. Clin. Cancer Res. 2004, 10, 3333–3341.
- Mabuchi, S.; Ohmichi, M.; Nishio, Y.; Hayasaka, T.; Kimura, A.; Ohta, T.; Saito, M.; Kawagoe, J.; Takahashi, K.; Yada-Hashimoto, N.; et al. Inhibition of NFκB Increases the Efficacy of Cisplatin in In Vitro and In Vivo Ovarian Cancer Models. J. Biol. Chem. 2004, 279, 23477–23485.
- Delma, C.R.; Thirugnanasambandan, S.; Srinivasan, G.P.; Raviprakash, N.; Manna, S.K.; Natarajan, M.; Aravindan, N. Fucoidan from Marine Brown Algae Attenuates Pancreatic Cancer Progression by Regulating P53–NFκB Crosstalk. Phytochemistry 2019, 167, 112078.
- Ko, L.J.; Prives, C. P53: Puzzle and Paradigm. Genes Dev. 1996, 10, 1054–1072.
- Levine, A.J. P53, the Cellular Gatekeeper for Growth and Division. Cell 1997, 88, 323–331.
- Cross, C.E.; Halliwell, B.; Borish, E.T.; Pryor, W.A.; Ames, B.N.; Saul, R.L.; McCORD, J.M.; Harman, D. Oxygen Radicals and Human Disease. Ann. Intern. Med. 1987, 107, 526–545.
- Kim, A.; Ha, J.; Kim, J.; Cho, Y.; Ahn, J.; Cheon, C.; Kim, S.H.; Ko, S.G.; Kim, B. Natural Products for Pancreatic Cancer Treatment: From Traditional Medicine to Modern Drug Discovery. Nutrients 2021, 13, 3801.
- Tripathi, S.K.; Biswal, B.K. Pterospermum acerifolium (L.) Wild Bark Extract Induces Anticarcinogenic Effect in Human Cancer Cells through Mitochondrial-Mediated ROS Generation. Mol. Biol. Rep. 2018, 45, 2283–2294.
- Ruiz-Torres, V.; Rodríguez-Pérez, C.; Herranz-López, M.; Martín-García, B.; Gómez-Caravaca, A.-M.; Arráez-Román, D.; Segura-Carretero, A.; Barrajón-Catalán, E.; Micol, V. Marine Invertebrate Extracts Induce Colon Cancer Cell Death via ROS-Mediated DNA Oxidative Damage and Mitochondrial Impairment. Biomolecules 2019, 9, 771.
- Xu, J.W.; Yan, Y.; Wang, L.; Wu, D.; Nai, K.Y.; Shi, H.C.; Li, F. Marine Bioactive Compound Dieckol Induces Apoptosis and Inhibits the Growth of Human Pancreatic Cancer Cells PANC-1. J. Biochem. Mol. Toxicol. 2021, 35, e22648.
- Dyshlovoy, S.A.; Honecker, F. Marine Compounds and Cancer: 2017 Updates. Mar. Drugs 2018, 16, 41.
- DrugBank Online|Database for Drug and Drug Target Info. Available online: https://go.drugbank.com/ (accessed on 28 April 2022).
- Stonik, V.A.; Fedorov, S.N. Marine Low Molecular Weight Natural Products as Potential Cancer Preventive Compounds. Mar. Drugs 2014, 12, 636–671.
- Dyshlovoy, S.A. Recent Updates on Marine Cancer-Preventive Compounds. Mar. Drugs 2021, 19, 558.
- Song, M.; Ou, F.-S.; Zemla, T.J.; Hull, M.A.; Shi, Q.; Limburg, P.J.; Alberts, S.R.; Sinicrope, F.A.; Giovannucci, E.L.; Van Blarigan, E.L.; et al. Marine Omega-3 Fatty Acid Intake and Survival of Stage III Colon Cancer According to Tumor Molecular Markers in NCCTG Phase III Trial N0147 (Alliance). Wiley Online Libr. 2019, 145, 380–389.
- Candela, C.G.; López, L.B.; Kohen, V.L. Importance of a Balanced Omega 6/Omega 3 Ratio for the Maintenance of Health. Nutritional Recommendations. Nutr. Hosp. 2011, 26, 323–329.
- Wendel, M.; Axel, R. Heller Anticancer Actions of Omega-3 Fatty Acids-Current State and Future Perspectives. Anti-Cancer Agents Med. Chem. 2009, 9, 457–470.
