The advances in transplant immunosuppression have reduced substantially the incidence of kidney graft rejection. The focus has moved from preventing rejection to preventing the long-term consequences of long-standing immunosuppression, including nephrotoxicity induced by calcineurin inhibitors (CNI), as well as infectious and neoplastic complications. Since the appearance in the late 1990s of mTOR inhibitors (mTORi), these unmet needs in immunosuppression management could be addressed thanks to their benefits (reduced rate of viral infections and cancer).
- kidney
- transplant
- kidney transplant
- immunosuppression
- mTOR
- mTOR inhibition
1. Introduction
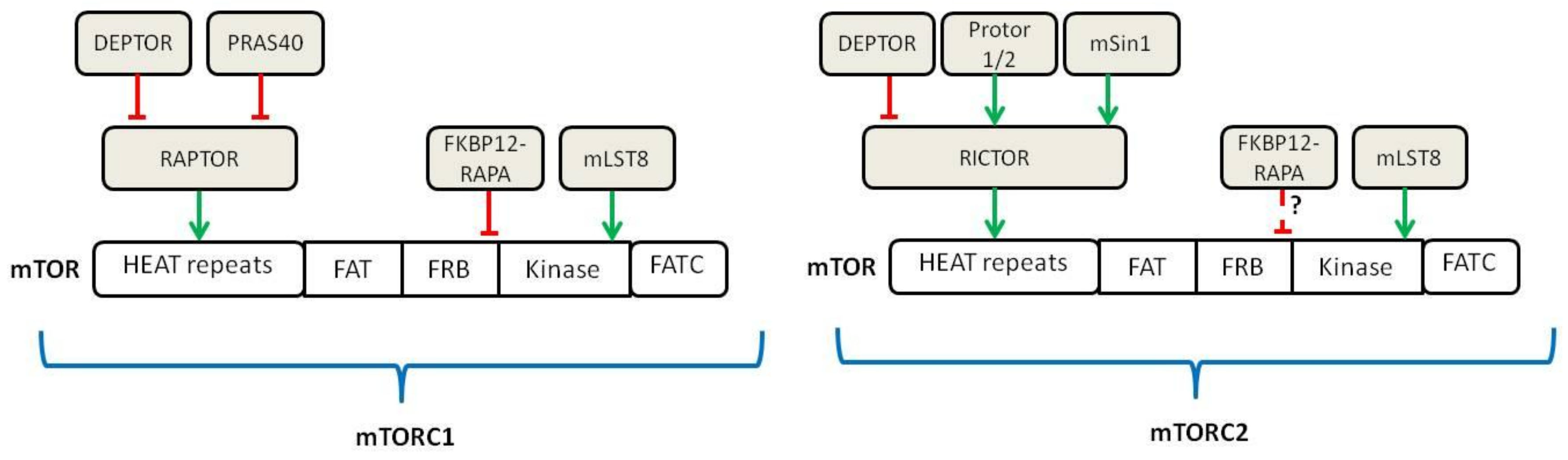
2. Pharmacology of mTOR Inhibitors
3. Use of mTOR Inhibitors in Graft-versus-Host Disease
4. Use of mTOR Inhibitors in Kidney Transplantation
5. Real-Life Use of mTOR Inhibitors in Renal Transplantation
6. Practical Use of mTOR Inhibitors in Kidney Transplantation—Troubleshooting
|
Side Effect |
Solution |
|---|---|
|
Neumonitis |
Discontinue mTORi. |
|
Thrombotic microangiopathy |
If clinically evident and in case of rejection, consider discontinuing mTORi. If it is only a finding in renal biopsy without clinical deterioration, consider reducing trough levels of either CNI or mTORi or both. In low-risk patients consider conversion from CNI to MPA. |
|
Surgical scar infection or late healing |
Switch to MPA until resolved and then switch back to mTORi. |
|
Lymphocele |
Switch to MPA until resolved and then switch back to mTORi. |
|
Productive surgical drainage |
Switch to MPA until resolved and then switch back to mTORi. |
|
Post-transplant diabetes mellitus |
Start of oral antidiabetic agent and/or insulin. Consider switching TAC to CsA. |
|
Hypertriglicerydemia |
Diet, weight loss, omega-3 fish oil. |
|
Hypercolesterolemia |
Diet, weight loss, statins, ezetimibe, fibrates. |
|
Proteinuria |
Consider using ACE inhibitors or Angiotensin Receptor Blockers. |
|
Edemas |
Consider using diuretics. In patients taking vasodilators (such as amlodipine), consider switching to another anti-hypertensive agent. |
This entry is adapted from the peer-reviewed paper 10.3390/ijms23147707
References
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO Clinical practice guideline for the care of kidney transplant recipients. Am. J. Transplant. 2009, 9, S1–S155.
- Vézina, C.; Kudelski, A.; Sehgal, S.N. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing strep-tomycete and isolation of the active principle. J. Antibiot. 1975, 28, 721–726.
- Martel, R.R.; Klicius, J.; Galet, S. Inhibition of the immune response by rapamycin, a new antifungal antibiotic. Can. J. Physiol. Pharmacol. 1977, 55, 48–51.
- Eng, C.P.; Sehgal, S.N.; Vézina, C. Activity of rapamycin (AY-22,989) against transplanted tumors. J. Antibiot. 1984, 37, 1231–1237.
- Chung, J.; Kuo, C.J.; Crabtree, G.R.; Blenis, J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell 1992, 69, 1227–1236.
- Bierer, B.E.; Mattila, P.S.; Standaert, R.F.; Herzenberg, L.A.; Burakoff, S.J.; Crabtree, G.; Schreiber, S.L. Two distinct signal transmission pathways in T lymphocytes are inhibited by com-plexes formed between an immunophilin and either FK506 or rapamycin. Proc. Natl. Acad. Sci. USA 1990, 87, 9231–9235.
- Kunz, J.; Henriquez, R.; Schneider, U.; Deuter-Reinhard, M.; Movva, N.; Hall, M.N. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell 1993, 73, 585–596.
- Sabatini, D.M.; Erdjument-Bromage, H.; Lui, M.; Tempst, P.; Snyder, S.H. RAFT1: A mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell 1994, 78, 35–43.
- Brown, E.J.; Albers, M.W.; Shin, T.B.; Ichikawa, K.; Keith, C.T.; Lane, W.S.; Schreiber, S.L. A mammalian protein targeted by G1-arresting rapamycin–receptor complex. Nature 1994, 369, 756–758.
- Sabers, C.J.; Martin, M.M.; Brunn, G.J.; Williams, J.M.; Dumont, F.J.; Wiederrecht, G.; Abraham, R.T. Isolation of a Protein Target of the FKBP12-Rapamycin Complex in Mammalian Cells. J. Biol. Chem. 1995, 270, 815–822.
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 6, 960–976.
- Mendonça, D.B.; Nguyen, J.T.; Haidar, F.; Fox, A.L.; Ray, C.; Amatullah, H.; Liu, F.; Kim, J.K.; Krebsbach, P.H. Mi-croRNA-1911-3p Targets MEAK-7 to Suppress MTOR Signaling in Human Lung Cancer Cells. Heliyon 2020, 6, e05734.
- Nguyen, J.T.; Ray, C.; Fox, A.L.; Mendonça, D.B.; Kim, J.K.; Krebsbach, P.H. Mammalian EAK-7 activates alternative mTOR signaling to regulate cell proliferation and migration. Sci. Adv. 2018, 4, eaao5838.
- Sancak, Y.; Thoreen, C.C.; Peterson, T.R.; Lindquist, R.A.; Kang, S.A.; Spooner, E.; Carr, S.A.; Sabatini, D.M. PRAS40 Is an Insulin-Regulated Inhibitor of the mTORC1 Protein Kinase. Mol. Cell 2007, 25, 903–915.
- Peterson, T.R.; Laplante, M.; Thoreen, C.C.; Sancak, Y.; Kang, S.A.; Kuehl, W.M.; Gray, N.S.; Sabatini, D.M. DEPTOR Is an mTOR Inhibitor Frequently Overexpressed in Multiple Myeloma Cells and Required for Their Survival. Cell 2009, 137, 873–886.
- Sarbassov, D.D.; Ali, S.M.; Kim, D.H.; Guertin, D.A.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and rap-tor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004, 14, 1296–1302.
- Frias, M.A.; Thoreen, C.C.; Jaffe, J.D.; Schroder, W.; Sculley, T.; Carr, S.A.; Sabatini, D.M. mSin1 Is Necessary for Akt/PKB Phosphorylation, and Its Isoforms Define Three Distinct mTORC2s. Curr. Biol. 2006, 16, 1865–1870.
- Pearce, L.R.; Huang, X.; Boudeau, J.; Pawłowski, R.; Wullschleger, S.; Deak, M.; Ibrahim, A.F.M.; Gourlay, R.; Magnuson, M.A.; Alessi, D.R. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem. J. 2007, 405, 513–522.
- Sarbassov, D.D.; Ali, S.M.; Sengupta, S.; Sheen, J.-H.; Hsu, P.P.; Bagley, A.F.; Markhard, A.L.; Sabatini, D.M. Prolonged Rapamycin Treatment Inhibits mTORC2 Assembly and Akt/PKB. Mol. Cell 2006, 22, 159–168.
- Sancak, Y.; Bar-Peled, L.; Zoncu, R.; Markhard, A.L.; Nada, S.; Sabatini, D.M. Ragulator-Rag Complex Targets mTORC1 to the Lysosomal Surface and Is Necessary for Its Activation by Amino Acids. Cell 2010, 141, 290–303.
- Lutz, M.; Mielke, S. New perspectives on the use of mTOR inhibitors in allogeneic haematopoietic stem cell transplantation and graft-versus-host disease. Br. J. Clin. Pharmacol. 2016, 82, 1171–1179.
- Zhou, R.Q.; Wang, X.; Ye, Y.B.; Lu, B.; Wang, J.; Guo, Z.W.; Mo, W.J.; Yang, Z.; Srisuk, P.; Yan, L.P.; et al. Prevention of acute graft vs. host disease by targeting glycolysis and mTOR pathways in activated T cells. Exp. Ther. Med. 2022, 24, 448.
- Kahan, B.D.; Napoli, K.L.; A Kelly, P.; Podbielski, J.; Hussein, I.; Urbauer, D.L.; Katz, S.H.; Van Buren, C.T. Therapeutic drug monitoring of sirolimus: Correlations with efficacy and toxicity. Clin. Transplant. 2000, 14, 97–109.
- MacDonald, A.S.; RAPAMUNE Global Study Group. A worldwide, phase III, randomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation 2001, 71, 271–280.
- Cucchiari, D.; Ríos, J.; Molina-Andujar, A.; Montagud-Marrahi, E.; Revuelta, I.; Ventura-Aguiar, P.; Piñeiro, G.J.; De Sousa-Amorim, E.; Esforzado, N.; Cofán, F.; et al. Combination of calcineurin and mTOR inhibitors in kidney transplantation: A propensity score analysis based on current clinical practice. J. Nephrol. 2019, 33, 601–610.
- Cucchiari, D.; Molina-Andujar, A.; Montagud-Marrahi, E.; Revuelta, I.; Rovira, J.; Ventura-Aguiar, P.; Piñeiro, G.J.; De Sousa-Amorim, E.; Esforzado, N.; Cofán, F.; et al. Use of de novo mTOR inhibitors in hypersensitzed kidney transplant recipients: Experience from clinical practice. Transplantation 2019, 104, 1686–1694.
- Qazi, Y.; Shaffer, D.; Kaplan, B.; Kim, D.Y.; Luan, F.L.; Peddi, V.R.; Shihab, F.; Tomlanovich, S.; Yilmaz, S.; McCague, K.; et al. Efficacy and Safety of Everolimus Plus Low-Dose Tacrolimus versus Mycophenolate Mofetil Plus Standard-Dose Tacrolimus in De Novo Renal Transplant Recipients: 12-Month Data. Am. J. Transplant. 2016, 17, 1358–1369.
- De Fijter, J.W.; Holdaas, H.; Øyen, O.; Sanders, J.S.; Sundar, S.; Bemelman, F.J.; Sommerer, C.; Pascual, J.; Avihingsanon, Y.; Pongskul, C.; et al. Early Conversion From Calcineurin Inhibitor- to Everolimus-Based Therapy Following Kidney Transplantation: Results of the Randomized ELEVATE Trial. Am. J. Transplant. 2017, 17, 1853–1867.
- Karayannapoulou, G.; Euvrard, S.; Kanitakis, J. Differential expression of p-Mtor in cutaneous basal and squamous cell carcinomas likely explains their different response to mTOR inhibitors in organ-transplant recipients. Anticancer Res. 2013, 33, 3711–3714.
- Euvrard, S.; Morelon, E.; Rostaing, L.; Goffin, E.; Brocard, A.; Tromme, I.; Broeders, N.; Del Marmol, V.; Chatelet, V. Sirolimus and secondary skin-cancer prevention in kidney transplantation. N. Engl. J. Med. 2012, 367, 329–339.
