Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The repair of critical bone defects is a hotspot of orthopedic research. With the development of bone tissue engineering (BTE), there is increasing evidence showing that the combined application of extracellular vesicles (EVs) derived from mesenchymal stem cells (MSCs) (MSC-EVs), especially exosomes, with hydrogels, scaffolds, and other bioactive materials has made great progress, exhibiting a good potential for bone regeneration.
- bone graft
- exosomes
- extracellular vesicles
- mesenchymal stem cells
1. Application of EVs Parent Cells
As key mediators of cell-to-cell communication, Exos play important roles in a variety of biological roles and injury repair processes, such as regulating immune response [1], promoting the repair of traumatic brain injury [2], and stimulating bone tissue regeneration [3]. Studies have shown that in the treatment of bone tissue regeneration, MSC-Exos stimulate osteoblast proliferation and angiogenesis and inhibit osteoclast maturation by delivering endogenous cargo [4]. Since Exos generally have similar therapeutic properties to their parent cells, Exos derived from different MSCs have different effects on bone regeneration and repair [5][6]. Therefore, in the strategy of applying MSC-Exos to bone regeneration, it is important to select an appropriate source of parental cell types [7].
The sources of parental MSCs of Exos mainly include BMSCs [8], ADSCs [9], UCMSCs [10], iPSCs [11], etc. As shown in Table 1, among these cells, BMSCs are the most commonly used cell source for tissue engineering [12]. Numerous studies have shown that BMSC-derived Exos (BMSC-Exos) can stimulate the proliferation and osteogenic differentiation of BMSCs, thereby promoting osteogenesis, angiogenesis, and bone mineralization in critical bone defect models [13]. Compared with BMSCs, human adipose mesenchymal stem cells (hADSCs), mainly derived from adipose tissue, are easier to obtain. hADSCs are widely distributed in the human body and proliferate rapidly, which is an ideal type of MSC for improving Exos production [14]. However, compared with BMSCs, hADSCs may have less osteogenic differentiation potential [15]. Some scholars compared the osteogenic differentiation potential of rat BMSC-Exos and ADSC-derived Exos (ADSC-Exos) in osteogenesis-induced (OI) conditions. The results showed that BMSC-OI-Exos significantly up-regulated the expression of osteogenesis-related genes and proteins, including Col I, Runx2, BSP, OCN, BMPR-IA, and BMPR-II, while ADSC-OI-Exos only up-regulated BSP, BMPR-IA and, BMPR-II, which suggested that ADSC-OI-Exos may have a lower osteoinductive ability compared with BMSC-OI-Exos [16]. In addition, hADSC-derived Exos (hADSC-Exos) have shown great therapeutic potential in the treatment of ischemic diseases, indicating that hADSC-Exos may also play a potential role in bone regeneration by promoting angiogenesis [17]. Human umbilical cord mesenchymal stem cells (hUCMSCs) have also attracted much attention in BTE. hUCMSCs are a primitive population of MSCs obtained from Wharton’s jelly, the main component of the human umbilical cord matrix [18]. hUCMSCs can indirectly promote bone regeneration by inducing angiogenesis rather than directly promoting osteogenic or chondrogenic differentiation. Compared with other MSCs derived from bone marrow or fat, hUCMSCs showed higher pluripotency [19][20] and stronger pro-angiogenesis properties [21]. In addition, hUCMSCs are derived from human postnatal waste tissues and were abundant in source, which is a rich source without ethical and moral controversies, showing greater clinical potential [22]. Similar to hUCMSCs, iPSCs also have no immune rejection or ethical issues and can proliferate indefinitely; however, iPSCs may have tumorigenic risks [23]. In recent years, studies have shown that MSCs derived from iPSCs (iPS-MSCs) have the advantages of both iPSCs and MSCs. iPS-MSCs can maintain their self-renewal ability after 40 passages. Importantly, iPS-MSCs are not tumorigenic [24]. In the treatment of bone defects, iPS-MSCs show strong proliferative and immunomodulatory abilities to promote bone regeneration [11]. Exos derived from iPS-MSCs also have similar properties to their parental cells. Therefore, iPS-MSCs are an excellent cell source for Exos to promote bone regeneration [25]. In addition to the above-mentioned MSCs, some local tissue-derived MSC-Exos also have good application potential. Stem cells from human exfoliated deciduous teeth (SHEDs), which are immature MSCs with multiple differentiation potential, can be easily obtained in a non-invasive way without ethical problems [26]. Compared with BMSCs, SHEDs have a stronger proliferative capacity due to the abundant secretion of growth factors such as fibroblast growth factor 2 (FGF2) and transforming growth factor-β2 (TGF-β2) [27]. Moreover, compared with pulp stem cells, SHEDs have a stronger capacity to promote bone mineralization [28]. SHED-derived Exos, in combination with tricalcium phosphate (β-TCP), have been shown to enhance alveolar bone regeneration by promoting angiogenesis and osteogenesis [29]. In addition to the source of parental MSCs, the therapeutic effect of Exos may also be influenced by some underlying diseases of the donor. A recent study showed that BMSC-Exos obtained from the T1DM rat model was significantly less effective in promoting BMSC osteogenic differentiation and endothelial cell angiogenesis than BMSC-Exos obtained from normal rats [6]. This suggests that Exos from donors with chronic underlying diseases have a poor therapeutic effect, or even the opposite therapeutic effect, which may not be suitable for regenerative medicine [30]. Further research is needed to explore the potential therapeutic effects of donor-derived Exos in disease states.
Table 1. Comparison of application characteristics and osteogenesis mechanism of common EVs parent cells.
| Parent Cells | Application Characteristics | Functions | References |
|---|---|---|---|
| BMSCs | Effective osteogenesis Easy to obtain Most widely used |
Osteogenesis differentiation | [12][13] |
| hADSCs | Easy to obtain Rapid multiplication; the best choice to increase yield The most widely distributed in humans Poor osteogenic ability and require additional substances to induce osteogenesis |
Osteogenesis differentiation | [14][15][16][17] |
| hUCMSCs | Higher pluripotency Strongest angiogenic properties Source from the waste organization, abundant sources There are no ethical and moral disputes Higher clinical potential |
Angiogenesis | [18][19][20][21][22] |
| iPS-MSCs | There are advantages both of iPSCs and MSCs Unlimited growth and self-renewal No longer tumorigenic There are no ethical and moral disputes Stronger proliferation capacity and immune regulation function |
Osteogenic differentiation and angiogenesis | [11][23][24][25] |
| SHEDs | Multiple differentiation potential Non-invasive means to obtain (easy access) There are no ethical issues Rich in growth factors such as FGF2, TGF-β2 Stronger proliferative capacity |
Osteogenic differentiation and angiogenesis | [26][27][28][29] |
2. Application of MSC-EVs in BTE
The regeneration of critical bone defects caused by bone tumors, injuries, and other bone diseases requires bone grafting to replace the defective bone. EV-mediated BTE therapy has become an attractive bone defect replacement strategy in recent years [31]. Accumulating studies have shown that EV-loaded hydrogels or scaffolds, alone or in combination, can significantly promote local bone regeneration (as shown in Figure 1).
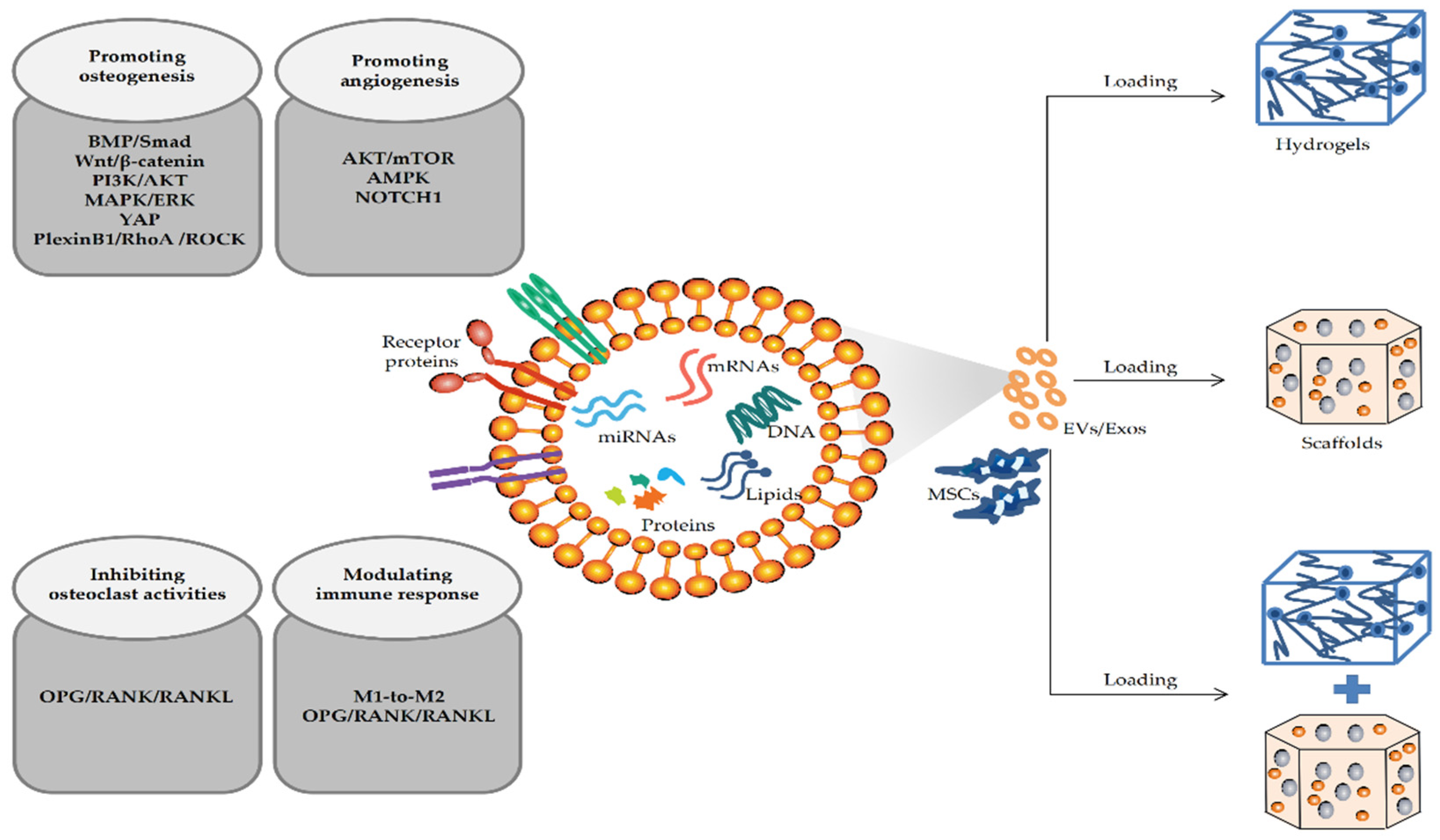
Figure 1. Mechanisms of MSC-EVs/MSC-Exos promoting the repair of bone defects in BTE.
2.1. Application of MSC-EVs Combined with Hydrogels
Hydrogels are physically or chemically cross-linked three-dimensional (3D) hydrophilic polymer networks that can adsorb large amounts of water without being dissolved [32]. Biomedical hydrogels are structurally similar to the natural extracellular matrix, which have attracted extensive attention in regenerative medicine due to their properties of tissue-like water content, good biocompatibility, and easy implantation. In regenerative medicine, hydrogels, as an effective carrier, are widely used in drug delivery and active molecule encapsulation matrix for tissue repair, including bone tissue repair [33]. In bone tissue regeneration, biopolymer hydrogels can be injected directly into bone defects and flow over complex and irregular bone surfaces, matching the gaps between implants and randomly shaped defects and increasing the contact area [34]. In addition, the porous structure of hydrogels can continuously release bioactive factors, including EVs/Exos, which is beneficial for repairing bone defects in BTE [34]. Studies have shown that Exos coated in biodegradable hydrogels can still exhibit the desired therapeutic effect [35][36]. Hydrogels provide a 3D matrix for Exos that prevents the dispersion of Exos and maintains its local concentration, which enables controlled release of Exos for sustained efficacy. Hydrogel-released Exos at bone defect sites can reduce Exos consumption and ectopic effects to promote angiogenesis and osteogenesis [37]. Some scholars found gelatin nanoparticle hydrogels combined with hADSC-Exos can accurately transport the hADSC-Exos to the target site and effectively promote bone healing [38]. In addition, the hydrogel 3D microenvironment can also enhance Exos activity and affect the interaction of EV integrin membrane protein between cells and the cell matrix to promote the proliferation and differentiation of osteoblasts and bone angiogenesis in a bone regeneration environment. Yu et al. [39] demonstrated that Exos derived from periodontal ligament stem cells (PDLSCs) encapsulated in a hydrogel 3D microenvironment exhibited enhanced osteoinductive ability and could significantly promote bone defect repair in rats. Another study demonstrated alginate hydrogels combined with EVs showed increased interactions with other cells, cell aggregation, and prolonged long-term viability, which in turn promoted osteogenesis [37]. Therefore, a variety of special properties of hydrogels can be combined with MSC-EVs to effectively treat bone defects.
At present, the commonly used biomedical hydrogels mainly include natural and synthetic polymer hydrogels, such as gelatin, hyaluronic acid hydrogel (HA-gel), chitosan, polyethylene glycol (PEG), etc. [40]. Compared to natural materials, synthetic polymers have basic structural units, well-defined properties, including porosity and degradation time, and known mechanical properties, making synthetic polymer hydrogels more suitable for specific bone tissue regeneration applications [41]. In addition, synthetic polymers are suitable delivery vehicles for EVs in bone defect repair due to their reliable source of material, long shelf life, and low risk of immunogenicity [42]. However, due to their fragile network structure, the mechanical properties, stability, and cell adhesion of traditional hydrogels are usually weak, which hinders their application as bone structural materials [43]. To overcome this limitation, hydrogels are often modified by chemical, physical, and biological methods to prepare high-performance hydrogels with enhanced mechanical properties, stability, cell adhesion, and other properties [40]. Ma et al. [44] used propionic acid (CA) with a catechol group to modify an injectable thermosensitive hydrogel through a covalent bond. The cross-linking network formed by the interaction between the catechol group of CA and the collagen molecules in hydrogels can effectively improve the mechanical properties of hydrogel [45]. In addition, the combination of two hydrogels to prepare new hydrogels is also a common way of hydrogel modification. Previous studies revealed that the combination of a gelatin derivative, gelatin methacrylate, and a PEG derivative, polyethylene glycol diacrylate, can prepare a hydrogel with good cell adhesion and stronger mechanical properties [46]. Hydrogels with good performance can be obtained by various methods; however, how to effectively load bioactive molecules such as EVs/Exos into hydrogels and improve their long-term retention at defect sites is also a challenge in BTE. Currently, the commonly used method is to directly mix Exos with hydrogels [47], which may lead to low loading efficiency, structural destruction of Exos, and hindered osteogenic potential [48]. Recent studies have demonstrated that substances such as peptides can be used to effectively fix EVs/Exos, showing excellent drug delivery ability, which can effectively deliver EVs/Exos [49]. Huang et al. [50] found that integrin membrane proteins in MSC-EVs could combine with collagen and fibronectin-derived peptides secreted by cells. Subsequently, the adhesive peptide (RGD) was added to alginate hydrogels containing EVs overexpressing bone morphogenetic protein 2 (BMP-2) to target cellular integrins. The results showed that EVs could be effectively fixed to hydrogels through the interaction of protein domains on the cell membrane [50]. In another study, some scholars incorporated Exos into an injectable thermosensitive hydrogel by constructing fusion peptides, which also enhanced the retention of Exos and improved the biological activity of Exos [44]. The above results indicated that the addition of bioactive substances such as peptides can enhance the fixation and retention of EVs without impairing the structural integrity and biological activity of EVs. This enhanced fixation and retention may prolong the presence of EVs at healing sites, leading to increased efficiency of delivery as well as functionality. It should be noted that the mechanical properties, gelation time, biocompatibility, and biological functions of hydrogels vary due to different raw materials and synthesis conditions in the practical application of hydrogels [51]. Some studies suggest that chitosan hydrogel has good biocompatibility, hemostasis, and chemical activity [52], while alginate is a biologically neutral material with adjustable mechanical properties and biodegradation [53]. In addition, since the efficacy of MSC-EVs encapsulated in hydrogels on bone regeneration largely depends on the design and function of hydrogels, attention should be paid to the different characteristics of hydrogels when they are used as delivery materials [51]. Zhang et al. [54] prepared an injectable thermosensitive chitosan hydrogel with good biodegradability and biocompatibility, which can improve the stability and retention of human placental MSC-Exos, and effectively enhance bone regeneration by promoting angiogenesis.
In summary, due to its special structure, hydrogels can effectively fill the bone defect area. When combined with EVs and other bioactive molecules, the porous hydrogels can realize the sustained release of EVs. In addition, on the one hand, effective encapsulation of hydrogels can maintain the concentration of EVs and provide a 3D microenvironment similar to the natural extracellular matrix, enhancing the activity of EVs and promoting injury repair; On the other hand, it reduces the possibility of contact with other tissues during transportation, which improves the accuracy and effectiveness of transportation, thereby maximizing the osteogenic effect of EVs. Traditional hydrogels have shortcomings such as insufficient mechanical properties, stability, and adhesion. Recent studies have found that hydrogels can be modified by adding active substances such as peptide sequences to improve their performance and achieve long-term retention of EVs. In addition, different types of hydrogels should be carefully selected to effectively exert their different properties in BTE. However, when applied to critical bone defects with large distances or poor biomechanical environment, it has to be admitted that the poor mechanical properties of hydrogels still cannot satisfy the effective repair of the damage, which makes the development and application of scaffold materials attract extensive attention in BTE.
2.2. Application of MSC-EVs Combined with Scaffolds
In the repair of bone defects, bioactive scaffolds are often used as bridging materials to promote bone regeneration [55]. BTE scaffolds are not only bioabsorbable and biodegradable but also can provide temporary mechanical support for the bone at the implant site, with good mechanical properties. In addition, scaffolds have a highly porous and 3D structure that ideally mimics the porosity, pore size, and interconnectedness of native bone. This specific structure promotes cell attachment and proliferation, providing space for the growth and vascularization of new tissue [56][57]. Palma et al. [58] reported the influence of different formulations of bone grafts in providing an adequate scaffold, thus emphasizing the importance of the type of carrier in the three-dimensional distribution of particles and also space provision in new bone formation. The results showed that the lyophilized form carrier created a more homogenous interparticle spacing, allowed a more suitable particle distribution and stabilization, and provided a required space which is crucial for proper cellular and vascular colonization, then promoting a faster bone regeneration with relevant clinical benefits [58]. Similarly, compared with compacted materials, a biocompatible 3D porous scaffold could ensure a uniform spacing and stable distribution of MSC-EVs [59]. The pores or the space provision of scaffolds can assure an adequate environment for growth factors and nutrients, realizing the constant flow of nutrients, cells, and growth factors from the outer portion to the core of the scaffold and promoting bone regeneration [60][61]. In addition, scaffold surface porosity and the related micro- and nano-morphology directly influence cell behavior, stimulating proper communication among the resident cells [59][62][63]. There is no consensus concerning the more appropriate porosity value or pore size currently. However, when mechanical properties are satisfied, over 90% of studies recommended high porosity values, with a wide range of pore sizes from 10 to at least 200 µm [62][64]. In BTE, bioscaffolds with good mechanical properties provide attachment for osteoblasts, which proliferate and differentiate into osteocytes. Subsequently, the implanted scaffold is gradually degraded and replaced by mature osteocytes, and finally, the typical bone structure is restored [65]. In addition, specific surfaces of some osteogenesis-induced scaffolds support osteoblastic cell differentiation and the expression of the osteoblastic phenotype [66]. Studies have shown that the classical β-TCP porous scaffolds have good biocompatibility and are widely used in the clinical application of bone repair and regeneration [67]. However, various scaffolds, including β-TCP scaffolds, have limited repair capacity for critical bone defects due to a lack of osteoinductive activity [68][69]. Only relying on the scaffold itself as a bone graft material cannot achieve satisfactory bone defect repair [56][70]. Accumulated studies have proved that MSC-EVs can effectively enhance the osteoinductive performance of scaffold materials as bioactive molecules. Therefore, as a bioactive material for carrying MSC-EVs, the combined application of bioscaffolds and MSC-EVs has a better regenerative effect in repairing bone defects [25]. Zhang et al. [25] demonstrated for the first time that human iPS-MSC-derived Exos (hiPS-MSC-Exos) can be used in combination with β-TCP to significantly promote osteogenesis in the rat calvarial defect model. Exos loaded on hiPS-MSC-Exos/β-TCP scaffolds are internalized into human bone mesenchymal stem cells (hBMSCs), which can stimulate the proliferation and differentiation of endogenous BMSCs and promote the recruitment of BMSCs to specific sites in bone defects to enhance new bone formation, indicating that Exos can enhance the osteogenic activity of β-TCP, and the potential mechanism of Exos promoting bone regeneration may be the activation of endogenous BMSCs at the bone defect sites. In addition, Xin et al. [71] demonstrated that hiPS-MSC-Exos/β-TCP combined with scaffolds can effectively promote neovascularization in the osteoporosis rat calvarial defect model, and the area of newly formed vessels increases with the increase of hiPSC-MSC-Exos concentration. Furthermore, MSC-Exos from gums or fat in combination with polymer or polymer/calcium silicate composite scaffolds also enhanced bone regeneration [72][73]. All the above results confirm the potential of MSC-EVs combined with scaffolds in repairing bone defects.
There are various types of scaffolds in BTE, including collagen sponge scaffolds [74], β-TCP scaffolds [25], hydroxyapatite (HA) scaffolds [10], calcium sulfate cement scaffolds [75], bioactive glass [16], polycaprolactone (PCL) scaffolds [76], and other innovative synthetic scaffolds [77], which should be selected appropriately according to their different properties when applied to the regeneration of bone defects. For example, compared with other bone substitutes, the translucent collagen sponge is an ideal scaffold for the detection of MSC-Exos in periodontal tissue regeneration and is commonly used as a scaffold material for carrying Exos in periodontal defect models [78]. In addition, native EVs have poor osteoinductive ability. The secretion of EVs and the expression profiles of their intrinsic cargo are influenced by different cellular microenvironments. Different delivery materials or culture conditions can modulate the microenvironment of parental cells and alter the abundance of EV cargo by giving appropriate physical, chemical, and mechanical stimuli, thereby affecting the function of EVs [79][80][81]. Therefore, currently, parental MSCs are often pretreated with hypoxia, chemical agents, or cytokines to obtain EVs with enhanced osteoinductive properties [82][83]. The functional engineered EVs prepared by various methods combined with different scaffold materials can effectively promote the repair of bone defects [84][85]. Liang et al. [86] found that angiogenesis was significantly promoted when dimethyloxalylglycine (DMOG)-pretreated MSC-Exos loaded by classical HA scaffolds in a rat calvarial defect model. DMOG is a small angiogenic molecule that regulates the stability of hypoxia-inducible factor-1α (HIF-1α) by inhibiting proline hydroxylase and can simulate cell hypoxia at normal oxygen levels [87][88]. However, hypoxia can improve the regulatory ability of MSCs to induce angiogenesis, osteogenic differentiation, and anti-apoptosis by activating the expression of HIF-1α, thereby changing the expression profile of MSC-Exo content [89]. Furthermore, preparing genetically modified hBMSCs by constitutively expressing osteogenic-related genes or proteins is also a common strategy to improve the osteoinductive properties of EVs. The functionally engineered EVs prepared by various methods combined with different scaffold materials can effectively promote the repair of bone defects [85][86]. Huang et al. [90] generated functionally engineered EVs with enhanced osteoinductive properties by constitutively expressing BMP-2 in hBMSCs, which showed a good effect on bone defect repair. Moreover, Ying et al. [91] loaded BMSC-Exos carrying mutant HIF-1α (BMSC-Exo-HIF-1α) into β-TCP scaffolds and found that the effect of BMSC-Exo-HIF-1α combined with β-TCP scaffolds in promoting angiogenesis and new bone regeneration was significantly better than that of BMSC-Exos combined with β-TCP scaffolds in the rat calvarial defect model. Although EVs with enhanced osteogenic capacity obtained by various means show good regenerative therapeutic effects, to date, most of the bioactive molecules loaded by carrier systems, including EVs, show explosive release, which may stimulate early bone resorption, leading to brittle bone formation or reduced bone formation, thereby impairing the osteogenic properties of EVs [92]. Therefore, to achieve the slow and sustained release of EVs required for treatment and improve the efficiency of drug administration, many explorations have been carried out. Through the synthesis of degradable copolymer poly(lactic-co-glycolic acid) (PLGA) and metal-organic frameworks (MOFs), Yue et al. [77] developed an Exo-functionalized PLGA/Mg-GA MOF (PLGA/Exo-MG-GA MOF) scaffold with a unique nanostructure that achieved the controlled release of cargo, thereby effectively promoting osteogenesis and angiogenesis and alleviating inflammation. In addition, some scholars have explored the application effect of the combination of synthetic scaffolds and reagents. Qayoom et al. [75] also achieved the controlled release of Exos with long-term effects by using independently synthesized calcium sulfate/nano-hydroxyapatite nano-cement (NC) as the carrier, combined with BMP and zoledronate (ZA), an anti-bone absorption agent. This is attributed to the bionic properties and interaction potential of the NC matrix with BMP and ZA. Previous studies have shown that the explosive release of Exos may be caused by direct adsorption of Exos on the scaffold due to the way of solution infusion. Some scholars believe that the explosive release of Exos may be controlled by surface chemical modification of the scaffold [9][71]. Lin et al. [93] designed a hierarchical mesoporous bioactive glass (MBG) scaffold, which was inherently osteoinductive and could provide structural bioactivity maintenance, which was applied as a carrier for lyophilized Exos in its micron-sized porosities. Further research found that the controlled release of BMSC-Exos and the maintenance of the biological activity were achieved by lyophilized delivery of BMSC-Exos onto graded MBG scaffolds through the sheltering of micropores on the surface of the scaffolds [16]. Recent studies have shown that active lyophilized Exos obtained by adding lyoprotectants may enable more stable and lower-cost long-term preservation of Exos during delivery, although this research has not been widely recognized [94]. The above results demonstrate that controllable release and even long-term preservation of EVs can be achieved by rationally modified scaffolds, thereby effectively improving the bone regeneration effect of EVs.
In summary, loading EVs onto various bioactive scaffold materials is a common strategy for bone defect repair in BTE. However, the osteogenic activity of native EVs may not be sufficient for critical bone defect repair. Engineered EVs, such as pre-treatment of MSCs by genetic engineering, combined with synthetic scaffolds can significantly improve the osteoinductive ability and promote angiogenesis and osteogenesis in bone defect models. In addition, the development of innovative scaffold materials or the application of lyophilized Exos may be a new strategy to achieve effective EV delivery and controlled release to maximize the osteogenic advantages of EVs in BTE. However, it has to be admitted that there are some problems in loading EVs with scaffolds alone: (1) it is still difficult to achieve precise sustained release; (2) the surface environment of the scaffolds may affect the osteogenic properties of EVs; (3) the filling effect and biocompatibility of scaffolds on bone defects are also inferior to hydrogels.
2.3. Application of EVs Combined with Hydrogels and Scaffolds
Bioscaffolds with good mechanical and stable properties can fill critical bone defects, while hydrogels with good hydrophilicity can effectively fill complex and irregular bone defects due to their gel-like properties. In addition, hydrogels with good biocompatibility and encapsulation properties have better performance in sustained-release EVs compared to bioscaffolds [32][56][57][95]. Therefore, the combination of hydrogels and scaffolds can not only provide good mechanical stability but also effectively fill bone defects based on irregular defect shapes and encapsulate EVs for controlled release. Currently, the application of EVs loaded by composite bioscaffolds combined with hydrogels is very attractive in bone regenerative medicine [96]. A recent study showed that the incorporation of a novel injectable hydrogel, polyethylene glycol maleate citrate (PEGMC), into β-TCP scaffolds effectively enhanced its composite strength and osteoinductive properties [97]. Subsequently, EVs were loaded into the composite PG/TCP (PEGMC + β-TCP) for application in bone defect repair. It was found that the novel composites loaded with EVs improved the microenvironment of BMSCs and induced high-rate and high-quality bone regeneration by promoting angiogenic activity [97]. Similarly, in another study, the combined use of HA-Gel hydrogel with a customized nano-hydroxyapatite/poly-ε -caprolactone (nHP) scaffold capable of matching the shape and size of the bone defects also significantly enhanced the angiogenic activity of UCMSC-Exos, demonstrating a strong ability to promote bone formation by inducing angiogenesis [10]. In this study, both above-mentioned composite scaffolds showed good biocompatibility. In addition, the customized nHP scaffold showed excellent mechanical support properties, while the HA-Gel hydrogel filled the pore structure of the scaffold and achieved the long-term sustained release of UCMSC-Exos. Moreover, to achieve the sustained release of Exos without compromising its biological activity, Swanson et al. [98] developed a PLGA-PEG-PLGA hydrogel microsphere as an Exos delivery system. Further, Exo-loaded PLGA-PEG-PLGA hydrogel microspheres were filled on poly (L-lactic acid) (PLLA) scaffolds, and it was found that the activity of Exos was not impaired and the repair of rat calvarial defects was significantly promoted. In addition, some studies have found that the new composite material obtained by the combination of solid scaffold materials and hydrogels not only has better mechanical properties but also shows some other unique properties such as self-healing [99]. Self-healing materials can repair themselves in a short time after being damaged, which can prevent the inhibitory effect of soft tissue infiltration on bone regeneration, thereby ultimately promoting bone damage repair [100]. Li et al. [22] successfully prepared a self-healing material (CHA/SF/GCS/DF-PEG) with good mechanical properties and plasticity by using CHA as the main component of the bone graft material, combined with silk fibroin (SF), glycol chitosan (GCS) and difunctionalized polyethylene glycol (DF-PEG). The CHA/SF/GCS/DF-PEG composite material is a promising scaffold for Exos because of its ideal structure and physical properties. The combined application of UCMSC-Exos with this composite material can effectively repair bone defects in rats by increasing BMP-2 and collagen deposition and promoting angiogenesis [22]. In addition, Liu et al. [95] prepared a composite hydrogel system with good adhesion and antibacterial properties modified by zeolitic imidazolate framework-8 (ZIF-8) nanoparticles, which can enhance the stability of the implanting environment after bone transplantation and promote bone repair. As a key member of MOFs, nanoscale ZIF-8, which sustainably releases Zn2+ and plays an active role in osteogenesis, angiogenesis, and antibacterial processes, is an effective modified material in BTE [101][102].
In summary, the combined application of hydrogels and scaffolds can obtain a composite bone graft material with good mechanical properties, biocompatibility, and encapsulation ability, which effectively makes up for the deficiency of hydrogels or scaffolds alone (As shown in Table 2). The combined application of the two materials, on the one hand, can provide the mechanical properties required for bone defect repair and fill critical bone defects with high stability. On the other hand, it can enhance the activity of EVs and make it possible to realize efficient delivery, long-term preservation, and sustained release of EVs, which is also of great significance for the effective repair of bone defects. In addition, the new graft material obtained by combining the hydrogels with the scaffolds also exhibits good self-healing, adhesion, and antibacterial properties, as well as other properties that are beneficial to the repair of bone defects. However, at present, the research on the application of complex composite materials in bone defect repair is insufficient due to the variety and complexity of raw materials. It should be noted that the above-mentioned new composite materials cannot be directly used for the treatment of clinical bone defects due to the high cost and complicated preparation process. Further research is needed to develop a low-cost and high-efficiency composite delivery system to deliver EVs in BTE.
Table 2. Summary of the application of different carrier materials in BTE.
| Materials | Advantages | Disadvantages | Common Types | Application | References |
|---|---|---|---|---|---|
| Hydrogels | Similar to the 3D environment in vivo Effectively encapsulate EVs to maintain local concentrations and enhance EVs performance Effectively fill irregular defect environment Release EVs slowly and sustainably Targeted transport, reducing loss and ectopic effect Good biocompatibility and chemical activity |
Poor mechanical properties Poor stability Inadequate adhesion of cell Failure of long-term retained of EVs |
Natural materials, (Gelatin; HA-Gel; chitosan) Synthetic polymers, (PEG)High-performance composite hydrogels, (modified injectable thermosensitive hydrogels; composite hydrogels with enhanced mechanical properties) |
Enhancing the performance of hydrogels(modifying hydrogels; combination application of different hydrogels) Improving the transport efficiency of EVs (adding fixed peptides; construction of fusion polypeptides) |
[33][34][35][36][37][38][40][41][42][43][44][49][50] |
| Scaffolds | The 3D pore structure is similar to natural bone and provides space for the growth and vascularization of new tissue Good mechanical properties Absorbable and biodegradable Specific inducible surface stimuli enhance the activity of EVs |
Failure of EVs Slow releasing Risk of missing the target Unable to provide similar living environments in vivo Poor effect of filling irregular voids |
Classical scaffold materials (collagen sponge, bone cement scaffold, BG; β-TCP, HA scaffolds; polymer scaffolds) Innovative synthetic scaffolds |
Enhancing the activity of EVs (preconditioning MSCs;inducing the expression of osteogenic related genes or proteins; combined with small molecule drugs and inducible factors such as siRNAs (externally and externally loaded)) Realizing the slow and sustained release of EVs(innovative synthetic scaffolds; scaffold materials combined with other materials;scaffold materials that provides EVs lyophilization protection) |
[25][56][57][67][68][69][71][77][78][82][83][84][85][86][91][93] |
| Hydrogels + Scaffolds | Effectively encapsulate EVs and enhance EVs activity Sustain and slow release of EVs Effective and efficient delivery of EVs Good effect of filling bone defects Stable mechanical properties Good biocompatibility Long-term retained of EVs |
The synthesis of composite materials is complicated The quality of application varies |
Hydrogels filling into scaffold materials (HA-Gel hydrogels combined with nHP scaffolds; PLGA-PEG-PLGA gel microspheres combined with PLLA scaffolds) Forming new composite materials (omposite material PG/TCP; Self-healing composites) |
Various new composite materials with good mechanical properties, such as self-healing, stability, adhesion and antibacterial abilities, were obtained | [22][32][56][57][95][97][98][99] |
This entry is adapted from the peer-reviewed paper 10.3390/membranes12070716
References
- Rahman, M.J.; Regn, D.; Bashratyan, R.; Dai, Y.D. Exosomes released by islet-derived mesenchymal stem cells trigger autoimmune responses in NOD mice. Diabetes 2014, 63, 1008–1020.
- Zhang, Y.; Chopp, M.; Meng, Y.; Katakowski, M.; Xin, H.; Mahmood, A.; Xiong, Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 2015, 122, 856–867.
- Yang, X.; Yang, J.; Lei, P.; Wen, T. LncRNA MALAT1 shuttled by bone marrow-derived mesenchymal stem cells-secreted exosomes alleviates osteoporosis through mediating microRNA-34c/SATB2 axis. Aging 2019, 11, 8777–8791.
- Qin, Y.; Sun, R.; Wu, C.; Wang, L.; Zhang, C. Exosome: A Novel Approach to Stimulate Bone Regeneration through Regulation of Osteogenesis and Angiogenesis. Int. J. Mol. Sci. 2016, 17, 712.
- Tan, S.S.H.; Tjio, C.K.E.; Wong, J.R.Y.; Wong, K.L.; Chew, J.R.J.; Hui, J.H.P.; Toh, W.S. Mesenchymal Stem Cell Exosomes for Cartilage Regeneration: A Systematic Review of Preclinical In Vivo Studies. Tissue Eng. Part B Rev. 2021, 27, 1–13.
- Zhu, Y.; Jia, Y.; Wang, Y.; Xu, J.; Chai, Y. Impaired Bone Regenerative Effect of Exosomes Derived from Bone Marrow Mesenchymal Stem Cells in Type 1 Diabetes. Stem Cells Transl. Med. 2019, 8, 593–605.
- Behera, J.; Tyagi, N. Exosomes: Mediators of bone diseases, protection, and therapeutics potential. Oncoscience 2018, 5, 181–195.
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016, 6, 21961.
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240–5254.
- Zhang, Y.; Xie, Y.; Hao, Z.; Zhou, P.; Wang, P.; Fang, S.; Li, L.; Xu, S.; Xia, Y. Umbilical Mesenchymal Stem Cell-Derived Exosome-Encapsulated Hydrogels Accelerate Bone Repair by Enhancing Angiogenesis. ACS Appl. Mater. Interfaces 2021, 13, 18472–18487.
- Fu, Q.L.; Chow, Y.Y.; Sun, S.J.; Zeng, Q.X.; Li, H.B.; Shi, J.B.; Sun, Y.Q.; Wen, W.; Tse, H.F.; Lian, Q.; et al. Mesenchymal stem cells derived from human induced pluripotent stem cells modulate T-cell phenotypes in allergic rhinitis. Allergy 2012, 67, 1215–1222.
- Garza, J.R.; Campbell, R.E.; Tjoumakaris, F.P.; Freedman, K.B.; Miller, L.S.; Santa Maria, D.; Tucker, B.S. Clinical Efficacy of Intra-articular Mesenchymal Stromal Cells for the Treatment of Knee Osteoarthritis: A Double-Blinded Prospective Randomized Controlled Clinical Trial. Am. J. Sports Med. 2020, 48, 588–598.
- Zhang, S.; Wong, K.L.; Ren, X.; Teo, K.Y.W.; Afizah, H.; Choo, A.B.H.; Lai, R.C.; Lim, S.K.; Hui, J.H.P.; Toh, W.S. Mesenchymal Stem Cell Exosomes Promote Functional Osteochondral Repair in a Clinically Relevant Porcine Model. Am. J. Sports Med. 2022, 50, 788–800.
- McIntosh, K.; Zvonic, S.; Garrett, S.; Mitchell, J.B.; Floyd, Z.E.; Hammill, L.; Kloster, A.; Di Halvorsen, Y.; Ting, J.P.; Storms, R.W.; et al. The immunogenicity of human adipose-derived cells: Temporal changes in vitro. Stem Cells 2006, 24, 1246–1253.
- Li, Q.; Yu, H.; Sun, M.; Yang, P.; Hu, X.; Ao, Y.; Cheng, J. The tissue origin effect of extracellular vesicles on cartilage and bone regeneration. Acta Biomater. 2021, 125, 253–266.
- Liu, A.; Lin, D.; Zhao, H.; Chen, L.; Cai, B.; Lin, K.; Shen, S.G. Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated Smad pathway. Biomaterials 2021, 272, 120718.
- Hu, G.W.; Li, Q.; Niu, X.; Hu, B.; Liu, J.; Zhou, S.M.; Guo, S.C.; Lang, H.L.; Zhang, C.Q.; Wang, Y.; et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res. Ther. 2015, 6, 10.
- Hendijani, F.; Sadeghi-Aliabadi, H.; Haghjooy Javanmard, S. Comparison of human mesenchymal stem cells isolated by explant culture method from entire umbilical cord and Wharton’s jelly matrix. Cell Tissue Bank. 2014, 15, 555–565.
- Fong, C.Y.; Chak, L.L.; Biswas, A.; Tan, J.H.; Gauthaman, K.; Chan, W.K.; Bongso, A. Human Wharton’s jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev. Rep. 2011, 7, 1–16.
- Wang, K.X.; Xu, L.L.; Rui, Y.F.; Huang, S.; Lin, S.E.; Xiong, J.H.; Li, Y.H.; Lee, W.Y.; Li, G. The effects of secretion factors from umbilical cord derived mesenchymal stem cells on osteogenic differentiation of mesenchymal stem cells. PLoS ONE 2015, 10, e0120593.
- Ando, Y.; Matsubara, K.; Ishikawa, J.; Fujio, M.; Shohara, R.; Hibi, H.; Ueda, M.; Yamamoto, A. Stem cell-conditioned medium accelerates distraction osteogenesis through multiple regenerative mechanisms. Bone 2014, 61, 82–90.
- Wang, L.; Wang, J.; Zhou, X.; Sun, J.; Zhu, B.; Duan, C.; Chen, P.; Guo, X.; Zhang, T.; Guo, H. A New Self-Healing Hydrogel Containing hucMSC-Derived Exosomes Promotes Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 564731.
- Jiang, Z.Z.; Liu, Y.M.; Niu, X.; Yin, J.Y.; Hu, B.; Guo, S.C.; Fan, Y.; Wang, Y.; Wang, N.S. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res. Ther. 2016, 7, 24.
- Lian, Q.; Zhang, Y.; Zhang, J.; Zhang, H.K.; Wu, X.; Zhang, Y.; Lam, F.F.; Kang, S.; Xia, J.C.; Lai, W.H.; et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation 2010, 121, 1113–1123.
- Zhang, J.; Liu, X.; Li, H.; Chen, C.; Hu, B.; Niu, X.; Li, Q.; Zhao, B.; Xie, Z.; Wang, Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res. Ther. 2016, 7, 136.
- Martinez Saez, D.; Sasaki, R.T.; Neves, A.D.; da Silva, M.C. Stem Cells from Human Exfoliated Deciduous Teeth: A Growing Literature. Cells Tissues Organs 2016, 202, 269–280.
- Nakamura, S.; Yamada, Y.; Katagiri, W.; Sugito, T.; Ito, K.; Ueda, M. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J. Endod. 2009, 35, 1536–1542.
- Wang, L.; Zhao, Y.; Shi, S. Interplay between mesenchymal stem cells and lymphocytes: Implications for immunotherapy and tissue regeneration. J. Dent. Res. 2012, 91, 1003–1010.
- Wu, J.; Chen, L.; Wang, R.; Song, Z.; Shen, Z.; Zhao, Y.; Huang, S.; Lin, Z. Exosomes Secreted by Stem Cells from Human Exfoliated Deciduous Teeth Promote Alveolar Bone Defect Repair through the Regulation of Angiogenesis and Osteogenesis. ACS Biomater. Sci. Eng. 2019, 5, 3561–3571.
- Gómez-Barrena, E.; Rosset, P.; Gebhard, F.; Hernigou, P.; Baldini, N.; Rouard, H.; Sensebé, L.; Gonzalo-Daganzo, R.M.; Giordano, R.; Padilla-Eguiluz, N.; et al. Feasibility and safety of treating non-unions in tibia, femur and humerus with autologous, expanded, bone marrow-derived mesenchymal stromal cells associated with biphasic calcium phosphate biomaterials in a multicentric, non-comparative trial. Biomaterials 2019, 196, 100–108.
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29.
- Leijten, J.; Seo, J.; Yue, K.; Santiago, G.T.; Tamayol, A.; Ruiz-Esparza, G.U.; Shin, S.R.; Sharifi, R.; Noshadi, I.; Álvarez, M.M.; et al. Spatially and Temporally Controlled Hydrogels for Tissue Engineering. Mater. Sci. Eng. R Rep. A Rev. J. 2017, 119, 1–35.
- Akbari, A.; Jabbari, N.; Sharifi, R.; Ahmadi, M.; Vahhabi, A.; Seyedzadeh, S.J.; Nawaz, M.; Szafert, S.; Mahmoodi, M.; Jabbari, E.; et al. Free and hydrogel encapsulated exosome-based therapies in regenerative medicine. Life Sci. 2020, 249, 117447.
- Choi, B.; Kim, S.; Lin, B.; Li, K.; Bezouglaia, O.; Kim, J.; Evseenko, D.; Aghaloo, T.; Lee, M. Visible-light-initiated hydrogels preserving cartilage extracellular signaling for inducing chondrogenesis of mesenchymal stem cells. Acta Biomater. 2015, 12, 30–41.
- Liu, B.; Lee, B.W.; Nakanishi, K.; Villasante, A.; Williamson, R.; Metz, J.; Kim, J.; Kanai, M.; Bi, L.; Brown, K.; et al. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat. Biomed. Eng. 2018, 2, 293–303.
- Zhang, Z.; Wang, X.; Wang, Y.; Hao, J. Rapid-Forming and Self-Healing Agarose-Based Hydrogels for Tissue Adhesives and Potential Wound Dressings. Biomacromolecules 2018, 19, 980–988.
- Holkar, K.; Kale, V.; Ingavle, G. Hydrogel-Assisted 3D Model to Investigate the Osteoinductive Potential of MC3T3-Derived Extracellular Vesicles. ACS Biomater. Sci. Eng. 2021, 7, 2687–2700.
- Li, R.; Li, D.; Wang, H.; Chen, K.; Wang, S.; Xu, J.; Ji, P. Exosomes from adipose-derived stem cells regulate M1/M2 macrophage phenotypic polarization to promote bone healing via miR-451a/MIF. Stem Cell Res. Ther. 2022, 13, 149.
- Yu, W.; Li, S.; Guan, X.; Zhang, N.; Xie, X.; Zhang, K.; Bai, Y. Higher yield and enhanced therapeutic effects of exosomes derived from MSCs in hydrogel-assisted 3D culture system for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2022; Online ahead of print. 112646.
- Zhang, T.; Cheng, Q.; Ye, D.; Chang, C. Tunicate cellulose nanocrystals reinforced nanocomposite hydrogels comprised by hybrid cross-linked networks. Carbohydr. Polym. 2017, 169, 139–148.
- Pishavar, E.; Luo, H.; Naserifar, M.; Hashemi, M.; Toosi, S.; Atala, A.; Ramakrishna, S.; Behravan, J. Advanced Hydrogels as Exosome Delivery Systems for Osteogenic Differentiation of MSCs: Application in Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 203.
- Lee, K.Y.; Alsberg, E.; Mooney, D.J. Degradable and injectable poly(aldehyde guluronate) hydrogels for bone tissue engineering. J. Biomed. Mater. Res. 2001, 56, 228–233.
- Sun, T.L.; Kurokawa, T.; Kuroda, S.; Ihsan, A.B.; Akasaki, T.; Sato, K.; Haque, M.A.; Nakajima, T.; Gong, J.P. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 2013, 12, 932–937.
- Ma, S.; Wu, J.; Hu, H.; Mu, Y.; Zhang, L.; Zhao, Y.; Bian, X.; Jing, W.; Wei, P.; Zhao, B.; et al. Novel fusion peptides deliver exosomes to modify injectable thermo-sensitive hydrogels for bone regeneration. Mater. Today. Bio 2022, 13, 100195.
- Lin, S.; Yang, G.; Jiang, F.; Zhou, M.; Yin, S.; Tang, Y.; Tang, T.; Zhang, Z.; Zhang, W.; Jiang, X. A Magnesium-Enriched 3D Culture System that Mimics the Bone Development Microenvironment for Vascularized Bone Regeneration. Adv. Sci. 2019, 6, 1900209.
- Vila, A.; Torras, N.; Castaño, A.G.; García-Díaz, M.; Comelles, J.; Pérez-Berezo, T.; Corregidor, C.; Castaño, Ó.; Engel, E.; Fernández-Majada, V.; et al. Hydrogel co-networks of gelatine methacrylate and poly(ethylene glycol) diacrylate sustain 3D functional in vitro models of intestinal mucosa. Biofabrication 2020, 12, 025008.
- Han, C.; Zhou, J.; Liang, C.; Liu, B.; Pan, X.; Zhang, Y.; Wang, Y.; Yan, B.; Xie, W.; Liu, F.; et al. Human umbilical cord mesenchymal stem cell derived exosomes encapsulated in functional peptide hydrogels promote cardiac repair. Biomater. Sci. 2019, 7, 2920–2933.
- Wang, J.; Li, W.; Zhang, L.; Ban, L.; Chen, P.; Du, W.; Feng, X.; Liu, B.F. Chemically Edited Exosomes with Dual Ligand Purified by Microfluidic Device for Active Targeted Drug Delivery to Tumor Cells. ACS Appl. Mater. Interfaces 2017, 9, 27441–27452.
- Yim, N.; Ryu, S.W.; Choi, K.; Lee, K.R.; Lee, S.; Choi, H.; Kim, J.; Shaker, M.R.; Sun, W.; Park, J.H.; et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat. Commun. 2016, 7, 12277.
- Huang, C.C.; Kang, M.; Shirazi, S.; Lu, Y.; Cooper, L.F.; Gajendrareddy, P.; Ravindran, S. 3D Encapsulation and tethering of functionally engineered extracellular vesicles to hydrogels. Acta Biomater. 2021, 126, 199–210.
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627.
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release Off. J. Control. Release Soc. 2006, 114, 1–14.
- Neves, M.I.; Moroni, L.; Barrias, C.C. Modulating Alginate Hydrogels for Improved Biological Performance as Cellular 3D Microenvironments. Front. Bioeng. Biotechnol. 2020, 8, 665.
- Zhang, K.; Zhao, X.; Chen, X.; Wei, Y.; Du, W.; Wang, Y.; Liu, L.; Zhao, W.; Han, Z.; Kong, D.; et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS Appl. Mater. Interfaces 2018, 10, 30081–30091.
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926.
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28.
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502.
- Palma, P.; Matos, S.; Ramos, J.; Figueiredo, M.; Krauser, J. New formulations for space provision and bone regeneration. Biodental Eng. I 2010, 1, 71–76.
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492.
- Liu, W.C.; Chen, S.; Zheng, L.; Qin, L. Angiogenesis Assays for the Evaluation of Angiogenic Properties of Orthopaedic Biomaterials—A General Review. Adv. Healthc. Mater. 2017, 6, 434.
- Chen, Y.; Chen, S.; Kawazoe, N.; Chen, G. Promoted Angiogenesis and Osteogenesis by Dexamethasone-loaded Calcium Phosphate Nanoparticles/Collagen Composite Scaffolds with Microgroove Networks. Sci. Rep. 2018, 8, 14143.
- Boyan, B.D.; Bonewald, L.F.; Paschalis, E.P.; Lohmann, C.H.; Rosser, J.; Cochran, D.L.; Dean, D.D.; Schwartz, Z.; Boskey, A.L. Osteoblast-mediated mineral deposition in culture is dependent on surface microtopography. Calcif. Tissue Int. 2002, 71, 519–529.
- Washburn, N.R.; Yamada, K.M.; Simon, C.G., Jr.; Kennedy, S.B.; Amis, E.J. High-throughput investigation of osteoblast response to polymer crystallinity: Influence of nanometer-scale roughness on proliferation. Biomaterials 2004, 25, 1215–1224.
- Perez, R.A.; Mestres, G. Role of pore size and morphology in musculo-skeletal tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 922–939.
- Ohgushi, H.; Dohi, Y.; Tamai, S.; Tabata, S. Osteogenic differentiation of marrow stromal stem cells in porous hydroxyapatite ceramics. J. Biomed. Mater. Res. 1993, 27, 1401–1407.
- Ripamonti, U.; Crooks, J.; Khoali, L.; Roden, L. The induction of bone formation by coral-derived calcium carbonate/hydroxyapatite constructs. Biomaterials 2009, 30, 1428–1439.
- Katagiri, W.; Osugi, M.; Kawai, T.; Hibi, H. First-in-human study and clinical case reports of the alveolar bone regeneration with the secretome from human mesenchymal stem cells. Head Face Med. 2016, 12, 5.
- Zhang, J.; Guan, J.; Zhang, C.; Wang, H.; Huang, W.; Guo, S.; Niu, X.; Xie, Z.; Wang, Y. Bioactive borate glass promotes the repair of radius segmental bone defects by enhancing the osteogenic differentiation of BMSCs. Biomed. Mater. 2015, 10, 065011.
- Han, P.; Xu, M.; Chang, J.; Chakravorty, N.; Wu, C.; Xiao, Y. Lithium release from β-tricalcium phosphate inducing cementogenic and osteogenic differentiation of both hPDLCs and hBMSCs. Biomater. Sci. 2014, 2, 1230–1243.
- Khaled, E.G.; Saleh, M.; Hindocha, S.; Griffin, M.; Khan, W.S. Tissue engineering for bone production- stem cells, gene therapy and scaffolds. Open Orthop. J. 2011, 5 (Suppl. 2), 289–295.
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int. J. Biol. Sci. 2016, 12, 836–849.
- Diomede, F.; Gugliandolo, A.; Cardelli, P.; Merciaro, I.; Ettorre, V.; Traini, T.; Bedini, R.; Scionti, D.; Bramanti, A.; Nanci, A.; et al. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: A new tool for bone defect repair. Stem Cell Res. Ther. 2018, 9, 104.
- Gandolfi, M.G.; Gardin, C.; Zamparini, F.; Ferroni, L.; Esposti, M.D.; Parchi, G.; Ercan, B.; Manzoli, L.; Fava, F.; Fabbri, P.; et al. Mineral-Doped Poly(L-lactide) Acid Scaffolds Enriched with Exosomes Improve Osteogenic Commitment of Human Adipose-Derived Mesenchymal Stem Cells. Nanomaterials 2020, 10, 432.
- Takeuchi, R.; Katagiri, W.; Endo, S.; Kobayashi, T. Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis. PLoS ONE 2019, 14, e0225472.
- Qayoom, I.; Teotia, A.K.; Kumar, A. Nanohydroxyapatite Based Ceramic Carrier Promotes Bone Formation in a Femoral Neck Canal Defect in Osteoporotic Rats. Biomacromolecules 2020, 21, 328–337.
- Wang, X.; Ao, J.; Lu, H.; Zhao, Q.; Ma, Y.; Zhang, J.; Ren, H.; Zhang, Y. Osteoimmune Modulation and Guided Osteogenesis Promoted by Barrier Membranes Incorporated with S-Nitrosoglutathione (GSNO) and Mesenchymal Stem Cell-Derived Exosomes. Int. J. Nanomed. 2020, 15, 3483–3496.
- Kang, Y.; Xu, C.; Meng, L.; Dong, X.; Qi, M.; Jiang, D. Exosome-functionalized magnesium-organic framework-based scaffolds with osteogenic, angiogenic and anti-inflammatory properties for accelerated bone regeneration. Bioact. Mater. 2022, 18, 26–41.
- Shirakata, Y.; Miron, R.J.; Nakamura, T.; Sena, K.; Shinohara, Y.; Horai, N.; Bosshardt, D.D.; Noguchi, K.; Sculean, A. Effects of EMD liquid (Osteogain) on periodontal healing in class III furcation defects in monkeys. J. Clin. Periodontol. 2017, 44, 298–307.
- Patel, D.B.; Gray, K.M.; Santharam, Y.; Lamichhane, T.N.; Stroka, K.M.; Jay, S.M. Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles. Bioeng. Transl. Med. 2017, 2, 170–179.
- Liu, L.; Liu, Y.; Feng, C.; Chang, J.; Fu, R.; Wu, T.; Yu, F.; Wang, X.; Xia, L.; Wu, C.; et al. Lithium-containing biomaterials stimulate bone marrow stromal cell-derived exosomal miR-130a secretion to promote angiogenesis. Biomaterials 2019, 192, 523–536.
- Moreno Madrid, A.P.; Vrech, S.M.; Sanchez, M.A.; Rodriguez, A.P. Advances in additive manufacturing for bone tissue engineering scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 631–644.
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345.
- Fan, J.; Lee, C.S.; Kim, S.; Chen, C.; Aghaloo, T.; Lee, M. Generation of Small RNA-Modulated Exosome Mimetics for Bone Regeneration. ACS Nano 2020, 14, 11973–11984.
- Yin, K.; Zhu, R.; Wang, S.; Zhao, R.C. Low-Level Laser Effect on Proliferation, Migration, and Antiapoptosis of Mesenchymal Stem Cells. Stem Cells Dev. 2017, 26, 762–775.
- Hu, C.; Li, L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J. Cell. Mol. Med. 2018, 22, 1428–1442.
- Liang, B.; Liang, J.M.; Ding, J.N.; Xu, J.; Xu, J.G.; Chai, Y.M. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res. Ther. 2019, 10, 335.
- An, S.Y.; Han, J.; Lim, H.J.; Park, S.Y.; Kim, J.H.; Do, B.R.; Kim, J.H. Valproic acid promotes differentiation of hepatocyte-like cells from whole human umbilical cord-derived mesenchymal stem cells. Tissue Cell 2014, 46, 127–135.
- Ge, T.; Yu, Q.; Liu, W.; Cong, L.; Liu, L.; Wang, Y.; Zhou, L.; Lin, D. Characterization of bone marrow-derived mesenchymal stem cells from dimethyloxallyl glycine-preconditioned mice: Evaluation of the feasibility of dimethyloxallyl glycine as a mobilization agent. Mol. Med. Rep. 2016, 13, 3498–3506.
- Crisostomo, P.R.; Wang, Y.; Markel, T.A.; Wang, M.; Lahm, T.; Meldrum, D.R. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am. J. Physiol. Cell Physiol. 2008, 294, C675–C682.
- Huang, C.C.; Kang, M.; Lu, Y.; Shirazi, S.; Diaz, J.I.; Cooper, L.F.; Gajendrareddy, P.; Ravindran, S. Functionally engineered extracellular vesicles improve bone regeneration. Acta Biomater. 2020, 109, 182–194.
- Ying, C.; Wang, R.; Wang, Z.; Tao, J.; Yin, W.; Zhang, J.; Yi, C.; Qi, X.; Han, D. BMSC-Exosomes Carry Mutant HIF-1α for Improving Angiogenesis and Osteogenesis in Critical-Sized Calvarial Defects. Front. Bioeng. Biotechnol. 2020, 8, 565561.
- Kaneko, H.; Arakawa, T.; Mano, H.; Kaneda, T.; Ogasawara, A.; Nakagawa, M.; Toyama, Y.; Yabe, Y.; Kumegawa, M.; Hakeda, Y. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone 2000, 27, 479–486.
- Lin, D.; Chai, Y.; Ma, Y.; Duan, B.; Yuan, Y.; Liu, C. Rapid initiation of guided bone regeneration driven by spatiotemporal delivery of IL-8 and BMP-2 from hierarchical MBG-based scaffold. Biomaterials 2019, 196, 122–137.
- Bari, E.; Perteghella, S.; Catenacci, L.; Sorlini, M.; Croce, S.; Mantelli, M.; Avanzini, M.A.; Sorrenti, M.; Torre, M.L. Freeze-dried and GMP-compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine 2019, 14, 753–765.
- Liu, Y.; Zhu, Z.; Pei, X.; Zhang, X.; Cheng, X.; Hu, S.; Gao, X.; Wang, J.; Chen, J.; Wan, Q. ZIF-8-Modified Multifunctional Bone-Adhesive Hydrogels Promoting Angiogenesis and Osteogenesis for Bone Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 36978–36995.
- Delawi, D.; Kruyt, M.C.; Huipin, Y.; Vincken, K.L.; de Bruijn, J.D.; Oner, F.C.; Dhert, W.J. Comparing autograft, allograft, and tricalcium phosphate ceramic in a goat instrumented posterolateral fusion model. Tissue Eng. Part C Methods 2013, 19, 821–828.
- Zhang, B.; Huang, J.; Liu, J.; Lin, F.; Ding, Z.; Xu, J. Injectable composite hydrogel promotes osteogenesis and angiogenesis in spinal fusion by optimizing the bone marrow mesenchymal stem cell microenvironment and exosomes secretion. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 111782.
- Swanson, W.B.; Zhang, Z.; Xiu, K.; Gong, T.; Eberle, M.; Wang, Z.; Ma, P.X. Scaffolds with controlled release of pro-mineralization exosomes to promote craniofacial bone healing without cell transplantation. Acta Biomater. 2020, 118, 215–232.
- Yang, J.; Han, C.R.; Duan, J.F.; Xu, F.; Sun, R.C. Mechanical and viscoelastic properties of cellulose nanocrystals reinforced poly(ethylene glycol) nanocomposite hydrogels. ACS Appl. Mater. Interfaces 2013, 5, 3199–3207.
- Chen, R.; Zhu, C.; Xu, L.; Gu, Y.; Ren, S.; Bai, H.; Zhou, Q.; Liu, X.; Lu, S.; Bi, X.; et al. An injectable peptide hydrogel with excellent self-healing ability to continuously release salvianolic acid B for myocardial infarction. Biomaterials 2021, 274, 120855.
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nyström, A.M.; Zou, X. One-pot Synthesis of Metal-Organic Frameworks with Encapsulated Target Molecules and Their Applications for Controlled Drug Delivery. J. Am. Chem. Soc. 2016, 138, 962–968.
- Zhang, X.; Chen, J.; Pei, X.; Wang, J.; Wan, Q.; Jiang, S.; Huang, C.; Pei, X. Enhanced Osseointegration of Porous Titanium Modified with Zeolitic Imidazolate Framework-8. ACS Appl. Mater. Interfaces 2017, 9, 25171–25183.
This entry is offline, you can click here to edit this entry!
