Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Veterinary Sciences
Gurltia paralysans is a neglected and re-emerging metastrongyloid angio-neurotropic nematode causing severe chronic meningomyelitis in domestic cats (Felis catus) as well as in free-ranging small wild felids such as kodkods (Leopardus guigna), margays (Leopardus wiedii) and the northern tiger cat (Leopardus triginus) in South America. Within these definitive hosts (DH), adult males and females of G. paralysans parasitize the leptomeningeal veins of the subarachnoid space and/or the meningeal veins of spinal cord parenchyma, inducing vascular alterations.
- Gurltia paralysans
- life cycle
- nematode
- feline
1. Introduction
Gurltia paralysans is a neglected metastrongyloid nematode (superfamily Metastrongyloidea, family Angiostrongylidae) causing severe chronic meningomyelitis in domestic cats (Felis catus) as well as in free-ranging wild felids of the genus Leopardus in South America. Historically, G. paralysans was first described by Kurt Wolfgang Wolffhügel (1933), a German scientist, naturalist, and parasitologist, who isolated adult nematodes from the vein system of the leptomeninges of 11 domestic cats suffering from chronic pelvic paraparesis within the provinces of Llanquihue and Puerto Varas in Southern Chile [1][2]. The etymology of the genus Gurltia is explained by Wolffhügel’s intention to honour Ernst Friedrich Gurlt (1794–1882), a German veterinary anatomist and teratologist. Wolffhügel (1933) initially described this neurological parasitosis associated with small felines as “paraplejia cruralis parasitaria felis” and placed the nematode species within the genus Hemostrongylus, later renamed Angiostrongylus [1]. One year later, Wolffhügel (1934) published an extended description of its geographic distribution, morphology, pathological findings, and clinical signs and speculated on its transmission and definitive host (DH) spectrum. This author showed the small wild felid species kodkod (Leopardus guigna; Figure 1) to be the main natural DH in Southern Chile and in the border regions of Argentina, locally known as “guiña” or “spotted tiger cat”. In addition, Wolffhügel (1993, 1934) proposed domestic cats as aberrant DH that were first introduced by European settlers into the South American continent and thereafter became exposed to this endemic nematode [3]. More recently, the spectrum of wild felid species acting as DH has increased, nowadays including the margay (Leopardus wiedii) and the northern tiger cat (Leopardus tigrinus) [4]. Additionally, within the genus Leopardus, other small wild felids have also been suggested as potential DH in South America [1][2][3][4][5][6][7]. Alongside the genus Leopardus, larger wild felids of South America, i.e., pumas (Puma concolor concolor), jaguars (Panthera onca), and jaguarondis (Herpailurus yagouaroundi), have also been suggested as potential DH, but this needs further investigation [1][5][7]. In a recent study on free-ranging guiñas in Chile, although no presence of G. paralysans was observed, the isolation of other closely related nematodes such as Angiostrongylus sp., Oslerus sp. and Troglostrongylus sp. was observed, indicating diversity, susceptibility, and the potential risk of lungworm infections in South America [8].
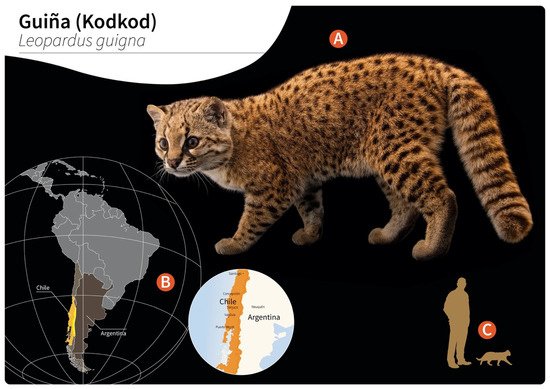
Figure 1. Distribution of wild guiñas (syn. huiñas, kodkods, spotted tiger cat) in South America. (A) Adult specimen of a guiña (Leopardus guigna) (image reprinted with permission from © Joel Sartore/Photo Ark, 2022). (B) Geographic distribution of guiña in Chile (orange) and Argentina (yellow). (C) Scale representation of an adult guiña.
In vivo, G. paralysans has a marked angio-neurotropism invading the venous system of leptomeninges, specifically the thoracic, lumbar, and sacral spinal cord segments. The distribution of G. paralysans adults within the meningeal veins of the subarachnoid space implies activation of the highly immunoreactive endothelium, as seen for Angiostrongylus vasorum [9], probably resulting in thrombophlebitis with thrombus formation, venous congestion, and meningeal haemorrhages due to endothelium damage, as observed in severe feline gurltiosis [10]. Clinical manifestations can include chronic symmetrical or asymmetrical pelvic limb ataxia, ambulatory paraparesis, uni- or bi-lateral hyperactive patellar reflexes, proprioceptive deficit of pelvic limbs, pelvic limb muscular atrophy, diarrhoea, weight loss, coprostasis, urinary and faecal incontinence, and death [1][2][3][4][5][6] (Table 1). Neurological signs are typically associated with neuroanatomical lesions observed in post mortem examinations and histopathological specimens within the spinal cord [1][10][11][12]. Recently, histological and immunohistochemical characterization of vascular alterations in naturally G. paralysans-infected domestic cats of Chile unveiled suppurative vasculitis, haemorrhages, vascular congestion, and varicosis of not only spinal cord but also cerebrum-, cerebellum- and brain stem-associated veins [13], thereby supporting endothelium-derived pro-inflammatory innate immune reactions.
Table 1. Reported cases of feline gurltiosis including age, geographic location, clinical presentation and diagnosis.
| Age | Location | Number of Cases |
Clinical Presentation | Diagnosis | Reference |
|---|---|---|---|---|---|
| 1–3 y | Chile (Los Ríos/Los Lagos regions) |
3 | Paraparesis (ambulatory) PL ataxia PL muscle atrophy Anal/urinary incontinence |
Post mortem (histopathology) |
[12] |
| 6–8 m | Colombia (Antioquia municipality) |
6 | Paraparesis (ambulatory) PL ataxia Spinal pain PL muscle atrophy Anal/urinary incontinence Decrease superficial/deep pain in PL |
Post mortem (histopathology, Myelo) |
[14] |
| 2 y | Argentina (Buenos Aires province) |
1 | Paraparesis (non-ambulatory) PL muscle atrophy Increase spinal reflexes in PL Decrease superficial/deep pain in PL |
Post mortem (histopathology) |
[15] |
| NA | Uruguay (Fray Bentos) |
2 | Paraparesis (ambulatory) Paraplegia PL ataxia |
Post mortem (histopathology) |
[16] |
| 1–3 y | Chile | 3 | Paraparesis (ambulatory) PL ataxia PL muscle atrophy Anal/urinary incontinence Spinal hyperaesthesia PL trembling Increase spinal reflexes in PL Paraplegia |
Post mortem (histopathology, specimens extracted from SSE) |
[11] |
| 8 m–10 y | Chile | 9 | Paraparesis (ambulatory) Paraparesis (non-ambulatory) Paraplegia PL ataxia Increase spinal reflexes in PL Spinal hyperaesthesia Anal/urinary incontinence |
Post mortem (histopathology, specimens extracted from SSE, Myelo, CT, MRI |
[17] |
| NA | Brazil (Río Grande do Sul) |
4 | Paraparesis (ambulatory) PL muscle atrophy Vesical atony Tail atony |
Post mortem (histopathology) |
[18] |
| NA | Argentina (Santa Fé) |
3 | Paraparesis (ambulatory) Paraplegia Decrease spinal reflexes in PL Decrease superficial pain in PL Skin lesions in the metatarsal region |
Post mortem (histopathology) |
[19] |
| 8 m | Chile (Ancud, Los Lagos regions) |
1 | Paraparesis (ambulatory) Anal/urinary incontinence Tail atony |
Myelo-CT, CSF (mononuclear pleocytosis), post mortem (histopathology) |
[10] |
| 2 y | Spain (Tenerife) |
1 | Uveitis in left eye | Specimen extracted from anterior chamber of the eye, PCR | [20] |
| NA | Brazil (Pernambuco) |
11 | Paraparesis (ambulatory) PL ataxia, PL muscle atrophy Skin lesions in metatarsal and phalangeal regions |
Post mortem (histopathology) |
[21] |
| 36 m | Chile | 10 | Paraparesis (ambulatory) Paraplegia PL ataxia Anal/urinary incontinence |
Post mortem (histopathology), IDEXX (Angio Detect), specimens extracted from SSE |
[5] |
2. Hypothetical Life Cycle of Gurltia paralysans (Angiostrongylidae)
Small felids of the genera Felis and Leopardus are considered as DH of G. paralysans, as sexual replication occurs within these carnivorous mammals. As such, intravascular gravid G. paralysans females passing non-embryonated eggs (i.e., those containing 16 blastomeres) within the subarachnoid leptomeningeal veins of spinal cords from domestic cats (F. catus) [12], kodkods (L. guigna) [1][2][3], margays (L. wiedii) and northern tiger cats (L. tigrinus) [4] have been reported. Within Chilean territories, other small wild felids of the genus Leopardus have been discussed as potential DH [1][2][6], such as the Andean cat (Leopardus jacobita) and the Pampas cat (Leopardus colocolo). In semiarid, subtropical and tropical regions of South America, the southern tiger cat (Leopardus guttulus), Geoffroy’s cat (Leopardus geoffroyi) and the ocelot (Leopardus pardalis) might also act as DH [1][2][3][4][22]. Moreover, in South, Central and North America, larger wild felids such as the cougar (P. concolor concolor), the jaguar (P. onca) and the jaguarundi (H. yagouarundi) are proposed as DH, but this needs further clarification [1][7].
Unfortunately, nothing is known on other aspects of the life cycle, such as the mode of DH infection, exogenous larval development in obligate intermediate hosts (IH), endogenous in vivo migration of infective L3, pre-patency, patency and post-patency [5][6][12]. Neither eggs nor first-stage larvae (L1) have been detected in faeces, blood, bronchial lavage and/or other body fluids of naturally G. paralysans-infected domestic cats, northern tiger cats and margays [1][4][22][23].
As reported for other nematodes of the family Angiostrongylidae, terrestrial/aquatic gastropods (snails, semi-slugs and slugs) acting as obligate IH as well as paratenic hosts (PH) have been proposed in the biology of G. paralysans. Therefore, larval development occurring through moults from first-stage larvae (L1) to second-stage larvae (L2) and to the final infective third-stage larvae (L3) (Figure 2) has recently been proposed [1][5][6][7]. The suspected PH in this life cycle, such as crustaceans, amphibians, reptiles, rodents and birds, might become infected after ingesting L3-carrying gastropod IH, as reported for closely related Angiostronglylus species [24][25]. To elucidate the presence of G. paralysans larval stages in obligate gastropod IH, a large-scale epidemiological survey was recently conducted in the southern parts of Chile [26]. In this study, 835 terrestrial gastropods from a previously well-recognized endemic focus surrounding the city of Valdivia, Chile [1][26], were collected, demonstrating that neither PCR, enzymatic digestion nor histological examinations revealed the presence of larvae [1][26]. Collected gastropods included slugs of the families Arionidae, Limacidae and Milacidae as well as snails of the family Helicidae. Nonetheless, neither terrestrial semi-slugs (family Helicarionidae) nor aquatic snails were included in this survey, and thus this needs further investigation [1][7][26].
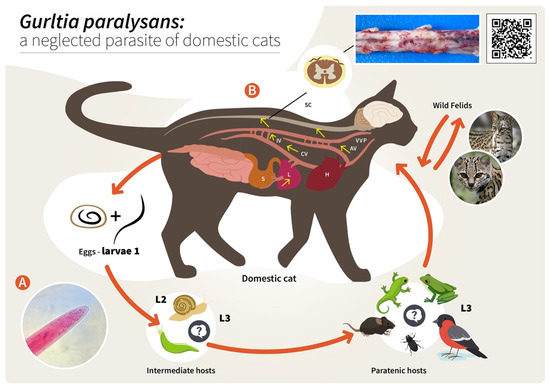
Figure 2. Proposed life cycle and migration pathways of Gurltia paralysans. (A) Cranial end of an adult specimen of G. paralysans. (B) Domestic cats (Felis catus) or wild felids (Leopardus spp.) acquire the L3 larvae by ingesting an infected obligate intermediate host (gastropods) or paratenic hosts (lizards, rodents, amphibians, birds or insects). Infective larvae penetrate the stomach and enter the hepatic portal system, and then the caudal vena cava and/or the azygous venous system. From these vein systems, the larvae migrate to the spinal cord via the intervertebral veins and the vertebral venous plexus. The larvae invade the veins of the subarachnoid space of the spinal cord, where they mature and lay eggs. It is still unknown on how domestic cats eliminate the eggs or the first-stage larvae (L1) into the environment, their further development into the L2 and L3 larval stages, or how the obligate intermediate hosts become infected with L1. AV: azygos vein; CV: caudal vena cava; IV: intervertebral veins; H: heart; L: liver; S: stomach; SC: spinal cord; VVP: vertebral venous plexus; L1: first-stage larvae; L2: second-stage larvae; L3: third-stage larvae. The inserted QR code shows a video of a G. paralysans-infected cat with clinical signs of paraparesis.
Proposed infection routes for felid DH are either after consumption of G. paralysans L3-infected gastropod IH or after consumption of L3-infected PH, as initially proposed by Wolffhügel (1934) [3]. As such, in his article, he referred to the colloquial name of feline gurltiosis used by locals, namely “lizard disease”, highlighting the pivotal role of PH in transmission. Alongside lizards, fish, frogs, toads, newts, snakes, turtles, birds, rodents, planarians, crustaceans, insects and myriapods have also been suspected in the life cycle of G. paralysans within South America [1][5][21][26]. Likewise, infective A. cantonensis-L3 larvae liberated from dead or living gastropods can survive outside IH for a short time, forming an important source of infection. The L3 larvae of A. cantonensis can enter new IH through the process known as intermediasis, which might occur in this life cycle as well, thereby extending the survival strategies of G. paralysans. These alternative transmission routes can occur with ease in neotropical South American rainforests, which have the highest biodiversity of protist, invertebrate and vertebrate species in the world [7].
Concerning the endogenous migration of G. paralysans L3 in felid DH in vivo, nothing is known so far. Hypothetically, L3 migration could be through the small intestinal mucosa in order to reach the mesenteric veins and/or lymphatic vessels of the abdominal viscera and thereafter via connections of either the azygos or the caval venous system (CVS) with the thoracic, lumbar or sacral intervertebral veins until they reach the vertebral venous plexus (VVP, Figure 2) [1]. The VVP is in direct communication with the cranial venous system, and because no valves exist in either of them, blood might flow cranially or caudally, depending on blood pressure [1][27]. G. paralysans could take advantage of the absence of valves in the VVP to reach either the spinal subarachnoid space or even the brain [1][12][28]. Similarly, in spinal schistosomiasis in humans, the dissemination of the parasite occurs via the intestinal veins to the VVP [29]. Spinal schistosomiasis usually involves the lower thoracic and lumbosacral spine, probably because the VVP connects the intra-abdominal veins with those of the lower spine [30]. The presence of fertile male and female nematodes, and gravid females passing eggs within the ventral VVP and basivertebral veins located in the vertebral bodies, were isolated during necropsies, confirming the marked angiotropism of G. paralysans in DH [1][12]. Moreover, parasitic localization within the VVP’s venous connections may explain the presence of eggs, L1, pre-adults and adults of G. paralysans in distant places, such as the cerebrum, cerebellum and anterior chamber of the eye of infected cats [1][20][27][31]. Nonetheless and in contrast to all Angiostrongylus species residing within arterial vessels, G. paralysans dwells within venous vessels. Thus, the adaptations of G. paralysans to the VVP’s venous connections might be associated not only with abiotic factors of the venous microenvironment, such as hypoxia and CO2 concentrations, but may also be linked to physical factors (e.g., temperature, blood flow velocity) and even nutrients, among others [1]. It seems indispensable for future investigations on the migratory pathways of G. paralysans to include not only vein tropism but also neuroanatomical localization within the subarachnoid VVP in felids [1]. During the patency period, gravid G. paralysans will then release un-embryonated eggs into the leptomeningeal vein system. Intravascular eggs will develop into L1, and hatching of the L1 will occur within the VVP, as demonstrated previously [1][12]. The free-released L1 will then breach the alveolar walls in order to access the bronchioles, bronchia and trachea, and are most likely expelled via faeces into the environment. Consistently, the life cycles of other closely related angio-neurotropic metastrongyloid genera of cervids (Elaphostrongylus and Parelaphostrongylus) might explain the final localizations of G. paralysans in the subarachnoid leptomeningeal veins of felid spinal cord. Likewise, adults of Elaphostrongylus alces occur in vessels of the epidural space of the vertebral canal and in the skeletal muscles of moose (Alces alces) [32][33]. Similar to G. paralysans, E. alces has obligate gastropod IH, causing neurological disorders in wild moose populations after ingestion of L3-carrying gastropods [32][33]. Earlier researchers suggested that E. alces L3 migrate directly from the gut into the epidural space of the caudal vertebral canal, where development to the adult stages takes place. During endogenous development, E. alces nematodes produce severe inflammation of the epidural tissue and spinal nerves [32]. In line with this, development of Elaphostrongylus rangiferi also takes place only within the brain and spinal cord vessels of reindeer (Rangifer tarandus), with subsequent migration of adult nematodes into the skeletal muscle [34]. In the case of Parelaphostrongylus tenuis, also known as “meningeal worm” or “brain worm”, which typically occurs in wild cervids (Cervidae), adult nematodes mate in the blood vessels of deer heads, and gravid females start releasing eggs into the circulatory system [35]. In fact, the release of eggs or larvae into the circulatory blood system might be linked with the appearance of P. tenuis larvae in the ventral portion of the anterior eye chamber [36], similar to a recent ophthalmic finding in a G. paralysans-infected domestic cat of Tenerife Island, Spain [20].
One of the most peculiar features of some Angiostrongylus species (Angiostrongylidae) is their strong neurotropism within warm-blooded DH or accidental/aberrant hosts (AH). As such, infectious A. cantonensis L3 must migrate through the central nervous system (CNS), where they develop further, reaching the L5 larval stage in the subarachnoidal space within two weeks post infection [37][38]. This part of the life cycle usually does not produce severe signs in DH (e.g., rats); however, infections in AH commonly result in eosinophilic meningitis, with several clinical scenarios [38], as was also the case for G. paralysans-infected domestic cats considered by Wolffhügel (1934) as AH. Clinical manifestations are common between G. paralysans and A. cantonensis due to the signs observed in infected animals resulting in increased intracranial pressure, neural tissue damage, the hosts’ pro-inflammatory response, congestion, thrombosis, thrombophlebitis, varices and thickening of the affected vessels [1][18][37].
This entry is adapted from the peer-reviewed paper 10.3390/pathogens11070792
References
- Gómez, M.; Moroni, M.; Muñoz, P.; Taubert, A.; Hermosilla, C.; Hirzmann, J.; Rojas, L. Gurltia paralysans: A neglected parasite of domestic cats. Austral J. Vet. Sci. 2021, 52, 33–45.
- Wolffhugel, K. Paraplegia cruralis felis causada por Gurltia paralysans nov. gen., n. sp. Rev. Chil. Hist. Nat. 1933, 37, 190–192.
- Wolffhugel, K. Paraplegia cruralis parasitaria felis durch Gurltia paralysans nov. gen., nov. sp. (Nematoda). 2. Infektkr. Haustiere 1934, 46, 28–47.
- Dazzi, C.; Santos, A.; Machado, T.; Ataíde, M.; Rodríguez, R.; Muller, A.; Sepúlveda, P.; Costa da Motta, A. First case report of nematode parasitic myelopathy in a wild feline in Brazil. Braz. J. Vet. Parasitol. 2020, 29, 1.
- Gómez, M.; García, C.; Maldonado, I.; Pantchev, N.; Taubert, A.; Hermosilla, C.; Moroni, M.; Muñoz, P.; Durán, A.; Mieres, M.; et al. Intra vitam diagnosis of neglected Gurltia paralysans infections in domestic cats (Felis catus) by a commercial serology test for canine angiostrongylosis and insights into clinical and histopathological findings-Four case reports. Pathogens 2020, 9, 921.
- Barrios, N.; Gómez, M.; Zanelli, M.; Rojas-Barón, L.; Sepúlveda-García, P.; Alabí, A.; Adasme, M.; Múller, A.; Rosenfeld, C.; González-Lagos, C.; et al. A Molecular Survey on Neglected Gurltia paralysans and Aelurostrongylus abstrusus Infections in Domestic Cats (Felis catus) from Southern Chile. Pathogens 2021, 10, 1195.
- Uribe, M.; López-Osorio, S.; Chaparro-Gutiérrez, J.J. The Neglected Angio-Neurotrophic Parasite Gurltia paralysans (Nematoda: Angiostrongylidae): Northernmost South American Distribution, Current Knowledge, and Future Perspectives. Pathogens 2021, 10, 1601.
- Acuña-Olea, F.; Sacristán, I.; Aguilar, E.; García, S.; López, M.J.; Oyarzún-Ruiz, P.; Brito, J.L.; Fredes, F.; Napolitano, C. Gastrointestinal and cardiorespiratory endoparasites in the wild felid guigna (Leopardus guigna) in Chile: Richness increases with latitude and first records for the host species. Int. J. Parasitol. Parasites Wildl. 2020, 13, 13–21.
- Grob, D.; Conejeros, I.; López-Osorio, S.; Velásquez, Z.D.; Segeritz, L.; Gärtner, U.; Schaper, R.; Hermosilla, C.; Taubert, A. Canine Angiostrongylus vasorum-Induced Early Innate Immune Reactions Based on NETs Formation and Canine Vascular Endothelial Cell Activation In Vitro. Biology 2021, 10, 427.
- Moroni, M.; Muñoz, P.; Mieres, M.; Gómez, M.; Vera, F. Severe spinal cord thrombophlebitis and meningoyelitis by Gurltia paralysans in a cat: A case report. Vet. Rec. Case Rep. 2016, 4, 1.
- Gómez, M.; Mieres, M.; Moroni, M.; Mora, A.; Barrios, N.; Simeone, C.; Lindsey, D. Meningomyelitis due to nematode infection in four cats. Vet. Parasitol. 2010, 170, 327–330.
- Moroni, M.; Muñoz, P.; Gómez, M.; Mieres, M.; Rojas, M.; Lillo, C.; Aguirre, F.; Acosta-Jamett, G.; Kaiser, M.; Lindsay, D. Gurltia paralysans (Wolffhugel, 1933): Description of adults and additional case reports of numerological diseases in three domestic cats from Southern Chile. Vet. Parasitol. 2012, 184, 377–380.
- Hartung, S.; Weyrich, A.; Moroni, M.; Gómez, M.; Herden, C. Histological and Immunohistochemical Characterization of Vascular Alterations in Meninges of Cats Infected with Gurltia paralysans. Pathogens 2022, 11, 88.
- Alzate, G.; Aranzazu, D.; Alzate, A.; Chaparro, J. Domestic cat paraplegia compatible with Gurltia paralysans nematode. First cases reported in Colombia. Rev. Colomb. Cienc. Pecu. 2011, 24, 663–669.
- Guerrero, I.; Paludi, A.; Saumell, L. Primera Descripción en Argentina de Gurltia paralysans en un Felino Doméstico. DVM Thesis, Universidad Del Centro De La Prov. Buenos Aires, Tandil, Argentina, 2011.
- Rivero, R.; Matto, C.; Adrien, M.; Nan, F.; Bell, T.; Gardiner, C. Parasite meningomyelitis in cats in Uruguay. Rev. Bras. Parasitol. Vet. 2011, 20, 259–261.
- Mieres, M.; Gómez, M.; Lillo, C.; Rojas, M.; Moroni, M.; Muñoz, P.; Acosta-Jamett, G.; Wiegand, R. Clinical, imaging and pathological characteristics of Gurltia paralysans myelopathy in domestic cats from Chile. Vet. Radiol. Ultrasound. 2013, 53, 1–8.
- Togni, M.; Panziera, W.; Souza, T.; Oliveira, J.; Mazzanti, A.; Barros, C.; Fighera, R. Epidemiological, clinical and pathological aspects of Gurltia paralysans infections in cats. Pesqui. Veterinária Bras. 2013, 33, 363–371.
- Bono, M.; Orcellet, V.; Marengo, R.; Bosio, A.; Junkers, E.; Plaza, D.; Marini, M.; Sánchez, A.; Rubio, M.; Candiotti, V. A description of three cases of parasitic meningomyelitis in felines of the province of Santa Fé, Argentina. Parasitaria 2016, 74, 1–4.
- Udiz-Rodríguez, R.; García-Livia, K.; Valladares-Salmerón, M.; Dorta-Almenar, M.; Martin-Carrillo, N.; Martin-Alonso, A.; Isquierdo-Rodríguez, E.; Feliu, C.; Valladares, B.; Foronda, P. First ocular report of Gurltia paralysans (Wolffhugel, 1933) in cat. Vet. Parasitol. 2018, 255, 74–77.
- Melo Neto, G.; da Silva, L.; Alves, R.; Olinda, R.; Dantas, A.; Torres, M. Infeccao por Gurltia paralysans em gatos domésticos no Estado de Pernambuco, Brasil. Acta Scientiae Veterinariae 2019, 47, 418.
- Oliveira, B. Politraumatismo em Gato-Maracajá (Leopardus wiedii) com Infeccao por Gurltia paralysans; Semana do Conhecimiento UPF: Passo Fundo, Italy, 2015; pp. 1–3.
- Peña, G. Hallazgos Clínicos, Hematológicos, Bioquímicos y de Lavado Broncoalveolar en 8 Gatos Domésticos (Felis catus) con Paraparesis/Plejia Producida por Gurltia paralysans. DVM Thesis, Universidad Austral De Chile, Valdivia, Chile, 2014.
- Morgan, E.R.; Modry, D.; Paredes-Esquivel, C.; Foronda, P.; Traversa, D. Angiostrongylosis in Animals and Humans in Europe. Pathogens 2021, 10, 1236.
- Segeritz, L.; Cardona, A.; Taubert, A.; Hermosilla, C.; Ruiz, A. Autochthonous Angiostrongylus cantonensis, Angiostrongylus vasorum and Aelurostrongylus abstrusus infections in native terrestrial gastropods from the Macaronesian Archipelago of Spain. Parasitol. Res. 2021, 120, 2671–2680.
- Sepúlveda-García, P.; Gómez, M.; Moroni, M.; Muñoz, P.; Muller, A. Evaluation of terrestrial gastropods intermediate hosts of Gutlia paralysans in southern Chile. Braz. J. Vet. Parasitol. 2021, 30, 1.
- Gómez, M.; Freeman, L. Revisión del plexo venoso vertebral en el perro (Review of the vertebral venous plexus in the dog). Int. J. Morphol. 2003, 21, 237–244.
- Katchanov, J.; Nawa, Y. Helminthic invasion of the central nervous system: Many roads lead to Rome. Parasitol. Int. 2010, 59, 491–496.
- Shahlaie, K.; Hawk, M.; Hu, B.; Theis, J.; Kim, K. Parasitic central nervous system infections: Echinococcus and Schistosoma. Rev. Neurol. Dis. 2005, 2, 76–85.
- Paz, J.; Valente, M.; Casella, E.B.; Marques-Dias, M.J. Spinal cord schistosomiasis in children: Analysis of seven cases. Arq Neuropisiquiatr. 2002, 60, 224–230.
- Figueroa, N. Descripción Histopatológica de Lesiones Encefálicas en Gatos Domésticos Infectados con Gurltia paralysans. DVM Thesis, Universidad Austral De Chile, Valdivia, Chile, 2017.
- Handeland, K.; Gibbons, L.; Skorping, A. Aspects of the life cycle and pathogenesis of Elaphostrongylus cervi in red deer (Cervus elaphus). J. Parasitol. 2000, 86, 1061–1066.
- Handeland, K. Cerebrospinal nematodiasis in a moose in Norway. J. Wildl. Dis. 2002, 38, 817–821.
- Hemmingsen, W.; Halvorsen, O.; Skorping, A. Migration of adult Elaphostrongylus rangiferi (Nematoda: Protostrongylidae) from the spinal subdural space to the muscles of reindeer (Rangifer tarandus). J. Parasitol. 1993, 79, 728–732.
- Lankester, M. Understanding the impact of meningeal worm, Parelaphostrongylus tenuis, on moose populations. Alces J. Devoted Biol. Manag. Moose 2010, 46, 53–70.
- Reinstein, S.; Lucio-Forster, A.; Bowman, D.; Eberhard, M.; Hoberg, E.; Pot, S.; Miller, P. Surgical extraction of an intraocular infection of Parelaphostrongylus tenuis in a horse. J. Am. Vet. Med. Assoc. 2010, 15, 196–199.
- Modrý, D.; Fecková, B.; Putnová, B.; Manalo, S.; Otranto, D. Alternative pathway in Angiostrongylus cantonensis (Metastrongyloidea: Angiostrongyloidea) transmission. Parasitology 2021, 148, 167–173.
- Ferdushy, T.; Hasan, M. Survival of first stage larvae (L1) of Angiostrongylus vasorum under various conditions of temperature and humidity. Parasitol Res. 2010, 107, 1323–1327.
This entry is offline, you can click here to edit this entry!
