Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell & Tissue Engineering
Synthetic 3D multicellular systems derived from patient tumors, or tumoroids, have been developed to complete the cancer research arsenal and overcome the limits of current preclinical models.
- preclinical models
- non-small-cell lung cancer
1. Introduction
Lung cancer is the deadliest cancer worldwide; non-small cell lung cancer (NSCLC) is the most common form, with 85% of all cases [1]. The survival rate over 5 years for patients with advanced stage lung cancer remains below 15% despite the diversity of therapeutic treatments and very important progress in the last two decades [2]. Treatment options mainly rely on surgery, complemented with radiotherapy, targeted chemotherapy, or immunotherapy, thanks to the development of specific markers of response [3]. The 5 year survival rate improves to 61.2% when diagnosis is performed at the stage of localized tumor, but drops to 9.9% when cancer is detected at the metastatic stage [4]. Both the large National Lung Screening Trial (NLST) conducted in the U.S. from 2002 to 2011, and the NELSON study in Europe, confirmed that the earlier the diagnosis, the higher the survival rate [5,6]. However, about 70% of lung cancer patients remain diagnosed at advanced stages, where heavy systemic treatment is necessary [7].
For patients at advanced stages, platinum-based chemotherapy regimen is the standard of care but is associated with severe toxicities [8]. In this respect, therapies targeting driver mutations in EGFR, or more recently, KRAS genes, have been a progress, but often face occurrence of resistance [9,10]. Immunotherapy has recently revolutionized the treatment of lung cancer [11]. The main strategy in immunotherapy is to target immune checkpoint pathways in order to escape local immune tolerance and to boost anti-tumor response [12]. Its therapeutic window is quite narrow, and the use of immune-checkpoint-blockers is associated with a high rate of immune-related adverse events (irAEs), reaching 26.82% of patients treated with PD-1 inhibitors [13]. These deleterious responses might affect multiple organs (skin, digestive tract, liver, endocrine gland, lung, thyroid, etc.) [14,15,16].
To better predict and assess the efficacy, resistance or toxicity of drug candidates during drug development, experimental advanced models have been designed. The most relevant are 3D models derived from both normal and tumoral lung epithelial cells. They aim at recapitulating the heterogeneity of tumoral cells, and at reproducing the complex network of interactions in the tumor microenvironment (TME). Taking advantage of the fast evolution of cell culture technologies and microsystems, 3D tumor models represent a major step forward in the characterization of new drug candidates and pave the way towards personalized medicine by using patient-derived tumor biopsies. To describe state-of-the-art lung cancer preclinical models, their limits in predicting drug efficacy in complex tumors or adverse events, and recent technological progresses that might rapidly benefit the search for safe and efficient drugs (Figure 1).
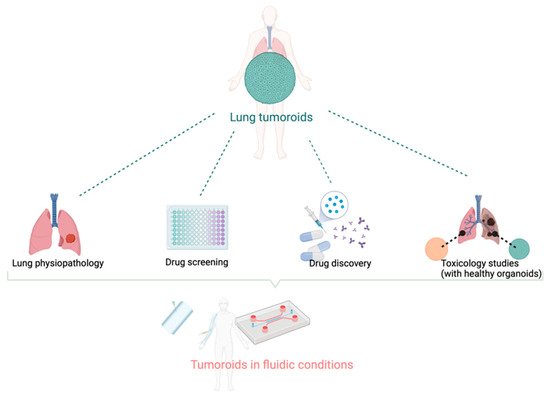
Figure 1. The variety of applications for tumoroids and organoids models. Directly derived from patient biopsy or surgery, synthetic tumor models can be used to study tumorigenesis, tumor growth, and interactions with the normal tissues. The drug discovery process should benefit from higher predictivity from these models than current preclinical models. The combination with microfluidic systems allows for better mimicry of the tumor dynamics and makes these tumor models suitable with high throughput/high content bioassays. Created with BioRender.com.
2. Current Experimental Models for Drug Development in NSCLC and Their Limits
2.1. Lung Cancer Cell Lines for In Vitro Studies
Lung cancer cell lines have been widely used in cancer research, the “historical” A549 cell counting itself 17,947 entries in PubMed (as of 17 March 2022). The NCI panel of cancer cell lines comprises more than 200 lung cancer cell lines derived from patients with either small-cell lung cancer (SCLC) or NSCLC [17,18]. Whereas monotypic cell cultures are suitable for the study of proliferative mechanisms and the study of signaling pathways, they have proven insufficient to understand some major interactions within the TME (e.g., with stromal cells, endothelial cells and/or immune cells). Pro-inflammatory cells and stromal cells were shown to be key in controlling tumor growth, metastasis and angiogenesis [18]. Another issue with the use of cancer cell lines is genetic variation; many authors have documented the loss of original phenotypic features from the primary tumor [19,20]. Despite these drawbacks, lung cancer cell lines still feed the vast majority of basic studies in cancer research, and of early drug screening campaigns [21].
2.2. Murine Models for In Vivo Studies
- (a)
-
Patient-derived tumor xenografts (PDXs)
PDXs have been used for understanding cancer metastasis and for drug screening. Biopsies and patient-derived tumor materials offer the advantage of encompassing multiple factors such as cellular heterogeneity, histological structures, malignant genotypes and phenotypes. Grafted onto immunodeficient mice, they tend to conserve essential features of the human primary tumor. In particular, somatic and genomic alterations and histological subtypes were found to be comparable between primary tumors and corresponding PDX [22,23,24]. Nevertheless, major limitations are reported. Genomic variation seems higher in PDXs, with an enrichment of aberrations in cancer associated genes [22]. Immunodeficient NOD/SCID or NOD/NSG mice are still largely used to avoid tumor rejection but they are not suitable for the assessment of immunotherapies [23]. Humanized PDX models are thus recommended in this perspective but are very expansive. The question of implantation site is also important, orthotopic grating or injection into the circulation are associated with a higher success rate, up to 30–40%, than subcutaneous grafting [25].
-
(b) Syngeneic murine models
These immunocompetent models turned out to be essential for understanding both tumor-host interactions and immune mechanisms [26]. Unfortunately, there is still a limited panel of murine lung cancer cell lines that can spontaneously form tumors in immunocompetent mice [27]. The development and validation of relevant immunocompetent syngeneic models for lung cancer will be a long process.
-
(c) Genetically engineered mouse models (GEMMs)
GEMMS were designed to approach genetic characteristics of human tumors that cannot be reflected in xenograft models, allowing disease modeling in immunocompetent environments. They are inducible models, enabling either overexpression, shutoff or functional replacement of selected genes of interest [17]. Lung cancer GEMMs targeting oncogenic drivers, such as KRAS or EGFR, are available for assessing response to targeted therapies, and discovering new pathways implicated in malignancy [28]. Resistant models to EGFR inhibitors were also reported [29]. A major hurdle in their development is that the establishment of GEMMs is rather expensive and long. Furthermore, validation of experimental procedures is important, as evolution of GEMMs might be highly variable within a cohort. Last, but not least, tumors with low malignancy may fail to recapitulate the tumor–host interactions in the course of cancer progression.
3. Limits of Current Preclinical Models in Lung Cancer Research
Current preclinical models fail to effectively mimic human responses [30]. These limitations, that impact both basic understanding of human tumor biology, as well as drug development processes, are summarized in Table 1. Major problems are: (i) murine stromal components replacing their human counterparts, (ii) the lack of immune system in most models, and (iii) the lack of the many interactions that characterize a fully functional TME [31]. Interestingly, the recent progress in 3D cultures of human cancer cells might help to overcome these limitations.
Table 1. Advantages and limits of the main preclinical lung cancer models.
| Technologies | Advantages | Limits | References | |
|---|---|---|---|---|
| In vitro | Cancer cell lines | Pure population of tumor cells Replicative ability Large diversity of genomic backgrounds |
|
[17,18,19,20] |
| In vivo | PDXs | Closer to patients’ primary tissues |
|
[17,22,23,24,25] |
| Syngeneic models | Functional immune system |
|
[27] | |
| GEMMs | Functional immune system Inducible model |
|
[17,28,29] | |
Interestingly, the recent progress in 3D cultures of human cancer cells might help to overcome these limitations. Numerous advantages have been reported compared with regular preclinical models, recapitulating complex structural features of natural tumors, and making them more predictive of patients’ individual responses. They also retain cancer general features such as hypoxia or necrotic domains, or substructures of drug resistant cells [32]. The 3D tumor models directly benefit from the large R&D effort in developing organoids and next-generation preclinical models, matching the ethical standards associated with the 3R approach.
4. Tumoroids: A Next-Generation Preclinical Model
Table 2 highlights representative examples of recent progress in the field. Kim et al. demonstrated that lung tumoroids can retain specific histological features of the primary tumor, as well as spontaneous TP53 and EGFR mutations [24]. The closely related concept “tumor-like organoids”, in other words, tumoroids, has spread to numerous laboratories [24,33,34,35,36,37]. All the 3D models cited use primary cells as starting material, with the aim of representing heterogeneity inter-patients, to better understand patient-specific drug responses.
Table 2. Current lung organoid/tumoroid models.
| Primary Tumor Histology, (Mutations) * | Technology Name | Culture Time | Applications | Ref. |
|---|---|---|---|---|
| NS | Patient-derived tumor spheroid (PDS) | 120 days | Mechanistic studies Resistant models Drug screening |
[38] |
| ADK, SCC, LCC | Lung cancer organoids | >1 year | Drug screening | [39] |
| ADK, SCC, LCC | Patient-derived lung cancer organoids | >6 months | Patient-specific drugs screening Living biobank as support to xenograft model |
[24] |
| ADK, SCC | NSCLC organoids | 3 months | Drug screening | [37] |
| ADK, SCC NSCLC (EGFR, KRAS) | Patient-derived organoids models (PDOs) | NS | Genomic analyses Production of treatment response |
[40] |
| NSCLC (EGFR, KRAS) | Patient lung-derived tumoroids (PLDTs) | NS | Drug screening | [41] |
| ADK, SCC, LCC, NSCLC | Lung cancer organoids | NS | Personalized medicine | [36] |
| NS | Patient-derived organoids (PDOs) | 2–3 months | Drug screening Comparative analysis |
[42] |
| ADK | Lung ADK (LADC)-derived organoid model | >50–200 days | Transcriptome analysis Biomarkers discovery Drug screening Living biobank |
[35] |
| ADK and SCC | Lung cancer organoids | 6 days | Drug screening | [43] |
| ADK and SCC primary or metastatic NSCLC | Patient-derived tumoroids (PDTs) | >13 months | Generation of cell lines | [44] |
| ADK | Patient-derived tumoroids (PDTs) | 4 days | Mimic the tumor vascular network PDTs ready to use in microfluidic device for drug screening |
[45] |
* ADK: adenocarcinoma; SCC: squamous cell carcinoma; LCC: large cell carcinoma, NS: non specified.
Among these different 3D models of lung cancer, we can observe a discrepancy in the definitions, that may lead to misunderstanding between the terms of “spheroids”, “organoids” and “tumoroids”. Spheroids are a monotypic cell system that concentrate more in structure than functionality. They retain less of the tissue architecture, compared with organoids, that represents the functionality of a healthy organ [46,47]. Similar to organoids, which are 3D self-organized cultures of organ-derived cells recapitulating major physiological functions, tumoroids are functional surrogates of native tumors. Derived from patients’ tumoral tissues, they have become widely used in understanding molecular pathways of carcinogenesis, in drug development and personalized medicine [35,47]. The nomenclature of the 3D models should be harmonized, and to make this clearer, we will refer to “Patient-derived tumoroids” for tumoral cells derived from patients that structurally and functionally represent the pathology.
Nevertheless, some limitations can be noted, including the lack of stromal and immune cells in the TME. The development of a relevant model for immuno-oncology is still needed [48].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10071677
This entry is offline, you can click here to edit this entry!
