Diabetic kidney disease (DKD) represents an important diabetes (DM) complication associated with significant impacts on morbidity, mortality, and quality of life. The evidence from cardiovascular and kidney outcome trials has dramatically impacted the standard of care for patients with DKD. While agents from the glucagon-like peptide-1 (GLP-1) receptor agonist class are known for their atherosclerotic cardiovascular disease (ASCVD) benefits, growing mechanistic and clinical evidence supports the benefit of GLP-1 receptor agonist therapy on progression of DKD. GLP-1 receptor activation is associated with anti-inflammatory and antifibrotic effects in the kidney, providing a plausible mechanism for kidney protection. Based on currently available clinical trial evidence, guidelines recommend the use of GLP-1 receptor agonists to mitigate ASCVD risk in patients with type 2 diabetes (T2D).
- diabetes
- GLP-1 receptor
- agents
1. Introduction
2. Proposed Mechanisms for Kidney Benefit with GLP-1 Receptor Agonists

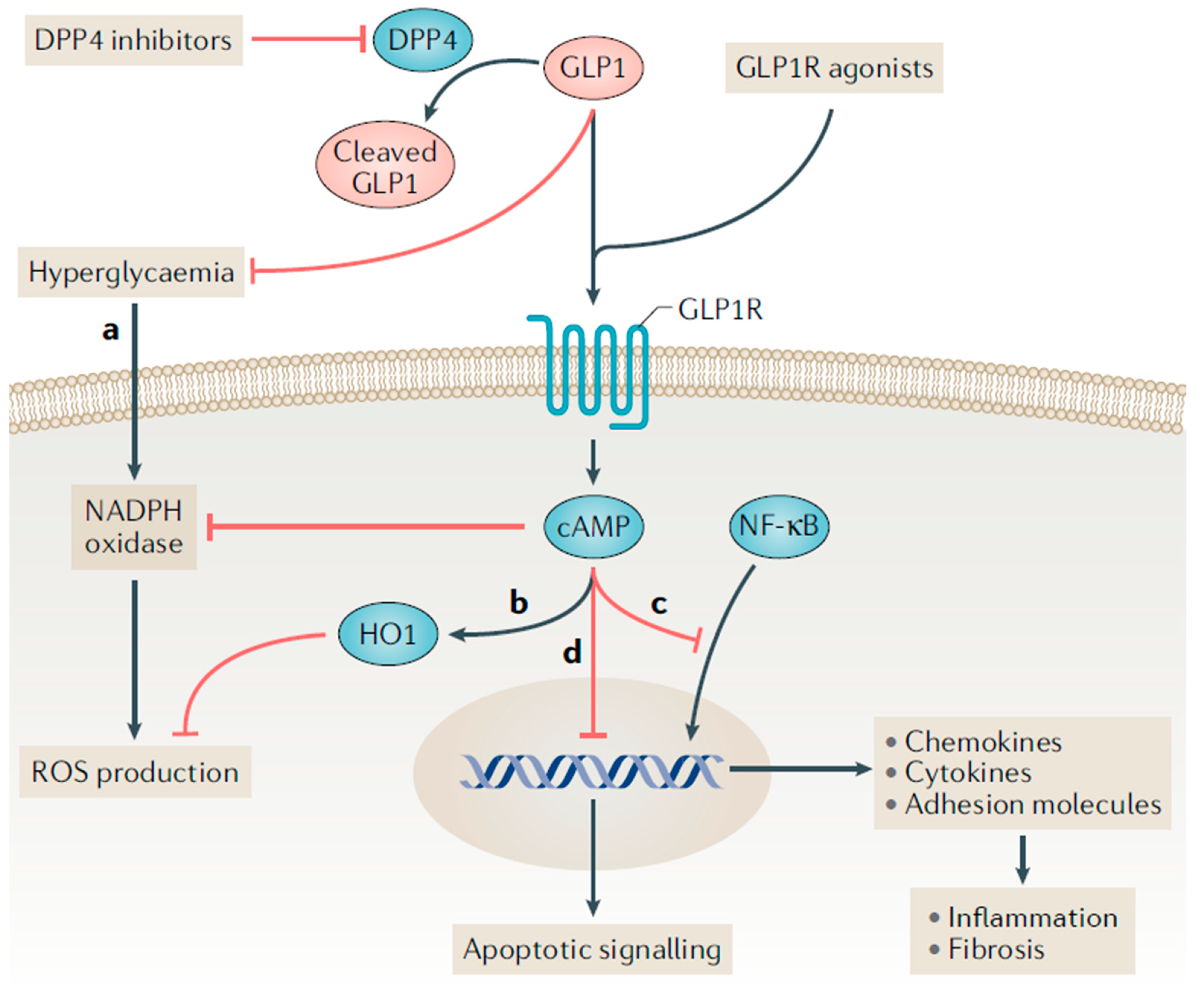
This entry is adapted from the peer-reviewed paper 10.3390/kidneydial2030034
References
- International Diabetes Foundation. IDF Diabetes Atlas: Tenth Edition 2021, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021.
- American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S17–S38.
- American Diabetes Association Professional Practice Committee. 6. Glycemic targets: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S83–S96.
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 487–493.
- American Diabetes Association Professional Practice Committee. 11. Chronic kidney disease and risk management: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S175–S184.
- Rao Kondapally Seshasai, S.; Kaptoge, S.; Thompson, A.; Di Angelantonio, E.; Gao, P.; Sarwar, N.; Whincup, P.H.; Mukamal, K.J.; Gillum, R.F.; Holme, I.; et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 2011, 364, 829–841.
- Afkarian, M.; Sachs, M.C.; Kestenbaum, B.; Hirsch, I.B.; Tuttle, K.R.; Himmelfarb, J.; De Boer, I.H. Kidney disease and increased mortality risk in type 2 diabetes. J. Am. Soc. Nephrol. 2013, 24, 302–308.
- American Diabetes Association Professional Practice Committee. 9. Pharmacological approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S125–S143.
- Trujillo, J.M.; Nuffer, W.; Smith, B.A. GLP-1 receptor agonists: An updated review of head-to-head clinical studies. Ther. Adv. Endocrinol. Metab. 2021, 12, 2042018821997320.
- ADVANCE Collaborative Group; Patel, A.; MacMahon, S.; Chalmers, J.; Neal, B.; Billot, L.; Woodward, M.; Marre, M.; Cooper, M.; Glasziou, P.; et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008, 358, 2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C., Jr.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559.
- Duckworth, W.; Abraira, C.; Moritz, T.; Reda, D.; Emanuele, N.; Reaven, P.D.; Zieve, F.J.; Marks, J.; Davis, S.N.; Hayward, R.; et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 2009, 360, 129–139.
- Pickup, J.C. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004, 27, 813–823.
- Lopez-Candales, A.; Hernandez Burgos, P.M.; Hernandez-Suarez, D.F.; Harris, D. Linking Chronic Inflammation with Cardiovascular Disease: From Normal Aging to the Metabolic Syndrome. J. Nat. Sci. 2017, 3, e341.
- Tuttle, K.R. Linking metabolism and immunology: Diabetic nephropathy is an inflammatory disease. J. Am. Soc. Nephrol. 2005, 16, 1537–1538.
- Pichler, R.; Afkarian, M.; Dieter, B.P.; Tuttle, K.R. Immunity and inflammation in diabetic kidney disease: Translating mechanisms to biomarkers and treatment targets. Am. J. Physiol. Renal. Physiol. 2017, 312, F716–F731.
- Matoba, K.; Takeda, Y.; Nagai, Y.; Kawanami, D.; Utsunomiya, K.; Nishimura, R. Unraveling the Role of Inflammation in the Pathogenesis of Diabetic Kidney Disease. Int. J. Mol. Sci. 2019, 20, 3393.
- Vilsboll, T.; Christensen, M.; Junker, A.E.; Knop, F.K.; Gluud, L.L. Effects of glucagon-like peptide-1 receptor agonists on weight loss: Systematic review and meta-analyses of randomised controlled trials. BMJ 2012, 344, d7771.
- Davies, M.; Færch, L.; Jeppesen, O.K.; Pakseresht, A.; Pedersen, S.D.; Perreault, L.; Rosenstock, J.; Shimomura, I.; Viljoen, A.; Wadden, T.A.; et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): A randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021, 397, 971–984.
- Wadden, T.A.; Bailey, T.S.; Billings, L.K.; Davies, M.; Frias, J.P.; Koroleva, A.; Lingvay, I.; O’Neil, P.M.; Rubino, D.M.; Skovgaard, D.; et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: The STEP 3 randomized clinical trial. JAMA 2021, 325, 1403–1413.
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rubio, M.A.; et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: The STEP 4 randomized clinical trial. JAMA 2021, 325, 1414–1425.
- Wegovy (Semaglutide) Injection ; Novo Nordisk Inc.: Plansboro, NJ, USA, 2021.
- Saxenda (Liraglutide) Injection ; Novo Nordisk Inc.: Plansboro, NJ, USA, 2021.
- Htike, Z.Z.; Zaccardi, F.; Papamargaritis, D.; Webb, D.R.; Khunti, K.; Davies, M.J. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis. Diabetes Obes. Metab. 2017, 19, 524–536.
- Alicic, R.Z.; Johnson, E.J.; Tuttle, K.R. Inflammatory Mechanisms as New Biomarkers and Therapeutic Targets for Diabetic Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 181–191.
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045.
- Yaribeygi, H.; Maleki, M.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. Anti-inflammatory potentials of incretin-based therapies used in the management of diabetes. Life Sci. 2019, 241, 117152.
- Alicic, R.Z.; Cox, E.J.; Neumiller, J.J.; Tuttle, K.R. Incretin drugs in diabetic kidney disease: Biological mechanisms and clinical evidence. Nat. Rev. Nephrol. 2021, 17, 227–244.
- Cox, E.J.; Alicic, R.; Neumiller, J.J.; Tuttle, K. Clinical evidence and proposed mechanisms for cardiovascular and kidney benefits from glucagon-like peptide-1 receptor agonists. US Endocrinol. 2020, 16, 80–87.
- Turner, J.E.; Becker, M.; Mittrucker, H.W.; Panzer, U. Tissue-Resident Lymphocytes in the Kidney. J. Am. Soc. Nephrol. 2018, 29, 389–399.
- Lee, S.B.; Kalluri, R. Mechanistic connection between inflammation and fibrosis. Kidney Int. 2010, 78 (Suppl. S119), S22–S26.
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. Inflammatory processes in renal fibrosis. Nat. Rev. Nephrol. 2014, 10, 493–503.
- Hogan, A.E.; Gaoatswe, G.; Lynch, L.; Corrigan, M.A.; Woods, C.; O’Connell, J.; O’Shea, D. Glucagon-like peptide 1 analogue therapy directly modulates innate immune-mediated inflammation in individuals with type 2 diabetes mellitus. Diabetologia 2014, 57, 781–784.
- Park, C.W.; Kim, H.W.; Ko, S.H.; Lim, J.H.; Ryu, G.R.; Chung, H.W.; Han, S.W.; Shin, S.J.; Bang, B.K.; Breyer, M.D.; et al. Long-term treatment of glucagon-like peptide-1 analog exendin-4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J. Am. Soc. Nephrol. 2007, 18, 1227–1238.
- Kodera, R.; Shikata, K.; Kataoka, H.U.; Takatsuka, T.; Miyamoto, S.; Sasaki, M.; Kajitani, N.; Nishishita, S.; Sarai, K.; Hirota, D.; et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 2011, 54, 965–978.
- Pyke, C.; Heller, R.S.; Kirk, R.K.; Ørskov, C.; Reedtz-Runge, S.; Kaastrup, P.; Hvelplund, A.; Bardram, L.; Calatayud, D. GLP-1 receptor localization in monkey and human tissue: Novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 2014, 155, 1280–1290.
- Bullock, B.P.; Heller, R.S.; Habener, J.F. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology 1996, 137, 2968–2978.
- Erdogdu, Ö.; Nathanson, D.; Sjöholm, Å.; Nyström, T.; Zhang, Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol. Cell. Endocrinol. 2010, 325, 26–35.
- Schlatter, P.; Beglinger, C.; Drewe, J.; Gutmann, H. Glucagon-like peptide 1 receptor expression in primary porcine proximal tubular cells. Regul. Pept. 2017, 141, 120–128.
- Crajoinas, R.O.; Oricchio, F.T.; Pessoa, T.D.; Pacheco, B.P.; Lessa, L.M.; Malnic, G.; Girardi, A.C. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am. J. Physiol.-Ren. Physiol. 2011, 301, F355–F363.
- Moreno, C.; Mistry, M.; Roman, R.J. Renal effects of glucagon-like peptide in rats. Eur. J. Pharmacol. 2002, 434, 163–167.
- Jensen, E.P.; Poulsen, S.S.; Kissow, H.; Holstein-Rathlou, N.H.; Deacon, C.F.; Jensen, B.L.; Holst, J.J.; Sorensen, C.M. Activation of GLP-1 receptors on vascular smooth muscle cells reduces the autoregulatory response in afferent arterioles and increases renal blood flow. Am. J. Physiol.-Ren. Physiol. 2015, 308, F867–F877.
- Muskiet, M.H.; Tonneijck, L.; Smits, M.M.; Kramer, M.H.H.; Diamant, M.; Joles, J.A.; van Raalte, D.H. Acute renal haemodynamic effects of glucagon-like peptide-1 receptor agonist exenatide in healthy overweight men. Diabetes Obes. Metab. 2016, 18, 178–185.
- Gutzwiller, J.P.; Tschopp, S.; Bock, A.; Zehnder, C.E.; Huber, A.R.; Kreyenbuehl, M.; Gutmann, H.; Drewe, J.; Henzen, C.; Goeke, B.; et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J. Clin. Endocrinol. Metab. 2004, 89, 3055–3061.
- Tonneijck, L.; Smits, M.M.; Muskiet, M.H.; Hoekstra, T.; Kramer, M.H.; Danser, A.H.; Diamant, M.; Joles, J.A.; van Raalte, D.H. Acute renal effects of the GLP-1 receptor agonist exenatide in overweight type 2 diabetes patients: A randomised, double-blind, placebo-controlled trial. Diabetologia 2016, 59, 1412–1421.
- Tonneijck, L.; Smits, M.M.; Muskiet, M.H.; Hoekstra, T.; Kramer, M.H.; Danser, A.J.; Ter Wee, P.M.; Diamant, M.; Joles, J.A.; Van Raalte, D.H. Renal effects of DPP-4 inhibitor sitagliptin or GLP-1 receptor agonist liraglutide in overweight patients with type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2016, 39, 2042–2050.
- Tonneijck, L.; Muskiet, M.H.; Blijdorp, C.J.; Smits, M.M.; Twisk, J.W.; Kramer, M.H.; Danser, A.J.; Diamant, M.; Joles, J.A.; Hoorn, E.J.; et al. Renal tubular effects of prolonged therapy with the GLP-1 receptor agonist lixisenatide in patients with type 2 diabetes mellitus. Am. J. Physiol.-Ren. Physiol. 2019, 316, F231–F240.
