Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Horticulture
Floral scents possess high ornamental and economic values to rose production in the floricultural industry. In the past two decades, molecular bases of floral scent production have been studied in the rose as well as their genetic inheritance. Some significant achievements have been acquired, such as the comprehensive rose genome and the finding of a novel geraniol synthase in plants.
- Rosa
- floral scents
- terpenoids
- phenylpropanoids
- benzenoids
- molecular breeding
1. Introduction
The floral scent is one of the most important traits in plants, which is essential to the fertilization of angiosperm plants by attracting and guiding pollinators [1]. Some volatile compounds in floral scents play important roles in plant defense against detrimental animals [2,3]. To humans, floral scent is an important flower trait; it brings mental pleasure. In addition, floral scent provides essential flavor to the food and perfume industries [4,5].
Most floral scent chemicals are produced by three general metabolic pathways, i.e., terpenoids, phenylpropanoids/benzenoids, and fatty-acid derivatives [1,6]. In plants, terpenes are the largest group of floral scent compounds, which are synthesized by two divergent pathways. One is the 2-C-Methyl-D-Erythritol-4-Phosphate (MEP) pathway, which is mainly located in plastids [7] and is responsible for the production of mono- and diterpenes [8]. The other is the mevalonate (MVA) pathway, which is mainly located in the cytosol, endoplasmic reticulum, and peroxisomes [9,10], and is responsible for the production of volatile sesquiterpenes. Phenylpropanoids/benzenoids represent the second-largest class of floral scent compounds [11], which are exclusively derived from L-phenylalanine (L-Phe). Most phenylpropanoids are not volatile unless they are further acylated or methylated at the C9 position, while benzenoids are volatile, which are synthesized through a branch of the phenylpropanoid pathway, i.e., the cinnamic acid pathway [12]. Fatty acid derivatives constitute the third largest class of flower volatiles, including low-molecular-weight alcohols, aldehydes, and lipids. Biosynthesis of volatile fatty acid derivatives is initiated by stereo-specific oxygenation of unsaturated C18 fatty acids, linolenic, and linoleic, and subsequently catalyzed by the lipoxygenase (LOX) pathway [13,14].
For centuries, roses have been one of the most important crops in the floriculture industry [15], and are highly popular worldwide as garden ornamental plants and cut flowers. Floral scent, as an important characteristic, not only improves the ornamental value of roses, but provides essential fragrances and flavorings for spices, perfumes, and cosmetics in related industries. Additionally, rose essential oil, which is composed of floral scent compounds, can be used as an analgesic or antispasmodic [16,17]. However, the development of fragrances in roses has been at a disadvantage in the breeding program, which has focused on the longevity and visual attributes of cut flowers for centuries [18]. The cause for the loss of fragrance in these flowers remains unknown, but it does not seem to conflict with the increase in the vase life [19]. Unraveling the molecular bases of floral scents is not only a fascinating topic in rose biology, but is also helpful for improving the yield of roses in the floriculture industry.
2. Scent Composition of Modern Roses
The evolution of the floral scent is a complex matter, which is influenced by various factors, including the dynamics between biosynthetic pathways and the balanced selection between pollinators and florivores, which may result in relatively rapid evolution [11].
Roses have been cultivated as early as 3000 BC in China, western Asia, and northern Africa [20]. Since the 14th century, when Chinese roses were first introduced to Europe, the Chinese and European roses began to hybridize extensively, forming the genetic basis of the ‘modern rose cultivars’ Rosa hybrida [21]. Although Rosa comprises approximately 200 species, only 8~20 contribute to the genetic make-up of modern roses, including Chinese rose R. chinensis, R. multiflora, and R. gigantea, and European rose R. moschata, R. gallica, R. canina, and R. Phoenicia [22,23]. Chinese and European roses differ greatly in scent composition [24,25,26,27,28,29,30]. Chinese roses principally produce lipid-derived alcohols and esters (such as hexenol and hexenyl acetate) and aromatic compounds (such as 3,5-dimethoxytoluene (DMT) and 1,3,5-trimethoxybenzene (TMB)), whereas the major scent components of European roses are 2-phenylethanol (2-PE) and a number of monoterpenes (such as rose oxide, geraniol, and nerol) (Table 1).
Table 1. Major components of rose floral scents.
| Compound Variety | Compounds | Odor | References |
|---|---|---|---|
| terpenes | β-cubebene | citrus, fruity, radish | [25,31] |
| β-elemene | herbal, waxy, fresh | [26,31] | |
| δ-cadinene | thyme, herbal, woody | [27] | |
| germacrene D | woody, spice | [31] | |
| geraniol | rose-like, sweet | [28,31,32] | |
| citronellol | fresh rosy | [27,28,31] | |
| nerol | lemon-like, floral | [27,28,32] | |
| linalool | citrus and floral | [27,32] | |
| farnesyl acetate | green-floral rose | [27] | |
| geranyl acetate | lavender | [27,31] | |
| citronellyl acetate | fresh, rose, fruity odor | [25,31] | |
| neryl acetate | rose and lavender-like | [25,31] | |
| citral | citrus and lemon | [25,27] | |
| dihydro-β-ionone | violet-like and earthy | [27] | |
| rose oxide | herbal, green floral, earthy | [30] | |
| Phenylpropanoids/ benzenoids |
2-phenylethanol | honey-like | [28,30,31] |
| 2-phenylethyl acetate | sweet, honey, rosy, with a slight yeasty honey note | [27,30,31,32] | |
| 1,3,5-Trimethoxybenzene (TMB) | phenolic spicy, earthy note | [28,33,34] | |
| dimethoxytoluene (DMT) | fresh, earthy, phenolic spicy | [28,33] | |
| benzyl acetate | floral, fruity, sweet, fresh | [27,32] | |
| eugenol | clove, carnation | [28,35] | |
| methyl eugenol | clove, carnation | [27,34] | |
| methyl isoeugenol | clove, carnation, woody | [27,34] | |
| fatty-acid derivatives | cis-3-hexenyl-1-alcohol | fresh and leafy green | [25,28,32] |
| 2-hexenyl acetate | fresh, fruity green | [27,31,32] | |
| cis-3-hexenyl acetate | fresh and leafy green | [27,31] |
Many modern rose flowers have earthy and spicy notes due to the presence of DMT [24,36]. DMT is a product of the phenolic methyl ethers (PME) synthesis pathway and is peculiar to Chinese ancient roses. The DMT synthesis pathway in modern roses is speculated to be obtained from Chinese roses, such as R. gigantean [36]. DMT is the basis of the ‘tea scent’ of modern roses. The tea scent is characterized by a combination of phenolic molecules from both Chinese and European lineages, which is reminiscent of ‘black tea’. It is brought to modern roses through an intermediate group of tea and hybrid tea roses, which are derived from the crosses of R. chinensis and R. gigantea with R. moschata. An array of methoxylated phenolics can be produced by these roses, such as DMT, TMB, methyleugenol, and methylisoeugenol, and a variety of alcohols and esters, such as 2-PE, citronellol, geraniol, 2-phenylethylacetate, and geranyl acetate, as well as mono- and sesquiterpenes (predominantly germacrene D), among which, DMT generally represents up to 90% of the total flower volatiles [25,27,28]. cis-3-hexenyl acetate and cis-3-hexenol are also derived from R. chinensis, which give a leafy green note to modern roses [27].
In modern roses, the main components of rose oil, including linalool, citronellol, nerol, and geraniol, are inherited from ancient European roses [27]. The damask rose (R. damascene) is an important intermediate, which is a progeny of ancient European roses by the crosses between R. gallica and R. Phoenicia. Iran is the center of diversity of the damask rose, from where the original oil-bearing cultivars are transferred to Turkey and Bulgaria [37,38,39,40]. The damask rose produces a floral scent characterized by rose oxide, which is rich in alcohols, such as 2-PE, geraniol, and nerol [28,30,41]. Among the components, 2-PE is a dominant aroma compound with a rose-like odor and characterized by the typical floral scent of modern roses [40].
3. Molecular Research Progress on Rose Scent Biosynthesis
Molecular and genetic approaches, including the candidate gene approach, transcriptomic analysis, and genetic mapping, have been used to identify scent-related genes and to unravel the gene expression network and floral scent inheritance in roses [31,33,42,43,44,45,46]. With these tools and approaches, dozens of scent-related genes have been identified and functionally validated, and many pathways of floral scent production have been uncovered in the rose (Figure 1).
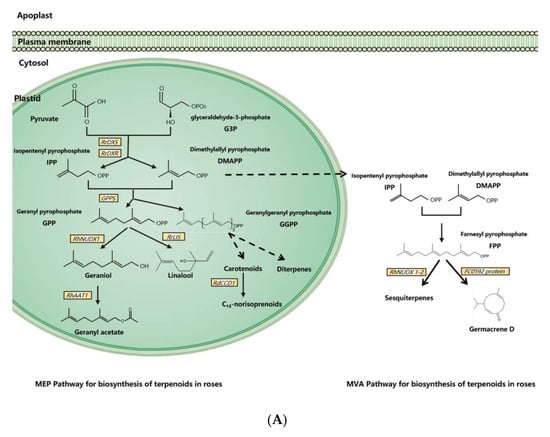
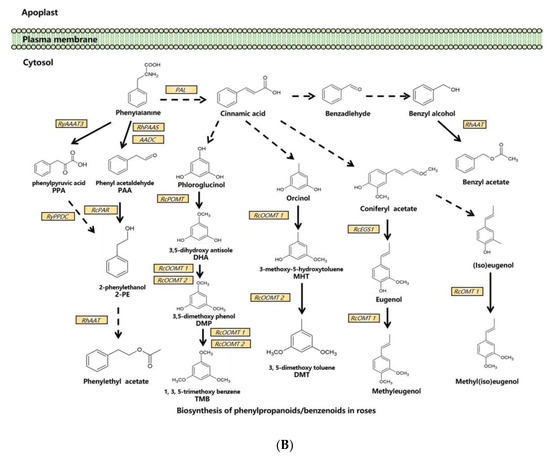
Figure 1. Synthesis pathways of floral scents in roses. (A) The biosynthesis pathway of terpenoids in roses. (B) Biosynthesis pathway of phenylpropanoids/benzenoids in roses. Solid lines indicate established biochemical reactions, and broken lines indicate possible steps. G3P: glyceraldehyde-3-phosphate; DXS: 1-deoxy-D-xylulose-5-phosphate synthase; DXR: 1-deoxy-D-xylulose-5-phosphate reductoisomerase; IPP: isopentenyl pyrophosphate; DMAPP: dimethylallyl pyrophosphate; GPP: geranyl pyrophosphate; GPPS: GPP synthase; GGPP, geranylgeranyl pyrophosphate; NUDX: nudix hydrolase; LIS: linalool synthase; AAT: alcohol acetyltransferase; CCD: carotenoid cleavage (di-)oxygenase; FPP: farnesyl pyrophosphate; PAL: phenylalanine ammonia lyase; PAAS: phenylacetaldehyde synthase; AADC: aromatic amino acid decarboxylase; AAAT: aromatic amino acid aminotransferase; PPA: phenylpyruvic acid; PAA: phenyl acetaldehyde; PAR: phenyl acetaldehyde reductase; PPDC: phenylpyruvate decarboxylase; 2-PE: 2-phenylethanol; POMT: phloroglucinol O-methyltransferase; OMT: O-methyltransferases; OOMT: orcinol O-methyltransferases; MHT: 3-methoxy-5-hydroxytoluene; DMT: 3,5-dimethoxy toluene; DHA: 3,5-dihydroxy antisole; DMP: 3,5-dimethoxy phenol; TMB: 1,3,5-trimethoxy benzene.
3.1. Biosynthesis of Terpenoids in Rose Floral Scents
Dimethylallyl pyrophosphate (DMAPP) and isopentenyl pyrophosphate (IPP) are synthesized from glyceraldehyde-3-phosphate (G3P) and pyruvate by a series of enzymes in plasmids [11]. As an enzyme for the synthesis of DMAPP and IPP, 1-deoxy-D-xylulose-5-phosphate reductoisomerase (RrDXR) is shown to play a key role in the production of volatile monoterpenes in R. rugosa [50]. Geranyl pyrophosphate (GPP) is synthesized from DMAPP and IPP by the MEP pathway in plastids [60]. GPP is a precursor of monoterpenes in plants, from which various monoterpenes are produced by a series of monoterpene synthases. In roses, a novel monoterpene synthase, designated as RhNUDX1, is responsible for geraniol synthesis. Recombinant RhNUDX1 only shows the diphosphohydrolase activity by transforming GPP to geranyl monophosphate (GP) in vitro. However, in a cytosolic context, RhNUDX1 is equivalent to geraniol synthase (GES), which is found in other plants responsible for geraniol production [18]. In rose cultivar Rosa× wichurana, another NUDX1 gene RwNUDX1-2 is involved in the biosynthesis of a group of sesquiterpenoids, especially E,E-farnesol. It is proposed that roses utilize different NUDX1 protein complexes to generate different derivatives [61].
Monoterpene alcohols are normally transformed to acetate esters by alcohol acetyltransferase (AAT), by which the acetyl moiety is transferred from acetyl-CoA to the alcoholic substrate [62,63]. In the rose, an AAT, RhAAT 1, is isolated, which shows limited substrate specificity in vitro. Its preferred substrate is geraniol, while it can also accept other alcohols as substrates, including citronellol, nerol, 1-octanol, 2-PE, and cis-3-hexen-1-ol [32]. Despite the fact that the preferred substrate is geraniol in vitro, the transgenic petunia flower of RhAAT1 mainly produces phenylethyl acetate and phenylmethyl acetate using 2-PE and benzyl alcohol as the substrates. When fed with geraniol or octanol, the transgenic flowers also produce acetates, suggesting its dependence on substrate availability in planta [51].
As a sesquiterpene, germacrene is a common ingredient of rose floral scents and an intermediate in the biosynthesis of other sesquiterpenes [64]. In R. hybrida, a gene (Clone FC0592) for sesquiterpenes has been identified and confirmed to produce germacrene D in an in vitro assay with farnesyl pyrophosphate (FPP) as the substrate [31].
Carotenoids are one class of tetraterpenoids, from which C13-norisoprenoids (such as monoterpenes β-damascenone, α-ionone, and β-ionone) are generated by degradation. A carotenoid cleavage (di-)oxygenase (CCD) gene RdCCD1 was confirmed to be responsible for the accumulation of C13-norisoprenoids in flowers of R. damascene and the cleavage of a variety of carotenoids in in vitro assays [48]. However, as the subclass 4 of CCD genes, RdCCD4 cannot utilize β-carotene as a substrate in in vivo assays, indicating that it is not involved in the production of β-ionone in R. damascene [49].
3.2. Biosynthesis of Phenylpropanoids/Benzenoids in Rose Floral Scents
In one branch pathway of phenylpropanoid synthesis, L-Phe is the direct precursor of 2-PE and its β-D-glucopyranoside (2-PEG) [41]. In roses, L-Phe can be converted into phenyl acetaldehyde (PAA) by both aromatic amino acid decarboxylase (AADC) and phenyl acetaldehyde synthase (PAAS) [52,65], and PAA is subsequently converted to 2-PE by phenyl acetaldehyde reductase (PAR) [53,58]. Another 2-PE biosynthetic pathway via phenylpyruvic acid (PPA) from L-Phe has been reported in roses. The aromatic amino acid aminotransferase (AAAT) catalyzes the production of PPA from L-Phe, and RNAi suppression of the AAAT gene RyAAAT3 decreases 2-PE production in rose protoplasts [54]. In this pathway, phenylpyruvate decarboxylase (RyPPDC) is also shown to be responsible for 2-PE production. However, RyPPDC, as a heat adaptation in the summer, is likely to participate in an alternative principal pathway for rose floral scent production [55].
Multiple branching pathways are involved in the synthesis of rose benzenoids. In the branching pathway for TMB, phloroglucinol O-methyltransferase (POMT) catalyzes the first methylation step of phloroglucinol (PLG) to 3,5-dihdroxyanisole (DHA) in R. chinensis [56]. DHA is subsequently converted to TMB by two orcinol O-methyltransferases, OOMT1 and OOMT2, through two final methylation reactions [28,35]. OOMT1 and OOMT2 are also responsible for DMT synthesis with orcinol as the initial substrate in the first and the second methylation steps, respectively [28,35]. Despite sharing a 96.5% similarity at the amino acid level, OOMT1 and OOMT2 exhibit different substrate specificities in PME biosynthesis. The main sequence difference is the single amino acid polymorphism in the phenolic substrate binding site. OOMT1 is mainly found in Chinese roses instead of European roses. It is speculated that OOMT1 may have evolved from an OOMT2-like gene, and its emergence is a critical step in the evolution of scent production in Chinese roses [36].
OOMTs also catalyze the production of methyleugenol and methyl(iso)eugenol by efficiently methylating eugenol and (iso)eugenol in R. chinensis [34].
In general, the rose floral scent is dominated by terpenoids, phenylpropanoids, and benzenoids. Only a few fatty acid derivatives are involved in rose floral scents. However, no related enzymes or genes have been isolated or characterized from roses to date.
3.3. Transcriptional Regulation of Rose Floral Scent Synthesis
Transcription factors (TFs) have been shown to participate in the coordinated regulation of the scent biosynthetic network [66,67]. Some TFs have been identified for the regulation of floral scents of phenylpropanoids/benzenoids in petunia, including activators of ODO1 [68], EOBI [69,70], EOBII [70,71,72], PH4 [73], and the repressor of PhMYB4 [74]. Recently, only three TFs have been isolated for transcriptional regulation of floral terpenoid production, including GaWRKY1 in Gossypium arboretum [75], MYC2 in Arabidopsis thaliana [76], and PbbHLH4 in Phalaenopsis bellina [77]. Interestingly, some TFs may act upstream of multiple metabolic pathways across terpenoids and phenylpropanoids/benzenoids. When the Arabidopsis transcription factor production of anthocyanin pigment 1 (PAP1) is introduced into the petunia and rose, phenylpropanoid- and terpenoid-derived scent compounds show elevated expressions compared to the control flowers [78,79].
In roses, only one R-type MYB TF, RhMYB1, may play a role in floral scent production, but its function has not been validated [59]. Sun found the expression of NUDX1 is transcriptionally regulated between scented R. chinensis ‘Old blush’ and unscented R.×wichurana, but failed to identify the functionary TFs in the regulation of NUDX1 [80]. In rose petals, the miR156-SPL9 regulatory hub is proposed to orchestrate the production of both colored anthocyanins and certain terpenes, by permitting the complexation of preexisting MYB-bHLH-WD40 proteins [15]. The maximum expression of GDS, which encodes the enzyme catalyzing germacrene D synthesis, is correlated with miR156 activation and with SPL9 downregulation. The miR156-SPL9 regulatory hub might also regulate the expression of terpene synthase genes directly. The absence of the expression of nerolidol synthase (NES) genes is correlated with the downregulation of SPL9 through activation of miR156.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23148014
This entry is offline, you can click here to edit this entry!
