Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Organic
Ionic liquids (ILs) are a new chemical medium and soft functional material developed under the framework of green chemistry and possess many unique properties, such as low melting points, low-to-negligible vapor pressures, excellent solubility, structural designability and high thermal stability.
- ionic liquids
- food analysis
- pesticides
1. Introduction
As an important chemical separation unit operation, the liquid–liquid extraction (LLE) method is widely used in the field of food separation and analysis. LLE involves the partitioning of an analyte(s) between two immiscible liquids (usually aqueous and organic), and partitioning depends on the degree of analyte solubility in the extraction solvent. Therefore, in the traditional LLE, the selection of appropriate extractant is critical. Normally, a large number of organic solvents are used as extractants in order to improve the extraction efficiency. This not only increases solvent recovery costs, but also brings to light environmental and security issues in the use of volatile, flammable, toxic organic solvents. In recent years, it has been a development trend for LLE to choose efficient and green solvents instead of organic solvents with the rise in the green chemical industry. Much attention is paid to very popular ILs. Compared to traditional organic solvents, ILs have the advantages of high melting points, low volatility and excellent thermal stability, which play an important role in LLE [55,56,57]. As ideal alternative extractants, ILs-based LLE has been applied in food separation and analysis. Although LLE is the most commonly used extraction, it is more time-consuming, uses large amounts of toxic organic solvents and is not sensitive enough for trace analysis. Liquid–liquid microextraction (LLME) can effectively avoid the use of an excess amount of organic solvents. However, there are still some shortcomings, such as long extraction time, low sensitivity and poor reproducibility. Thus, the development of effective extraction methods that overcome these drawbacks are very necessary.
At present, the LLME technique is the most widely used in food analysis. In the last few decades, a number of LLME modes and their applications have been developed, making it very difficult to choose a correct mode, let alone choosing an appropriate extractant for a particular application. To recognize this process, LLME can be classified into three major micro solvent extraction modes: single drop microextraction (SDME) [58,59], hollow fiber liquid–liquid microextraction (HF-LLME) [60] and dispersive liquid–liquid microextraction (DLLME) [61,62,63,64]. Among them, DLLME is one of the most common LLME methods. As an improvement of the LLME, DLLME was first proposed by Rezaee and coworkers in 2006 [65]. Since then, massive research achievements related to the application of the DLLME method have been made [66,67]. DLLME involves the distribution of the target analyte between a sample solution and a small volume of extractants. In DLLME, the emulsion system of water/dispersant/extractant is formed by adding micro-upgrade of extractants and milli-upgrade of dispersants to the aqueous phase sample matrix. After centrifugation, the extraction layer can be absorbed and directly injected for analysis. Thus, the mechanism of DLLME is mainly based on the partitioning of an analyte(s) between two immiscible liquids, and partitioning depends on the degree of analyte solubility in the extraction solvent. DLLME is a ternary solvent system in which the disperser solvent serves as a bridge between the sample solution and extractant, due to its excellent solubility/miscibility with most polar and non-polar solvents [68]. Furthermore, the volume of the disperser solvent must be higher than the extractant to obtain satisfactory extraction results. Except for the disperser solvents, syringes are used to inject rapidly the mixture of disperser solvent and extractant, which can offer air-assisted dispersion through the sample solution. DLLME has great application value and extensive prospects due to its simplicity, rapidness, low sample volume, cost effectiveness, high precision, excellent enrichment capacity and recovery for analytes [69]. Recent advancements in DLLME methods are mainly focus on the use of greener solvents to meet the requirements of green analytical chemistry. ILs belong to the class of green solvents, and many researchers have used ILs-based DLLME during the pre-concentration in food samples. Several techniques have been developed and discussed briefly in each of the following sub-classified DLLME modes. Some application examples of IL in DLLME for the extraction of food analysis are shown in Table 1.
Table 1. Some representative applications of ILs in DLLME.
| Matrix | Target Analytes | ILs | Extraction Technique | Recoveries (%) | Reference |
|---|---|---|---|---|---|
| Milk and coffee | Polychlorinated biphenyls and acrylamide |
[C4MIM][Br]; [C8MIM][Br]; [BeBIM][Br]; [BeEOHIM][Br]; [HeOHMIM][Cl] | In situ IL-DLLME | - | [32] |
| Honey | Triazine herbicides | [C6MIM][PF6] | Conventional IL-DLLME | 94.2–103.4% | [50] |
| Chocolates | Nickel and cobalt | [C6MIM][FAP] | Vortex-assisted IL-DLLME | - | [61] |
| Beverage | Parabens | [C8MIM][PF6] | Conventional IL-DLLME | 58.8–89.2% | [70] |
| Honey | Chlorophenol compounds | [C4MIM][NTf2] | In situ DLLME | 91.60–114.33% | [71] |
| Soft noncarbonated vitaminized drink | Red 2G, azorubine, allura red and fast green dyes | TOALS; THADHSS | In situ DLLME | 100% | [72] |
| Honey | Pyrethroid pesticides | [N8881][Tf2N] | Conventional, ultrasound-assisted, and temperature-assisted IL-DLLME | 101.2–103.0% | [73,74] |
| Infant formula milk powders |
sulfonamides | [C2MIM][BF4]; [C4MIM][BF4]; [C4MIM][PF6]; [C6MIM][PF6]; [C8MIM][PF6] |
Microwave-assisted IL-DLLME | 79.8–91.7% | [75] |
| Vegetable | Aryloxyphenoxypropionate herbicides | [C6MIM][PF6]; [C4MIM][PF6]; [C8MIM][PF6] |
Air-assisted IL-DLLME | 76–83% | [76] |
| Juice | Fungicides | [C6MIM][NTF2]; [C8MIM][PF6]; [C6MIM][PF6] | Air-assisted IL-DLLME | 74.9–115.4% | [77] |
| Wine | Sulfonylurea herbicides | [C6MIM][PF6] | Vortex-assisted IL-DLLME | - | [78] |
| Soy milk and soy sauce | Aryloxyphenoxy-propionate herbicides | [(C6)3C14P][NTf2] | Conventional IL-DLLME | - | [79] |
| Vegetable oils |
Triazine herbicides | [C6MIM][FeCl4] | MIL-DLLME | 81.8–114.2% | [80] |
| Oilseeds | Triazine herbicides | [C4MIM][FeCl4] | MSPD-MIL-DLLME | - | [81] |
| Rice | Inorganic selenium | [C4MIM][FeCl4] | MIL-UDSA-DLLME | 94.9–104.8% | [82] |
| Milk and juice | Bisphenols | [C4MIM][PF6]; [C6MIM][PF6]; [C8MIM][PF6]; [C4MIM][BF4]; [C4MIM-SH][Br] |
Ultrasonic-assisted IL-DLLME | 91.6–107.9% | [83] |
| Juice | Triazine herbicides | [C2MIM][BF4]; [C4MIM][BF4]; [C4MIM][PF6]; [C6MIM][PF6]; [C8MIM][PF6] | Microwave-assisted IL-DLLME | 76.7–105.7% | [84] |
| Milk | Sulfonamides | [C2MIM-TEMPO][PF6], [C3MIM-TEMPO][PF6], [C4MIM-TEMPO][PF6]; [C5MIM-TEMPO][PF6] | In situ MIL-DLLME | - | [85] |
| Honey | Neonicotinoid insecticides | [C4MIM][BF4]; [C4MIM][Cl]; [C4MIM][Br]; [C6MIM][Br]; [C8MIM][Br] |
In situ IL-DLLME | 81.0–103.4% | [86] |
| Vegetable oils | Nickel (II) and copper (II) ions | [TBP][PO4] | Conventional IL-DLLME | - | [87] |
| Mango juice, tempe, budu, and canned sardine fish |
Biogenic amines | [C4MIM][PF6] | In situ ultrasonic-assisted IL-DLLME | 70.7–118.4% | [88] |
| Herbal tea | Pyrethroid pesticides | [C6MIM][PF6] | Ultrasound enhanced temperature-assisted IL-DLLME | 74.02–109.01% | [89] |
| Solid bean | Auramine O | [BBIM][Tf2N]; [HHIM][Tf2N] | Air-assisted IL-DLLME | 90% | [90] |
| Vegetable protein drinks | Triazine and phenylurea pesticide | [P4 4 4 12][BF4] | Temperature-assisted IL-DLLME | 81.26–118.42% | [91] |
| Wheat | Aflatoxins | [C6MIM][PF6], [C8MIM][PF6]; [C10MIM][PF6]; [C6MIM][Tf2N]; [C8MIM][Tf2N] | Conventional IL-DLLME | - | [92] |
| Milk | Estrogens | [P6,6,6,14][FeCl4]; [P6,6,6,14]2[MnCl4; [P6,6,6,14]2[CoCl4];[P6,6,6,14]2[NiCl4] | MIL-DLLME | - | [93] |
| Fish | Green and violet dyes | [C8MIM][PF6] | Conventional IL-DLLME | - | [94] |
| Honey | Cr(III) Species | [P6,6,6,14][FeCl4] | MIL-DLLME | - | [95] |
| Wheat | Organophosphorus pesticides | [C8MIM][Tf2N] | Ultrasonic-assisted IL-DLLME | 74.8–115.5% | [96] |
2. Conventional IL-DLLME
In the conventional DLLME method, a dispersive agent is used to form a “dispersed phase” or “emulsion” to improve mass transfer and facilitate the contact between the extraction solvent and the compound to be extracted. However, the extraction solvent spreads over the surface of the sample, leading to difficulty in solvent removal. To overcome this problem, special narrow neck centrifuge tubes and extraction solvents with the melting points below room temperature are recommended. After centrifugation, the centrifuge tube is placed in an ice-water bath to solidify or precipitate the extraction solvent. Very few extraction solvents are appropriately melted to meet the requirements. However, there are many ILs with melting points in the ranges necessary, which prove useful in this technique. As the simplest IL-DLLME method, it only utilizes ILs as extractants instead of organic solvents. The samples containing analytes were extracted/preconcentrated by simply mixing the sample aqueous with the IL and the dispersive solvent. Usually, the dispersive solvents used in this method are organic solvents, especially methanol [95]. This conventional IL-DLLME method was often used for pesticide residue analysis in food samples. As is well-known, herbicides can control the growth of grasses and broadleaf weeds in agricultural field, which are extensively used around the world. However, their residues have been found in the environment and pose a threat to public health problems due to their high toxicity. In consideration of the long-term persistence of herbicides in the environment, it is very likely that they can be introduced to our lives by food during its production. Therefore, an analytical method with high sensitivity is favored by researchers.
As previously indicated, ILs can be used as extraction solvents in DLLME that are also increasingly being employed in food analysis due to their lower toxicity and volatility compared to conventional solvents. However, in the conventional IL-DLLME method, the anion of most ILs used for this purpose is [PF6]−. They should have low solubility in water. Thus, food analysis by IL-DLLME usually requires the use of disperser solvents. In this sense, IL-DLLME was used for the first time to extract multiclass pesticides from different matrices (i.e., bananas, grapes and plums) [97]. In the long term, the use of pesticides such as insecticides, fungicides and herbicides for agriculture can contaminate the environment. Pesticide residues may reach humans through the food chain and cause chronic exposure and long-term toxicity effects. As a kind of natural food, today’s honey is produced in an environment polluted by different sources of contamination, which results in the direct or indirect pollution of honey. Since honey is a complicated matrix containing organic and inorganic constituents, especially saccharides, pre-concentration steps for the extraction and enrichment of analytes are very important to obtain reliable results. Therefore, the IL 1-hexyl-3-methylimidazolium hexafluorophosphate ([C6MIM][PF6]) is often used as an extractant in conventional IL-DLLME combined with high-performance liquid chromatography (HPLC) to detect the triazine herbicides in honey [98]. The extraction procedure is as follows: a mixture of IL and dispersant is rapidly injected into the sample solution. After shaking for 10 min, high recovery and enrichment factor can be obtained under the optimal conditions. On the one hand, the imidazolium cation of the IL is conducive to the formation of the interactions between IL and triazine compounds, mainly including hydrogen bonding, electrostatic forces and π-π interaction. On the other hand, when the anion of the IL is [PF4], it tends to be hydrophobic. Moreover, the hydrophobicity of IL increases with the length of the alkyl chain on the cation. Thus, the selected IL is suitable for the extraction of triazine herbicides, which is beneficial to the separation of extraction phase and raffinate phase.
In addition to imidazolium-based ILs, other types of ILs can also be used as extractants. For example, the IL trihexyl(tetradecyl)phosphonium bistriflamide [(C6)3C14P][NTf2] was employed as the extractant for the analysis of aryloxyphenoxy-propionate herbicides in soy-based foods. The density of the used IL is higher than that of the water. Therefore, the convenience of this work is that it is easy to take the extractant from the bottom of a conical tube due to the low viscosity, very low water solubility and higher density [79]. Meanwhile, compared to ILs with the anion [PF6], the ILs with [NTf2] as the anion are generally more hydrophobic. Even more to the point, a novel IL tetra butyl phosphonium phosphate ionic liquid ([TBP][PO4]) was used as an extraction solvent in DLLME for the preconcentration of nickel (II) and copper (II) ions from vegetable oils [87]. Generally, phosphate ions have strong coordination ability and can form soluble complexes with many metal ions. These ILs with the anion [PO4] are often applied to the extraction of metal ions from the food matrix. Finally, the scheme of conventional IL-DLLME is depicted in Figure 1A.
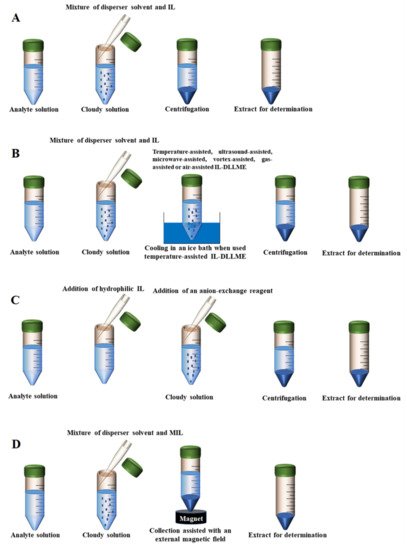
Figure 1. The scheme of different DLLME modes. (A) Conventional IL-DLLME; (B) External assisted IL-DLLME; (C) in situ IL-DLLME; (D) MIL-DLLME.
3. External Assisted IL-DLLME
Although small volumes of liquid samples (1~20 mL) and extraction solvents (0.5~25 µL) are expected in the DLLME method, relatively large volumes (20~50 mL) of water samples, with correspondingly large volumes of extraction solvents and dispersion solvents (200~500 µL) are often used in published DLLME procedures, especially in solvent dispersion-assisted DLLME [99]. Large volumes of samples are used to not only lower the limits of quantification, but also lead to the excessive use of extraction or dispersion solvents, such as acetone, chloroform, acetonitrile and methanol. If these solvents cannot be recycled, they may result in serious environmental pollution. In addition, ILs are usually so viscous that it is necessary to use an additional dispersal method to completely disperse these solvents [100]. IL-DLLME modes not requiring solvent dispersion assistance mainly include the following types: temperature-assisted IL-DLLME, ultrasound-assisted IL-DLLME, microwave-assisted IL-DLLME, vortex-assisted IL-DLLME, gas-assisted IL-DLLME and air-assisted IL-DLLME. Compared with conventional IL-DLLME, these modes can facilitate the mixing of the IL and the sample solution. In the temperature-assisted IL-DLLME method, the heating of a mixture containing the sample solution analytes and the hydrophobic IL is required to ensure the adequate formation of fine droplets. Additionally, the applications of vortex, microwaves, ultrasounds, gas and air are also accompanied by an increase in temperature. Notably, the dispersive solvents are not needed in most cases.
Drug residue is the most serious problem in food safety. As a kind of dietary food for humans, milk may be contaminated by residual drugs, such as oxytetracycline, estrogen and sulfonamides. Massive consumption of milk which contains residual drugs may have a tremendous influence on human health, especially on the endocrine system. There is an urgent need to establish a rapid, effective and highly sensitive method for the detection of contaminants in milk. The most common sample pretreatment methods reported for IL-DLLME are ultrasound-assisted, microwave-assisted and vortex-assisted procedures. In the study reported by Gao et al., two ILs were used in the same ultrasound-assisted IL-DLLME for the extraction of sulfonamides in infant formula milk powder samples, where the hydrophobic ILs ([C4MIM][PF6], [C6MIM][PF6] and [C8MIM][PF6]) served as the extractant and the hydrophilic ILs ([C2MIM][BF4] and [C4MIM][BF4]) served as the disperser solvent [101]. According to the experimental results, the solubility of ILs in water may affect their extraction recoveries when used as extractants. The solubility of [C4MIM][PF6], [C6MIM][PF6] and [C8MIM][PF6] in water was 18.8, 7.5 and 2.0 mg L−1, respectively. The extraction recovery obtained with [C4MIM][PF6] was lower than those obtained with [C6MIM][PF6] and [C8MIM][PF6]. That is, the higher the solubility, the worse the extraction effect. For the selection of the dispersion solvent, the main criterion is its miscibility with the extractant and aqueous solution. The hydrophilic IL is miscible with the hydrophobic IL and water. When the hydrophilic IL is added into the aqueous solution containing hydrophobic IL, a distinct cloudy solution can be formed in a short time. Therefore, hydrophilic ILs are suitable as dispersion solvents when hydrophobic ILs are employed as extractants. Zhang et al. compared the extraction efficiency of conventional, ultrasound-assisted and temperature-assisted IL-DLLME methods for pyrethroid pesticides in honey samples [73]. The results indicated that ultrasound-assisted IL-DLLME had the best extraction efficiency. The experimental conditions, especially ultrasonic time, were examined. Under the optimized conditions, the mixture underwent ultrasonic treatment for only 2 min and was required to obtain high enrichment factors (506~515) and good recoveries (101.2~103.0%) when 1-octyl-3-methylimidazolium hexafluorophosphate [C8MIM][PF6] was used as the extraction solvent and methanol was used as the disperser solvent. For same analytes, Wang and co-workers used microwave-assisted IL-DLLME by using the ILs trioctylmethylammonium bis(trifluoromethylsulfonyl)imide ([N8881][NTf2]) and HPLC to separate and detect them. Similarly, under the optimal microwave extraction conditions of 200 W applied for 60 s, excellent recoveries were achieved compared to DLLME alone [74]. Most recently, a simple and effective method, namely ultrasound-enhanced temperature-controlled IL-DLLME, was developed for the extraction of five pyrethroid residues in herbal tea [84]. The use of ultrasonication and heating was found to improve the ability of the IL ([C6MIM][PF6]) to extract the analytes. The above results have shown that the presence of ILs in externally assisted IL-DLLME methods can significantly improve extraction efficiencies for their applications. However, these studies did not investigate the mechanism between the external energy and IL interactions, which led to higher extraction efficiencies. Based on the ILs structure containing organic cations and inorganic or organic anions, they can efficiently absorb and transfer microwave/ultrasound energy and, consequently, rapidly warm the solvent and the sample, suggesting very high heating rates [102,103,104]. Additionally, external energy (temperature, microwave or ultrasound) can accelerate or improve the dispersion of ILs in the extraction system. Therefore, ILs are very suitable for microwave or ultrasound assisted chemistry.
Additionally, vortex-assisted, gas-assisted and air-assisted IL-DLLME methods are also widely used for the extraction of food samples before analysis [90,91,92,93]. In the vortex-assisted IL-DLLME technique, mechanical dispersion of the extraction solvent can be most easily attained by simply vortexing the sample. For the gas-assisted IL-DLLME method, dispersion is conducted by bubbling fine bubbles of air or injecting an inert gas into the sample/extraction solvent mixture. Additionally, bubbles are created by adding an acid solution to the sample containing a carbonate to produce CO2 or passing compressed gas through the solution. The sample and extraction solvent are rapidly pulled into and forced out of a syringe in air assisted IL-DLLME. In the process, the vacuum is created by withdrawing the syringe plunger to produce the shearing forces and dissolved air bubbles, which will disrupt the surface tension in the water and solvent, leading to the formation of dispersion. An example involving the air assisted IL-DLLME technique used 1-hexyl-3-methylimidazolium hexafluorophosphate ([C6MIM][PF6]) as an extractant to extract and preconcentrate aryloxyphenoxypropionate herbicides from aqueous and vegetable samples [76]. In addition, the viscosities of ILs used in this method should not be too high due to the incompatibilities with the syringe plunger movement. For instance, a novel and simple air-assisted IL-based DLLME technique combined with HPLC was developed for the analysis of five fungicides in juice samples by You and co-workers [77]. In their research, three ILs commonly applied in DLLME, including 1-hexyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C6MIM][NTF2]), 1-octyl-3-methylimidazolium hexafluorophosphate ([C8MIM][PF6]) and 1-hexyl-3-methylimidazolium hexafluorophosphate ([C6MIM][PF6]), were employed as extraction solvents. The results showed that [C6MIM][NTF2] had better extraction efficiency than the other two ILs, which might have resulted from its better solvation capabilities toward the target analytes. Moreover, the viscosity of [C6MIM][NTF2] was lower than the other two ILs, allowing it to more easily form a cloudy solution. The scheme is depicted in Figure 1B.
3. In Situ IL-DLLME
The in situ IL-DLLME, also termed in situ solvent formation microextraction based on ILs, was first proposed by Bahdadi and Shemirani in 2009 [105]. In the in situ IL-DLLME method, a hydrophilic IL is usually utilized as an extractant solvent of analytes in food samples. The hydrophilic IL can be transformed into a hydrophobic IL by a metathesis reaction, in which an anion-exchange reagent is added to facilitate the reaction. During this process, the analytes contained in sample solution are precipitated along with the hydrophobic IL [32]. This method can effectively avoid or reduce the use of the dispersion organic solvents. Moreover, the extraction is completed in a short time. In order to improve the kinetics of the metathesis reaction, vortex, microwaves, ultrasound, or shaking is generally utilized in this method.
The in situ IL-DLLME technique has been applied towards the analysis of many analytes from food samples. For instance, Fan et al. used the in situ IL-DLLME technique as the pretreatment method for the extraction of chlorophenol compounds in honey samples. The IL 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([C4MIM][NTf2]) was employed as the extractant and formed in situ by the addition of hydrophilic IL 1-butyl-3-methylimidazolium tetrafluoroborate ([C4MIM][BF4] and LiNTf2. At first, an appropriate amount of IL [C4MIM][BF4] was added to the honey sample solution and the mixture was manually stirred to ensure complete mixing. Then, an anion exchange reagent (LiNTf2) was quickly added into the above mixture to form fine droplets of [C4MIM][NTf2] [71]. Zhang and co-workers developed a simple, rapid and sensitive in situ IL-DLLME method coupled to headspace gas chromatography (GC) for the analysis of polychlorinated biphenyls and acrylamide at trace levels from food samples [29]. The results indicated that the optimized in situ IL-DLLME method exhibited good analytical precision towards the analytes. Moreover, the matrix-compatibility of the developed method was also investigated by quantitative analysis of acrylamide in brewed coffee samples.
Among the newest publications related to in situ IL-DLLME, particular attention should be paid to the research of Smirnova et al. [72]. In their study, two tetraalkylammonium-based ILs, including tetraoctylammonium N-lauroylsarcosinate (TOALS) and tetrahexylammonium dihexylsulfosuccinate (THADHSS), were obtained in the course of extraction by metathesis reaction occurring upon the mixing of IL cation (tetra-n-octylammonium bromide, i.e., TOABr or tetran-hexylammonium bromide, i.e., THABr) and anion suppliers (sodium N-lauroylsarcosinate, i.e., NaLS or sodium dihexylsulfosuccinate, i.e., NaDHSS) in an aqueous solution containing analytes. The TOALS and THADHSS formed in situ were, respectively, employed as extractants of in situ IL-DLLME to extract different dyes in a food sample. Generally, extraction with ILs formed in situ is carried out without the dispersing agents during the extraction procedure. The extraction efficiency of analytes is mainly affected by the molar ratio of cationic to anionic suppliers. Moreover, mechanism studies indicate that the recovery of anionic dyes for in situ IL formation is closely related to ion exchange of dye anion in organic phase and bromide. In this work, it is supposed that bromide emerged in the organic phase from the precursor, TOABr or THABr. However, such a mechanism may take place even for extraction, using pre-synthesized ILs because bromide may be presented in the product as an admixture. Additionally, an in situ derivatization combined with the ultrasound-assisted IL-DLLME method was developed for the extraction of biogenic amines in foods [88]. The IL 1-butyl-3-methylimidazolium hexafluorophosphate ([C4MIM][PF6]) was used as the extraction solvent and dispersed into the aqueous sample solution as fine droplets by ultrasonication. Results indicated that the developed method was eco-friendly, sensitive, rapid and cost-effective for the determination of biogenic amines in a wide range of food sample matrices. The scheme is depicted in Figure 1C.
4. Magnetic IL-DLLME
The use of ILs in DLLME has boosted the rise of a wide variety of modalities of the technique. However, the ILs are difficult to recycle after use. The high cost of ILs and their unknown toxicity to the environment, either by themselves or by their degradation products, greatly restrict the further application of ILs. Recently, a new idea has emerged involving introducing magnetism into the IL-DLLME. Many DLLME techniques related to magnetism have been reported, named in situ magnetic retrieval-IL-DLLME, magnetic effervescent tablet-assisted IL-DLLME (META-IL-DLLME) and magnetic ionic liquid-based DLLME (MIL-DLLME) [36].
In the in situ magnetic retrieval-IL-DLLME technique, the magnetic nanoparticles (MNPs) of iron oxide (i.e., Fe3O4) are usually used for the retrieval of the in situ created IL [70]. Iron oxides used as magnetic sorbents retrieve the IL that contained the analytes. Then, the analyte is desorbed prior to analysis. Due to the large surface area, MNPs can be easily isolated from a sample solution with the assistance of an external magnetic field. Fast mass transfer resulting from the large interfacial area between the IL and the sample solution can occur [106,107]. Additionally, a dispersing solvent and centrifugation are not often required in this method to extract the analytes and separate the extracting phase. Therefore, the application of MNPs in in situ IL-DLLME is considered as another rapid, simple, effective and eco-friendly microextraction technique, which is proved to be a widely used in the pre-treatment method. In the work reported by Fan et al., the in situ IL-DLLME combined with ultra-small Fe3O4 MNPs was developed to detect pyrethroid pesticides from water samples [108]. The microextraction performance was enhanced by optimizing the experimental conditions, especially anion-exchange reagents. In fact, the proposed method is nanometer-level microextraction with the high sensitivity.
Although the magnetic retrieval of the IL was achieved by the introduction of MNPs, the solvent dispersion and recovery steps do not run synchronously. To solve this problem, a novel a novel IL-DLLME assisted by magnetic effervescent tablets named META-IL-DLLME was proposed by Yang and co-workers. This method combined IL-DLLME with the magnetic retrieval of the extractant. A magnetic effervescent tablet composed of Fe3O4 magnetic nanoparticles, sodium carbonate, sodium dihydrogen phosphate and 1-hexyl-3-methylimidazolium bis(trifluoromethanesulfonimide) was used for extractant dispersion and retrieval. Two ILs, including 1-hexyl-3-methylimidazolium bis(trifluoromethanesulfonimide) ([C6MIM]NTF2) and 1-octyl-3-methylimidazolium bis(trifluoromethanesulfonimide) ([C8MIM][NTF2]), were employed as extractants, which was dispersed by the effervescing agent and then retrieved by ferroferric oxide [109]. The proposed method successfully combines effervescence dispersion and magnetic recovery and reduces some limitations of the classic IL-DLLME. As a result, the dispersion and collection of the green extractant can be completed almost simultaneously, which is timesaving and environmentally friendly. Thus, this method may be a promising sample preparation technique in the field of trace analysis from food samples.
Another innovative approach for the recovery and collection of ILs is to use magnetic ionic liquids (MILs) in DLLME. MILs have an excellent response to an external magnetic field and are easy to recycle [110]. In recent decades, the development of MILs urged a new wave of research due to their unique physical and chemical properties, as well as their potential abundance of opportunities for the development of sample preparation techniques [111,112]. As effective extraction solvents, MILs have attracted interest to replace routine nonmagnetic extraction solvents in DLLME. An example of MIL-DLLME was reported by Wang and co-workers in 2014. A MIL, precisely 1-hexyl-3-methylimidazolium tetrachloroferrate [C6MIM][FeCl4], was used as the extractant for the preconcentration of triazine herbicides from vegetable oils. After extraction, phase separation was rapidly achieved by intrinsic magnetism of the MIL and external magnetic field in this method [80]. Soon afterwards, the same research group proposed a novel matrix solid-phase dispersion combined with magnetic ionic liquid dispersive liquid–liquid microextraction (MSPD-MIL-DLLME) for the extraction of six triazine herbicides from oilseeds. In this method, the MIL 1-butyl-3-methylimidazolium tetrachloroferrate [C4MIM][FeCl4] was employed as the extraction solvent to simplify the extraction procedure by magnetic separation. As a result, the elution and cleanup can be accomplished in one step by this method [81]. Additionally, among the most recent publications devoted to MILs, the research of Wang et al. seems to be very promising. The authors developed a novel and sensitive MIL-based up-and-down-shaker-assisted DLLME for the separation and preconcentration of inorganic selenium from various rice matrixes. As the first microextraction step, the MIL, 1-butyl-3-methylimidazolium tetrachloroferrate ([C4MIM][FeCl4]) was selected as the extractant to extract the complex of Se(IV) and 2,3-diaminonaphthalene from sample aqueous solution with the assistance of an up-and-down-shaker vortex agitator. After microextraction, the MIL containing target analytes was collected at the bottom of the tube by applying an external magnetic field around the test tube. Under the optimal extraction condition, the proposed method provides good precision and reproducibility [82]. Beiraghi et al. developed a new centrifuge-less IL-DLLME technique for the selective preconcentration of trace amounts of potassium from oil samples. In their study, a new task specific magnetic polymeric ionic liquid (TSMPIL) was employed as a chelating and extraction solvent [113]. More important points are that the proposed method provides excellent preconcentration factors in a relatively short extraction time without the need of a complexing agent and a centrifuge step. Most recently, Yao and Du proposed a novel in situ MIL-DLLME method for the simultaneous determination of sulfonamides in milk. In this method, four organic MILs, such as [C2MIM-TEMPO][PF6], [C3MIM-TEMPO][PF6], [C4MIM-TEMPO][PF6] and [C5MIM-TEMPO][PF6], were in situ formed. Compared to other DLLME methods, the extraction process of in situ MIL-DLLME is rapid, completely free of any organic solvents and realizes magnetic-assisted phase separation [85]. Several phosphonium-based MILs, including [P6,6,6,14+][FeCl4−], [P6,6,6,14+]2[MnCl42−], [P6,6,6,14+]2[CoCl42−] and [P6,6,6,14+]2[NiCl42−] combined with DLLME were synthesized and applied for the extraction of six estrogens in milk [93]. Indeed, an increasing number of MIL-DLLME applications have been reported and reviewed in recent years [114,115]. Due to the inherent magnetism, MILs gradually become excellent candidates for performing magnetic separation. Normally, the DLLME promoted by MILs is carried out without the use of centrifugation, decantation, and solidification stages. After extraction, the MIL containing analytes can be harvested by magnetic separation, making it more feasible, straightforward, and throughput the DLLME procedures. Thus, the combination of the MILs and DLLME is a powerful analytical methodology and shows the potentials of practical applications in the treatment of food samples. The scheme of MIL-DLLME is depicted in Figure 1D.
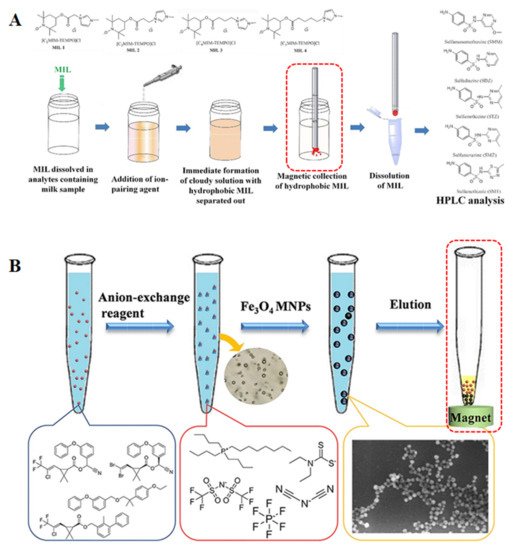
As far as we know, ILs have been successfully employed as both extraction and/or dispersive solvents in several DLLME applications. In the most classical DLLME mode, centrifugation is applied as the last step to separate the extraction solvent from the sample matrix. A microsyringe is often needed to manually collect the final microdroplet containing the preconcentrated analytes for further analysis. In order to increase sample throughput in IL-DLLME, the centrifugation should be avoided. The main strategies are as follows: the use of tailor-made dedicated extraction devices, parallel extraction, magnetic-based separation, semi-automatic or fully automatic flow injection, which involves the use of microfluidic devices and robotic equipment. Among them, magnetic separation is often performed as in the case of DLLME with magnetic retrieval. As shown in Figure 2, after application, both MILs and MNPs can be recovered by magnetic separation without centrifugation. New trends in food sample preparation using DLLME are geared towards employing ILs with greener properties to comply with green analytical chemistry requirements. In fact, ILs are not totally environmentally friendly and pollution-free. Therefore, the recycling and reuse of ILs after extraction is a non-negligible process, which is very crucial for the “green” feature.
In conclusion, LLME techniques have found an important place in sample preparation due to their inherent advantages over conventional procedures. As one of the important forms of LLME, DLLME is a well-established method and commonly used in food analysis. In these methods, microliter volumes are used, and the effect of unintended solvent evaporation is magnified, which results in inaccurate quantitative results. In a word, the volatility of extraction solvents is a great concern because it affects the enrichment factor and repeatability of these methods. Thus, solvents with densities higher than water (ρ > 1) are preferred to overcome volatility issues, as they settle below sample aqueous solution. In the previous literature, more than 40% of reported DLLME methods were carried out with solvents with densities greater than 1.0 g/cm3 [114]. ILs have many of the same advantages as ILs, especially low volatility, indicating that the IL-DLLME method is a very flexible and a promising tool for trace analysis in complex food samples. Its flexibility lies in the ability to be coupled to various agitation methods (vortex, ultrasonic, microwave, etc.) and instrumental detection systems. Currently, in the IL-DLLME method, 1-alkyl-3-methylimidazolium hexafluorophosphate ([CnMIM]PF6) is the IL most commonly employed, with 2–8 carbons in the alkyl chain, being hexyl and octyl the most usual alkyl groups. Their synthesis is relatively simple and they are already commercially available. Additionally, imidazolium-based ILs are conventionally more stable compared to other ILs. Moreover, they can offer a variety of properties, mainly tunable viscosity and solubility, depending on the alkyl-chain length of the imidazolium ring and the counter anion. In addition, the imidazolium cation is easier to form the interactions with target analytes, mainly including hydrogen bonding, electrostatic forces and π-π interaction. On the other hand, such ILs are usually hydrophobic, which is conducive to the separation of extraction phase and raffinate phase. Overall, the main advantages of the developed ILs-based DLLME method are ease of operation, cost-effective, eco-friendly and high extraction factor for target analytes. Nevertheless, some ILs suffer from more or less drawbacks such as toxicity, poor biodegradability and high costs. These incomplete data on their disadvantages over advantages prove the need for continuous interest and development in this area. More efforts are still needed to solve the above issues.
This entry is adapted from the peer-reviewed paper 10.3390/separations9070170
This entry is offline, you can click here to edit this entry!
