Single-targeted chimeric antigen receptor (CAR) T cells tremendously improve outcomes for patients with relapsed/refractory hematological malignancies and are considered a breakthrough therapy. However, over half of treated patients experience relapse or refractory disease, with antigen escape being one of the main contributing mechanisms. Dual-targeting CAR T-cell therapy is being developed to minimize the risk of relapse or refractory disease. Preclinical and clinical data on five categories of dual-targeting CAR T-cell therapies and approximately fifty studies were summarized to offer insights and support the development of dual-targeting CAR T-cell therapy for hematological malignancies. The clinical efficacy (durability and survival) is validated and the safety profiles of dual-targeting CAR T-cell therapy are acceptable, although there is still room for improvement in the bispecific CAR structure. It is one of the best approaches to optimize the bispecific CAR structure by boosting T-cell transduction efficiency and leveraging evidence from preclinical activity and clinical efficacy.
- CAR T-cell therapy
- antigen escape
- dual-targeting
- bispecific CAR
- hematological malignancies
1. Introduction
2. Common Dual CAR Strategies
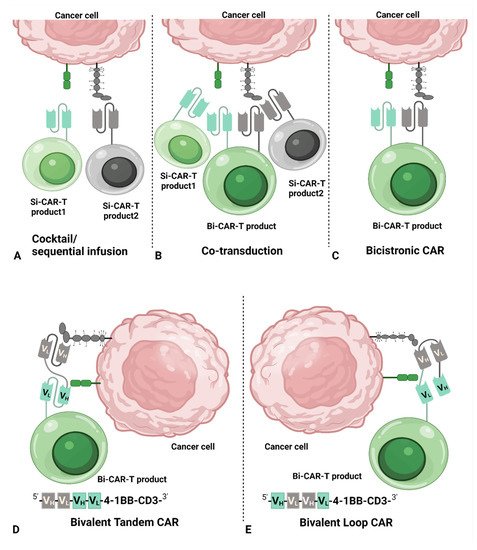
- (i)
-
Cocktail/sequential infusion of two separate Si-CAR T-cell products
- (ii)
-
Heterogeneous cell products of Si-CART and Bi-CART resulted from co-transduction of two separate vectors
- (iii)
-
One Bi-CAR T-cell product with bicistronic CAR (Bicistronic Bi-CART)
- (iv)
-
One Bi-CAR T-cell product with bivalent tandem CAR (Tandem Bi-CART)
- (v)
-
One Bi-CAR T-cell product with bivalent loop CAR (Loop Bi-CART)
| CAR Strategy | Advantages | Disadvantages |
|---|---|---|
| Cocktail/ sequential infusion of two Si-CAR T-cell products |
|
|
| Co-transduction with two Si-CAR vectors |
|
|
| Bicistronic Bi-CART |
|
|
| Bivalent Tandem Bi-CART |
|
|
| Bivalent Loop Bi-CART |
|
|
| Ref.: First Author | Target | Stage | Construct/CAR Strategy | Transduction Efficiency | IL-2 | IFN-ɣ | Cytotoxicity | In Vivo |
|---|---|---|---|---|---|---|---|---|
| Zah [21] | CD19/ CD20 |
Preclinic | Tandem19-20 long (CD19-LinkerG4S-CD20-HingeCH2CH3-CD28tm-4-1BB-CD3-T2A-EGFRt; HingeCH2CH3=229 aa) | NA | ∼0 (CD19 K562); ∼200 pg/Ml (CD20 K562) | ∼1000 pg/Ml (CD19 K562); ∼2200 pg/Ml (CD20 K562) | ∼11% (E:T = 10:1, CD20 K562) | (Only comparing Si-CART with Bi-CART) |
| Tandem20-19 long (CD20-LinkerG4S-CD19-HingeCH2CH3-CD28tm-4-1BB-CD3-T2A-EGFRt; HingeCH2CH3=229 aa) | ∼0 (CD19 K562); ∼10 pg/mL (CD20 K562) | ∼1800 pg/mL (CD19 K562); ∼2000 pg/mL (CD20 K562) | ∼13% (E:T = 10:1, CD20 K562) | |||||
| Tandem19-20 short (CD19-LinkerG4S-CD20-Hinge-CD28tm-4-1BB-CD3-T2A-EGFRt; Hinge=12 aa) | ∼1400 pg/mL (CD19 K562); ∼200 pg/mL (CD20 K562) | ∼3800 pg/mL (CD19 K562); ∼600 pg/mL (CD20 K562) | ∼21% (E:T = 10:1, CD20 K562) | |||||
| Tandem20-19 short (CD20-LinkerG4S-CD19-Hinge-CD28tm-4-1BB-CD3-T2A-EGFRt; Hinge=12 aa) | ∼1500 pg/mL (CD19 K562); ∼100 pg/mL (CD20 K562) | ∼4200 pg/mL (CD19 K562); ∼2100 pg/mL (CD20 K562) | ∼35% (E:T = 10:1, CD20 K562) | |||||
| Tandem20-19 short=ii (CD20-LinkerG4Sx4-CD19-Hinge-CD28tm-41BB-CD3-T2A-EGFRt; Hinge=12 aa) * | ∼150 pg/mL (CD19- Raji); highest in CD19- Raji | ∼2200 pg/mL (CD19- Raji) | ∼60% (E:T = 10:1, CD19- Raji) # | |||||
| Schneider [26] | CD19/CD20 | Preclinic | Tandem1920 (CD19-LinkerGS-CD20-CD8tm-41BB-CD3) | 85% | ∼2000 pg/mL | ∼4000 pg/mL | Tandem2019 > Tandem1920 in various cell lines | Tumor burden 25 days after inoculation: No difference between 2019, 1920 and 19 + 20 co-administration; Survival 25 days after inoculation: 2019 > 19 + 20 co-administration |
| Tandem2019 (CD20-LinkerGS-CD19-CD8tm-41BB-CD3) * | 89% | ∼2200 pg/mL | ∼4500 pg/mL | |||||
| Shah [27] | CD19/ CD20 |
Clinic | Tandem (CD20-CD19-CD8 hinge-4-1BB-CD3) | 7.4–28% | NA | NA | NA | NA |
| Tong [28] | CD19/ CD20 |
Preclinic & Clinic | TanCAR1 (CD19VL-CD19VH-LinkerEA-CD20VH-CD20VL-CD8-4-1BB) | 22% | <3500 pg/mL | ∼1500 pg/mL | <40% | (Only comparing Si-CART with Bi-CART) |
| TanCAR2 (CD19VL-CD19VH-LinkerG4S-CD20VH-CD20VL-CD8-41BB) | 19% | ∼4000 pg/mL | ∼1600 pg/mL | <60% | ||||
| TanCAR3 (CD19VH-CD19VL-LinkerEA-CD20VL-CD20VH-CD8-41BB) | 33% | <3500 pg/mL | ∼1600 pg/mL | <60% | ||||
| TanCAR4 (CD19VH-CD19VL-LinkerG4S-CD20VL-CD20VH-CD8-41BB) | 39% | <3500 pg/mL | ∼1600 pg/mL | <60% | ||||
| TanCAR5 (CD20VL-CD20VH-LinkerEA-CD19VH-CD19VL-CD8-41BB) | 17% | <3500 pg/mL | ∼1500 pg/mL | <60% | ||||
| TanCAR6 (CD20VL-CD20VH-LinkerG4S-CD19VH-CD19VL-CD8-41BB) | 33% | <3500 pg/mL | ∼1500 pg/mL | <60% | ||||
| TanCAR7 (CD20VH-CD20VL-LinkerEA-CD19VL-CD19VH-CD8-41BB) * | 35% (10.1–35.1% in patients’ PBMC) | ∼3500 pg/mL | ∼1600 pg/mL | >60% (Raji) | ||||
| TanCAR8 (CD20VH-CD20VL-LinkerG4S-CD19VL-CD19VH-CD8-41BB) | 33% | <3500 pg/mL | ∼1600 pg/mL | <60% | ||||
| Dai [29] | CD19/ CD22 |
Clinic | TanCAR (CD22m971-LinkerEAAAK-CD19FMC63-CD8-4-1BB-CD3) | 10.32–16.91% | 1700 pg/mL | 4000 pg/mL | NA | |
| Wang [30] | CD19/ CD22 |
Clinic | TanCAR (CD19VL-CD19VH-CD22VL- CD22VH-4-1BB-CD3) | 60.1 (30–75.1)% | NA | NA | NA | NA |
| Zhang [31] | CD19/ CD22 |
Clinic | Loop (CD22VL-CD19VL-CD19VH-CD22VH-4-1BB-CD3) | 20 to ∼78% | NA | NA | NA | NA |
| Cordoba [32] | CD19/ CD22 |
Clinic | Bicistronic | 17.7% (8.6–39.3%) | NA | NA | ∼100% | Tumor burden in CD19- mice: Bi-CAR-T < CD19 Si-CART |
| Qin [33] | CD19/ CD22 |
Preclinic | Co-transduction with two Si-CAR vectors | 23% | NA | NA | NA | Tumor burden 13 days after inoculation: TanCAR1 < TanCAR4; (For LoopCAR, only comparing Si-CART with Bi-CART) |
| TanCAR1 (CD22VH-Linker1G4Sx1-CD22VL-L5G4Sx5-CD19VL-Linker6TKPE-CD19VH-CD8-4-1BB) | 60% | ∼11,000 pg/mL (CD19CD22 K562) | ||||||
| TanCAR2 (CD19VL-Linker6TKPE-CD19VH-Linker5G4Sx5-CD22VH-Linker1G4Sx1-CD22VL-CD8-4-1BB) | 29% | NA | ||||||
| TanCAR3 (CD22VH-Linker6TKPE-CD22VL-Linker5G4Sx5-CD19VL-Linker6TKPE-CD19VH-CD8-4-1BB) | 0% | NA | ||||||
| TanCAR4 (CD22VH-Linker1G4Sx1-CD22VL-Linker4G4Sx4-CD19VL-Linker6TKPE-CD19VH-CD8-4-1BB) | 56% | ∼26,000 pg/mL (CD19CD22 K562) | ||||||
| LoopCAR1 (CD19VL-Linker3G4Sx3-CD22VH-Linker1G4Sx1-CD22VL-Linker3G4Sx3-CD19VH-CD8-4-1BB) | 19% | ∼<2000 pg/mL (CD19CD22 K562) | ||||||
| LoopCAR2 (CD19VL-Linker3G4Sx3A-CD22VH-Linker6TKPE-CD22VL-Linker3G4Sx3B-CD19VH-CD8-4-1BB) | 42% | ∼2800 pg/mL (CD19CD22 K562) | ||||||
| LoopCAR3 (CD19VL-Linker2G4Sx2-CD22VH-Linker6TKPE-CD22VL-Linker2G4Sx2-CD19VH-CD8-491BB) | 24% | ∼25000 pg/mL (CD19CD22 K562) | ||||||
| LoopCAR4 (CD22VH-Linker2G4Sx2-CD19VL-Linker2G4Sx2-CD19VH-Linker2G4Sx2-CD22VL-CD8-4-1BB) | 63% | ∼5000–26,000 pg/mL (CD19CD22 K562) | ||||||
| LoopCAR5 (CD19VL-Linker3G4Sx3C-CD22VH-Linker2G4Sx2-CD22VL-Linker3G4Sx3D-CD19VH-CD8-4-1BB) | 49% | ∼10,000 pg/mL (CD19CD22 K562) | ||||||
| LoopCAR6 (CD19VL-Linker1G4Sx1-CD22VH-Linker6TKPE-CD22VL-Linker1G4Sx1-CD19VH-CD8-4-1BB) * | 82% | ∼22,000 pg/mL (CD19CD22 K562) | ||||||
| Spiegel [34] | CD19/ CD22 |
Clinic | Loop (CD19VH-CD22VL-CD22VH-CD19VL-CD8-4-1BB) * | 60.1% | NA | NA | NA | NA |
| Yang [35] | CD19/ CD22 |
Preclinic & Clinic | Loop GC022C | 67.50% | NA | NA | 75% (1:1) | NA |
| Loop GC022F | 53.60% | NA | NA | 55% (1:1) | NA | |||
| Wang [16] | CD19/ CD22 |
Clinic | Cocktail/Sequential infusion of two Si-CAR-T products with separate Si-CAR vectors | 40.4% ± 18.4% (CAR19); 42.8% ± 19.6% (CAR22) | ∼3500 pg/mL (Raji) | ∼15,000 pg/mL(Raji) | ∼60% CD22;∼50% CD19 (E:T = 10:1; Raji) | Reducing Leukemia burden: infusion of one Si-CAR-T product ∼ co-infusion of two Si-CAR-T products |
| Pan [15] | CD19/ CD22 |
Clinic | Sequential infusion of two Si-CAR-T products with separate Si-CAR vectors | 10.4%∼74.7% (CAR19); 8.3%∼69.8% (CAR22) | NA | NA | NA | NA |
| Ruella [36] | CD19/ CD123 |
Preclinic | Bicistronic | 46% | NA | NA | NA | NA |
| Kang [37] | BCMA/ CD19 |
Preclinic | Tandem (BCMA-CD19-CD8tm-CD28-CD3) | 46% to 55% | NA | NA | NA | NA |
| Mei [38] | BCMA/ CD38 |
Preclinic | Tandem 38BM (CD38-BCMA-CD8-4-1BB-CD3) | 60.1% | NA | BM38 > 38BM | BM38 > 38BM | Survival: BM38 > 38BM |
| Tandem BM38 (BCMA-CD38-CD8-4-1BB-CD3) | 59.4% | |||||||
| Clinic | Tandem BM38 (BCMA-CD38-CD8-4-1BB-CD3) | 12% to 60% | NA | NA | NA | NA | ||
| de Larrea [39] | BCMA/ GPRC5D |
Preclinic | Co-infusion of two Si-CAR-T products with separate Si-CAR vectors | 60% to 70% | NA | NA | Efficacy: Bicistronic = separate Si-CAR vectors > Tandem in BCMA-GPRC5D+ models; Tandem > Bicistronic > separate Si-CAR vectors in BCMA+ GPRC5D+ models |
|
| Bicistronic (BCMA-4-1BB-GPRC5D-41BB) | 60% to 70% | NA | NA | ∼80% (BCMA-/GPRC5D+) | ||||
| Bicistronic (BCMA-4-1BB-GPRC5D-CD28) | 60% to 70% | NA | NA | ∼65% (BCMA-/GPRC5D+) | ||||
| Tandem (GPRC5D-BCMA-4-1BB) | 60% to 70% | NA | NA | ∼65% (BCMA-/GPRC5D+) | ||||
| Globerson [40] | CD138/ CD38 |
Preclinic | Bicistronic (CD138VL-Linker-CD138VH-CD28-CD38VL-CD38VH-CD8-FcγR) | 72% | 2000–3000 pg/mL | ∼90%(E:T = 1:1) | 97.4 days (n = 26) | |
| Dai [41] | CD5/ CD7 |
Preclinic | bicistronic (CD7-4-1BB-CD3-P2A-CD5-4-1BB-CD3-T2A-EGFRt) | 12.4%, 34.2% | NA | NA in concentrations | Tan5-7 =Tan7-5 > bicistronic | Expansion and persistence: Tan5-7 = Tan7-5 > bicistronic |
| Tan5-7 (CD5-Linker-CD7-4-1BB-CD3-T2A-EGFRt) | 58.1%, 62.2% | NA | NA in concentrations | |||||
| Tan7-5 (CD7-Linker-CD5-4-1BB-CD3-T2A-EGFRt) | 49%, 57.6% | NA | NA in concentrations | |||||
| Zah [42] | BCMA/ CS1 (SLAMF7) |
Preclinic | TanCS1-BCMA (CS1-LinkerG4S-BCMA-Hinge-CD28tm-41BB-CD3-T2A-EGFRt, 1122aa) | ∼41% | NA | NA | Si-CART < Bi-CART | Survival: TanCS1-BCMA = TanBCMA-CS1 |
| TanBCMA-CS1 (BCMA-LinkerG4S-CS1-Hinge-CD28tm-41BB-CD3-T2A-EGFRt, 1121aa) | ∼35% | NA | NA | |||||
| bicistronic (CS1-BCMA, 1194aa and 1411aa) | 0.97% to 2.56% | NA | NA | |||||
| Chen [43] | Preclinic | bicistronic (BCMA-CS1) | 19.89% | NA | NA | NA | NA |
3.1. Clinical Efficacy of Dual-Targeting CAR T-Cell Therapy for Hematological Malignancies
Currently, investigations reveal that there are four main mechanisms responsible for relapse due to antigen escape: i) receptor genetic mutations [9,10,48], ii) cell lineage switch [9,10,48], iii) epitope masking [9,10,48], and iv) trogocytosis [49]. The loss of receptors on the membrane is attributed to CD19 mutants in exons 2–5 arising from DNA genetic alteration and alternative RNA splicing, which were detected in 19 patients in the clinics and prevented the recognition of CD19 Si-CAR T cells [50,51]. This mechanism led to the rationale for dual-targeting CAR T-cell therapy that could eliminate CD19-negative malignant B cells, which retain CD20 or CD22. Lineage switching, such as transformation from a lymphoblastic lineage to a myeloid lineage [52,53] or from chronic lymphoblastic leukemia to plasmablastic lymphoma [54], has been identified, resulting in the loss of CD19 and even other B-cell antigens, including CD20 and CD22 expression. To overcome this mechanism, Bi-CAR T cells, targeting unusual antigens other than B-cell antigens, needs to be explored during early discovery. Ruella et al. (2018) reported a rare case of epitope masking caused by unintentionally transducing B cells with CAR construct against CD19; the expression of CAR on the resulting CAR-transduced B cell leukemia cells (CARB) bound to the CD19 epitope of the same CARB, thus, blocking the binding of CD19 Si-CART to CARB [36]. This was caused by CAR T cell manufacturing [36], which cannot be solved by dual-targeting CAR T-cell therapy. In recent years, tumor cells are found to be able to transfer the target antigen to CAR T cells via trogocytosis, resulting in diminished antigen expression on tumor cells and fratricide of CAR T cells [49].Published clinical data on relapse after CD19 Si-CAR-T therapy until 2018 were eloquently summarized by Majzner and Mackall [10]. In four trials, 37 of 220 patients with ALL experienced CD19-negative relapse after treatments with CD19 Si-CAR T-cell therapy [10], with median follow-up ranging from 12 [55], 13.1 [3], 22.6 [56], to 29 months [57], respectively. The level of CD19 expression in NHL after treatments of Si-CAR-T and dual-targeting CAR-T therapy has not been well summarized, possibly due to false negativity since tumor tissue heterogenicity or sampling that can lead to an unreliable conclusion. Some trials reported CD19 expression as negative or positive [4,28,58], while one trial reported percentages in which no specific number was interpreted as CD19-negative or CD19-low expression [27]. In a meta-analysis study on CD19 Si-CAR T-cell therapy, the median progression-free survival (PFS) of subjects with B cell malignancies was 7 months [59], whereas time to CD19-negative or CD19-low relapse has not been well analyzed. The time to CD19-negative or CD19-dim relapse was reported to be around 2–3 months in five patients, 4–6 months in four patients, 8–9 months in five patients, and 14 months in one patient [50,51]. Despite the difficulty in sampling in clinical trials, it may be of value to gather more data on the time to CD19-negative or CD19-low relapse to serve as a parameter for future investigation.
The advantage of dual-targeting CAR T-cell therapy over Si-CAR-T-cell therapy is its ability to decrease antigen escaping of tumor cells. Clinical studies of Si-CAR T-cell therapy have already shown >90% complete response (CR) [30,60], leaving little room for improvement in terms of the initial response to dual-targeting CAR T-cell therapy. Therefore, the expectation for dual-targeting CAR T-cell therapy is not only to improve the durability of the response but also to reinduce the response in patients who relapsed or were refractory after treatments with Si-CAR T-cell therapy. Tables 3 and 4 provide data questioning whether dual-targeting CAR T-cell therapy can override Si-CAR T-cell therapy in durability and long-term clinical benefit, e.g., longer duration of response (DOR) and overall survival (OS). It seems that dual-targeting CAR T-cell therapy has demonstrated better DOR and OS than Si-CAR T-cell in a small number of studies. However, there were no head-to-head studies and, therefore, the conclusions should be interpreted with caution due to differences, such as disposition of patients and supportive care between studies. Similar results were found when comparing the data from different studies. In ALL, 6-month RFS and OS were similar between CD19 Si-CAR T-cell product tisagenlecleucel [3] and CD19/CD22 Bi-CAR T-cell therapy [61]. Likewise, the 12-month PFS for NHL patients was close among tisagenlecleucel in diffuse large B-cell lymphoma [58], brexucabtagene autoleucel in mantle-cell lymphoma [4], and CD19/CD20 Tandem Bi-CAR T-cell therapy in B-cell lymphoma [28]; however, the comparison should be viewed with caution among different clinical entities. In particular, in one trial with a head-to-head comparison of CD19 Si-CAR T cells with CD19/CD22 Bi-CAR T cells, the median leukemia-free survival (LFS) in patients without hematopoietic stem cell transplantation (HSCT) after CAR T-cell treatment was 2 months for CD19 Si-CAR T cell treatment, while LFS was 3 months for CD19/CD22 Bi-CAR T cell treatment, demonstrating a better DOR of Bi-CAR T-cell therapy [30].
Significant differences were observed during the comparison. For example, OS for ALL patients treated with CD19 Si-CAR T cells [3,30] was close to those with CD22 Si-CAR T cells [62], which was shorter than those treated with CD19/CD22 Bi-CART cells [30]. OS for ALL patients treated with cocktail infusion of CD19/CD22 Si-CAR T cells [16] was the longest among those treated with CD19 Si-CAR T cells, CD22 Si-CAR T cells and Bi-CAR T cells. In ALL, CD22 Si-CAR T cells performed poorer than CD19/CD22 Bi-CAR T cells with regard to the 6-month RFS. In NHL, the percentages of PFS and OS in a trial on CD19/CD20 Bi-CAR T-cell threapy [28] were higher than those in a trial with CD19 Si-CAR T-cell therapy [1], despite the ten-fold enrollment in the latter. Whether results from a small sample size can be reproduced in an expanded cohort with head-to-head comparision remains to be determined.
Table 3. Comparison of dual-targeting CD19/CD22 CAR T-cell therapy with the respective Si-CAR T-cell therapy with respect to duration of response, survival, and expansion in ALL.
Ref.: First Author |
Target |
CAR Strategy |
Sample Size (CR Patients) |
Durability |
OS (mon and %) |
In Vivo Expansion |
|
Maude [60] |
CD19 |
One Si-CAR-T product |
30 (27 CR) |
NA |
78% (6-mon OS) |
Median Cmax: 39.8% Cmax: >5000 copies/μg gDNA (>15,000 copies/μg gDNA in 26 pts) |
|
Maude [3] |
CD19 |
One Si-CAR-T product |
75 (61 CR) |
73% (6-mon RFS), 50% (12-mon RFS) |
19.1 mon (median OS), 90% (6-mon OS), 76% (12-mon OS) |
Median Tmax: 10 days Cmax: NA |
|
Grupp [63] |
CD19 |
One Si-CAR-T product |
79 (65 CR) |
66% (18-mon PFS); Responses were ongoing in 29 pts (max DOR, 29 mon and ongoing) |
70% (18-mon OS) |
NA |
|
Shah [62] |
CD22 |
One Si-CAR-T product |
56 (40 CR) |
31.6 mon (EFS), 6 mon (RFS in CR), 11 remain in remission with a median f/u of 9.7 mon |
13.4 mon (median OS) |
Tmax: days 14 ~ 21 Median Cmax: 77% CAR+T cells; 480.5 CAR-T/μL |
|
Wang [30] |
CD19 |
One Si-CAR-T product |
35 (31 CR) |
~2 mon (median LFS in 19 non-HSCT pts) |
~12 mon (median OS in all pts) |
Tmax: day 10.5 Median Cmax: 590.4 CAR-T/μL |
|
Wang [30] |
CD19/CD22 |
One Tandem Bi-CAR-T product |
15 (13 CR) |
~3 mon (median LFS in 13 non-HSCT pts) |
~21 mon (median OS in all pts) |
Tmax: day 9 Median Cmax: 448.2 CAR-T/μL |
|
Wang [16] |
CD19/CD22 |
Cocktail/Sequential infusion of two Si-CAR-T products |
51 (48 CR) |
52.9% (12-mon PFS) 13.6 mon (median PFS) |
62.8% (12-mon OS) 31 mon (median OS) |
Median Tmax and Mean/Median Cmax NA |
|
Pan [15] |
CD19/CD22 |
Cocktail/Sequential infusion of two Si-CAR-T products |
20 (20 CR) |
79.5% (12-mon LFS) |
92.3% (12-mon OS) |
Median Tmax and Mean/Median Cmax NA |
|
Schultz [64] |
CD19/CD22 |
One Bivalent Bi-CAR-T product |
12 (10 CR) |
NA |
92% (9.5-mon median f/u) |
Median Cmax: 11.13% (Dose Level 1) and 29.1% (Dose Level 2) |
|
Dai [29] |
CD19/CD22 |
One Tandem Bi-CAR-T product |
6 (6 CR) |
≥ 5 mon (RFS in 5 CR, 3 ongoing > 8 mon, 1 relapse after 3 mon) |
NA |
Median Tmax and Mean/Median Cmax NA |
|
Yang [35] |
CD19/CD22 |
One Loop Bi-CAR-T product |
16 (>6/7 CR) |
3 mon (median observed time without relapse) |
NA |
Median Cmax: 109,000 copies/μg gDNA |
|
Tang [61] |
CD19/CD22 |
One Tandem Bi-CAR-T product |
22 (22 CR) |
76.9% (6-mon RFS), 67.3% (12-mon RFS) |
94.4% (6-mon OS), 57.2% (12-mon OS) |
NA |
|
Spiegel [34] |
CD19/CD22 |
One Loop Bi-CAR-T product |
17 (15 CR) |
5.8 mon (PFS) |
11.8 mon (median OS) |
Median Cmax : 36 CAR-T/μL 1794 copies/50ng gDNA Tmax: days 10–14 |
|
Cordoba [32] |
CD19/CD22 |
One Bicistronic Bi-CAR-T product |
15 (13 CR) |
48% (6-mon EFS), 32% (12-mon EFS) |
80% (6-mon OS), 60% (12-mon OS) |
Cmax > 30,000 copies/μg DNA Median Tmax: 12 days |
Ref.: First Author |
Target |
CAR Strategy |
Sample Size (CR Patients) |
Durability |
OS (mon and %) |
In Vivo Expansion |
|
Locke [65] |
CD19 |
One Si-CAR-T product |
7 |
3 ongoing CR at 12+mon |
NA |
Median Tmax and Mean/Median Cmax NA |
|
Locke [66] |
CD19 |
One Si-CAR-T product |
108 |
11.1 mon (Median DOR), 44% (12-mon PFS) |
59% (12-mon OS) |
Median Tmax and Mean/Median Cmax NA |
|
Schuster [67], [58] |
CD19 |
One Si-CAR-T product |
93–99 |
Median DOR NR (10 mon-NR), 66% (12-mon PFS) |
49% (12-mon OS) |
Median Tmax and Mean/Median Cmax NA |
|
Jacobson [68] |
CD19 |
One Si-CAR-T product |
109 |
65.6% (18-mon PFS) |
87.4% (18-mon OS) |
Median Tmax: 9 days Median Cmax NA |
|
Abramson [1] |
CD19 |
One Si-CAR-T product |
269 |
6.8 mon (PFS), 51.4% (6-mon PFS), 44.1% (12-mon PFS); Median DOR NR (8.6-NR) |
74.7% (6-mon OS), 57.9% (12-mon OS) |
Median Tmax: 12 days Median Cmax: 23,928.2 copies/μg gDNA |
|
Wang [4] |
CD19 |
One Si-CAR-T product |
60 |
61% (12-mon PFS) |
83% (12-mon OS) |
Median Tmax: 15 days Median Cmax NA |
|
Zhang [69] |
CD20 |
One Si-CAR-T product |
11 |
>6 mon (PFS), 1 CR for 27 mons |
NA |
Median Tmax: ~28 days Median Cmax NA |
|
Tong [28] |
CD19/CD20 |
One Tandem Bi-CAR-T product |
27 |
79% (6-mon PFS), 64% (12-mon PFS) |
82% (6-mon OS), 71% (12-mon OS) |
Mean Cmax: 496 CAR-T/μL Median Tmax: NA |
|
Shah [27] |
CD19/CD20 |
One Tandem Bi-CAR-T product |
22 |
12 CR > 6 mon; 6 CR > 12 mon; 8 CR ongoing |
NA |
Median Tmax and Mean/Median Cmax NA |
|
Tholouli [70] |
CD19/CD22 |
One Bicistronic Bi-CAR-T product |
35 |
4 CR > 10 mon; 4 CR > 5 mon. |
NA |
Median Tmax and Mean/Median Cmax NA |
|
Wang [16] |
CD19/CD22 |
Cocktail/Sequential infusion of two Si-CAR products |
36 |
9.9 mon (median PFS) 50.0% (12-mon PFS) |
18.0 mon (median OS) 55.3% (12-mon OS) |
Median Tmax and Mean/Median Cmax NA |
|
Zhang [31] |
CD19/CD22 |
One Loop Bi-CAR-T product |
32 |
40.0% (12-mon PFS) 66.7% (12-mon PFS in CR at 3 mon) |
63.3% (12-mon OS) 100% (12-mon OS in CR at 3 mon) |
Median Tmax: 12 days Geometric mean Cmax: 286,294.4copies/μg DNA |
|
Spiegel [34] |
CD19/CD22 |
One Loop Bi-CAR-T product |
21 |
3.2 mon (median PFS) |
22.5 mon (median OS) |
Cmax: 36 CAR-T/μL 1794 copies/50ng gDNA Tmax: days 10–14 |
Abbreviations: Cmax, peak of CAR-T/Peak CAR; CR, complete response; EFS, event-free survival; f/u, follow-up; FL follicular lymphoma; gDNA, genomic DNA; LFS, leukemia-free survival; mon, month(s); NA, not available; NR, not reached; non-HSCT, no hematopoietic stem cell transplantation; NHL, non-Hodgkin lymphoma; OS, overall survival; PFS, progression-free survival; pts, patients; Ref., reference; RFS, relapse-free survival; Tmax, the median time to maximum expansion.
Strategies involving CD19/CD20 or CD19/CD22 to design Bi-CAR T-cell therapy are based on the hypothesis that targeting CD20 or CD22 would benefit patients with a loss or reduction in CD19 to overcome antigen escape. After careful extraction of data from published clinical trials, details of CD19 expression and the related efficacy were not identified. Only patients with positive CD19 expression were enrolled in several studies, resulting in limited data concerning whether reinduction of CR can be achieved by targeting CD20 or CD22 after the failure of targeting CD19. There were a small number of patients (exact number undisclosed) with CD19-negative/dim expression after treatments with CD19 Si-CAR T-cell therapy who responded to CD22 Si-CAR T-cell therapy [62]. As shown in Table 5, regardless of the small number to date, targeting CD22 or CD20 with Si-CAR T-cell therapy or Bi-CAR T-cell therapy could have helped over twenty patients with CD19 escape achieved CR, among which four patients remained in CR for more than 6 months with 12-month remission in one patient [14,27,28,71]. In particular, seven patients with prior exposure to CD19 Si-CAR T-cell therapy were enrolled in two studies on CD19/CD20 Bi-CAR T-cell therapy [27,28], among whom five patients managed to achieve CR after Bi-CAR T-cell therapy [28]. There were patients with CD19 antigen escape or prior usage of CD19 Si-CAR T-cell therapy who achieved CR after administration of alternative Bi-CAR T cells targeting CD19/CD20 or CD19/CD22. Therefore, clinical data are available to support the targeting of CD10/CD20 or CD19/CD22 in relapsed patients due to resistance to CD19 Si-CAR T-cell therapy.
Table 5. Outcomes in patients with negative or low-CD19 expression after treatments with CD22 Si-CAR T-cell therapy and CD19/CD20 Bi-CAR T-cell therapy.
|
Ref.: First Author |
Target |
Characteristics of CD19 and CD22 Expression |
Outcome |
|
Fry [71] |
CD22 |
10 ALL pts with CD19neg or CD19dim |
CR: 6/10 *, 4 in CR for ≥ 6 mon; 1 in CR for 12 mon; 1 in CR for 9 mon ongoing |
|
Tong [28] |
CD19/CD20 |
4 NHL pts with CD19neg |
CR: 2/4; PR: 1/4; PD:1/4 |
|
Shah [27] |
CD19/CD20 |
4 NHL pts with < 40% CD19 |
CR: 3/4; PR: 1/4 |
|
Gardner [14] |
CD19/CD22 |
13 ALL pts with diverse expression of CD19 and CD22 |
CR: approximately 9–11/13 |
*: amount of CR/number of CD19neg pts. Abbreviations: ALL, acute lymphoblastic leukemia; CR, complete response; mon, month(s); NA, not available; NR, not reached; neg, negative; NHL, non-Hodgkin lymphoma; OS, overall survival; PR, partial response; PD, progressive disease; Ref., reference; pts, patients.
Overcoming BCMA-negative or BCMA-low escape has been proposed as capable of reversing the resistance of malignant plasma cells to BCMA Si-CAR T-cell therapy [72]. However, after examining more than 200 patients treated with BCMA Si-CAR T-cell therapy in several trials [8,73–79], BCMA-negative cells were detected in only two patients who relapsed. A BCMA-negative plasma cell population was present in one patient [75], while BCMA-negative and BCMA-positive plasma cells were present in the other patient [80]. By comparison, 10 patients relapsed with BCMA-positive expression or BCMA expression returning to the baseline level [78,81]. Therefore, evidence of relapse resulting from loss or down-regulation of BCMA expression derived from current clinical data is scarce, making the evidence of BCMA-negative or BCMA-low escape not as robust as that for CD19.
Despite limited evidence on the failure of response due to BCMA escape among trials with Si-CAR T-cell therapy targeting BCMA, the combination of BCMA CAR and a second CAR is still being explored in MM [11,12], most of which adopt cocktail/sequential infusion of BCMA Si-CAR T cells and other Si-CAR T cells. Bi-CAR T-cell therapy with bivalent CAR recognizing BCMA/CD19 [82] and BCMA/CD38 [38] have advanced into clinics, while BCMA/CS1(SLAMF7) [42,43] and BCMA/GPRC5D Bi-CAR T-cell therapies [39] are forthcoming, as they were found to be effective in preclinical models. BCMA/CD19 Bi-CAR T-cell therapy showed exciting efficacy in a small group of patients [82]. ORR in five patients was 100%, similar to that reported in most early trials with fewer than 20 patients [74,82]. Only one grade 3 cytokine release syndrome (CRS) occurred without the incidence of neurotoxicity (NT). Data on the DOR and PFS for BCMA/CD19 Bi-CAR T-cell therapy are pending, as data only revealed that the response in one patient with stringent CR (sCR) was >4 months [82]. In addition, BCMA/CD38 Bi-CAR T-cell therapy has also received greater attention due to encouraging clinical results in recent years. After treatments with BCMA/CD38 Bi-CAR T-cell therapy, a responder remained in sCR for >12 months, and five of eight patients with sCR maintained sCR at a median follow-up of 9 months, with the 9-month PFS being 75% [83]. Given that the DOR of present BCMA Si-CAR T-cell therapy in MM is far from satisfactory, Bi-CAR T-cell therapy targeting other antigens together with BCMA might warrant further investigation. Although clinical efficacy, such as response and survival, has been reported to be irrelevant to BCMA expression [73,84], the data on the detailed expression pattern over time in responders who relapsed are limited. Meanwhile, it is unclear whether patients with reduced BCMA expression have been enrolled in current trials of Bi-CAR T-cell therapy. It may be worthwhile to design trials that include the tracking of BCMA expression and the related response in the individual patient during the clinical course, especially among patients treated with Bi-CAR T cells after relapse of BCMA Si-CAR T-cell therapy, to identify patients with reduced BCMA expression compared to baseline who could benefit from BCMA Bi-CAR T-cell therapy.
3.2. Expansion of Dual-Targeting CAR T Cells in Hematological Malignancies
In vivo expansion of CAR T cells in patients with hematological malignancies has been summarized in Tables 3 and 4 to assess if there are any obvious differences in cell proliferation between Si-CAR T cells and dual-targeting CAR T cells. Time to maximum expansion is comparable in ALL and NHL between Si-CAR T cells and dual-targeting CAR T cells, ranging from 9 to 14 days. Similarly, maximum expansion of CAR T cells detected by polymerase chain reaction is comparable in NHL between one CD19 Si-CAR T-cell product [1] and two CD19/CD22 Loop Bi-CAR T-cell products [31,34]. Unfortunately, the disclosed data are insufficient to make a full comparison on the maximum expansion of CAR T cells among different dual-targeting CAR T cells and Si-CAR T cells.
In particular, CD22 CAR T cells in Bi-CAR T cells produced by co-transduction of two vectors expanded poorly[14], which was consistent with the findings from the other groups [33]. When simulating the cocktail of CD19 Si-CAR T cells and CD20 Si-CAR T cells by coculture of two Si-CAR T cells with tumor cells, it was found that CD19 Si-CAR T cells are preferentially amplified over CD20 Si-CAR T cells in vitro [21], which could lessen the effect of CD20 Si-CAR T cells in eliminating CD19-negative cells. However, poor Si-CAR T-cell expansion after the second infusion of Si-CAR T cells was not observed in clinical studies on cocktail/sequential infusion of two Si-CAR T-cell products, one with CD22 Si-CAR T cells after CD19 Si-CAR T cells [15] and the other with mainly CD22 Si-CAR T cells prior to infusion of CD19 Si-CAR T cells [16].
3.3. Clinical Safety Profile of Dual-Targeting CAR T-Cell Therapy in the Treatment of Hematological Malignancies
Theoretically, dual-targeting CAR T-cell therapy can be stimulated by two antigens, raising the question of whether stronger activation of CAR T cells in patients than Si-CAR T-cell therapy would occur. Whether dual stimulation in T cells would lead to increased activation of T cells in patients and, therefore, a greater incidence of adverse events than Si-CAR T-cell therapy, requires investigation. Results from early trials enrolling less than 10 subjects to large trials with an enrollment of more than 200 subjects are listed in Tables 6 and 7. CRS and NT are the main focus in the present review.
Table 6. Comparing CRS and NT in dual-targeting CAR T-cell therapy with Si-CAR-T therapy in ALL.
|
Ref.: First Author |
Target |
Enrollment |
CRS Gr1-2 |
CRS Gr3-4 |
NT Gr1-2 |
NT Gr3-4 |
|
Maude [60] |
CD19 |
30 |
22/30 (73%) |
8/30 (27%) |
13/30 (43%) |
None |
|
Maude [3] |
CD19 |
75 |
77% |
~25% |
30/75 (40%) |
None |
|
Wang [30] |
CD19 |
35 |
19/35 (54.3%) |
16/35 (45.7%) |
2/35 (5.7%) |
None |
|
Fry [71] |
CD22 |
21 |
16/21 (76%) |
None |
Mild/transient/mild-moderate >2/21 (10%) |
|
|
Shah [62] |
CD22 |
58 * |
45/58 (90%). |
12/58 (24%) |
minimal/transient |
|
|
Dai [29] |
CD19/CD22 |
6 |
100% |
None |
None |
None |
|
Schultz [64] |
CD19/CD22 |
12 |
9/12 (75%) |
1/12 (8%) |
2/12 (17%) |
1/12 (8%) |
|
Wang [30] |
CD19/CD22 |
15 |
13/15 (86.7%) |
2/15 (13.3%) |
None |
None |
|
Wang [16] |
CD19/CD22 |
51 |
40/51 (78.4%) |
11/51 (21.6%) ∫ |
11/51 (12%) |
1/51 (1%) |
|
Pan [15] |
CD19/CD22 |
20 |
17/20 (85%) |
1/20 (5%) |
3/20 (15%) |
1/20 (5%) |
|
Spiegel [34] |
CD19/CD22 |
17 |
12/17 (70.6%) |
1/17 (5.9%) |
2/17 (11.8%) |
3/17 (17.6%) |
|
Cordoba [32] |
CD19/CD22 |
15 |
12/15 (80%) |
0 |
4/15 (26.7%) |
0 |
* 56 ALL, 1 diffuse large B-cell lymphoma, 1 chronic myeloid leukemia. ∫ denotes Gr 3-5. Abbreviations: ALL, acute lymphoblastic leukemia; CRS, cytokine release syndrome; Gr, grade; NT, neurotoxicity.
Table 7. Comparison of CRS and NT in dual-targeting CAR T-cell therapy with Si-CAR-T therapy in NHL.
|
Ref.: First Author |
Target |
Enrollment |
CRS Gr1-2 |
CRS Gr3-4 |
NT Gr1-2 |
NT Gr3-4 |
|
Locke [65] |
CD19 |
7 |
5/7 (71%) |
1/7 (14%) |
100% |
4/7 (57%) |
|
Jacobson [68] |
CD19 |
148 |
111/148 (75%) |
10/148 (7%) |
59/148 (40%) |
28/148 (19%) |
|
Abramson [85] |
CD19 |
28 |
10/28 (36%) |
None |
5/28 (18%) |
4/28 (14%) |
|
Abramson [1] |
CD19 |
269 |
~40% |
6/269 (2%) |
~30% |
27/269 (10%) |
|
Zhang [69] |
CD20 |
11 |
None severe |
|||
|
Shah [86] |
CD19/CD20 |
11 |
6/11 (55%) |
None |
3/11 (27%) |
None |
|
Shah [27] |
CD19/CD20 |
22 |
14/22 (64%) |
1 (5%) |
7/22 (32%) |
3 (14%) |
|
Tong [28] |
CD19/CD20 |
28 |
~30% |
4/28 (14%) |
~14% |
None |
|
Zhang [87] |
CD19/CD20 |
87 |
61% |
10% |
NA |
2% |
|
Tholouli [70] |
CD19/CD22 |
35 |
12/35 (34%) |
None |
1/35 (3%) |
2/35 (5.7%) |
|
Wang [16] |
CD19/CD22 |
38 |
30 (78.9%) |
8 (21.1%)∫ |
NA |
NA |
|
Zhang [31] |
CD19/CD22 |
32 |
20 (62.5%) |
9 (28.1%) |
1 (3.1%) |
4 (12.5%) |
|
Spiegel [34] |
CD19/CD22 |
21 |
15/21 (71.4%) |
1/21 (4.8%) |
8/21 (38.1%) |
1/21 (4.8%) |
∫ denotes Gr 3-5. Abbreviations: CRS cytokine release syndrome; Gr grade; NT neurotoxicity; NHL, non-Hodgkin lymphoma.
Grade 3–4 CRS was absent in the few studies on both Si-CART [62,71,85] and Bi-CART [29,70,86], while no grade 3-4 NT was reported in trials on both Si-CART [3,30,60,62,71] and Bi-CART [28–30,86]. For studies with available ASTCT scales for CRS and NT, no grade 3–4 CRS was reported in two of nine (22.2%) trials of Si-CAR T-cell therapy or in three of eight (37.5%) trials of Bi-CAR T-cell therapy. Meanwhile, no grade 3–4 NT was reported in three out of seven trials (42.9%) of Si-CAR T-cell therapy or in four of eight (50%) trials on Bi-CAR T-cell therapy. In conclusion, a higher incidence of grade 3–4 CRS and NT occurred in Si-CAR T-cell therapy than in Bi-CAR T-cell therapy.
The incidence of grade 1–2 CRS was similar between Si-CAR T-cell therapy and Bi-CAR T-cell therapy. All patients experienced a grade 1–2 NT in one trial on CD19 Si-CAR T-cell therapy [65], and all patients experienced grade 1–2 CRS in one trial on CD19/CD22 Bi-CAR T-cell therapy [29], both of which enrolled less than 10 subjects. The incidence of grade 1–2 NT for Si-CAR T-cell therapy was higher than that for Bi-CAR T-cell therapy.
Taken together, these findings indicated that Bi-CAR T-cell therapy is less likely to cause severe CRS and NT than Si-CAR T-cell therapy. There seems to be a difference in the safety profile with respect to the occurrence of CRS and NT between Bi-CAR T-cell therapy and Si-CAR T-cell therapy by simply looking at the numbers; however, considering the sample size, different clinical sites, and possible inadequate management of CRS and NT during early development of CAR T-cell therapy, it seems more investigations are needed to confirm this conclusion.
4. Comparison of Different Dual-Targeting CAR T-Cell Therapies
As shown in Figure 2, different dual CAR strategies have been translated into six clinical trials on Tandem Bi-CAR T cells targeting CD19/CD20 [27,28], CD19/CD22 [29,30,64], and BCMA/CD38 [38]; four trials on cocktail/sequential infusion of two separate Si-CAR T cells of targeting CD19 or CD22 [15,16] and BCMA or CD19 [88,89]; three trials on Loop Bi-CAR T cells targeting CD19/CD22 [31,34,90]; two trials on bicistronic Bi-CAR T cells targeting CD19/CD22 [32,70]; and two trials on CD19/CD22 Bi-CAR T cells produced by co-transduction of two separate vectors [14,91]. Because the exact construct (tandem or loop) for DOR and survival of a dual BCMA/CD19 targeted FasT CAR-T GC012F [92] has not been disclosed so far, the trial was not included in this section.
Figure 2. Clinical trials of different dual-targeting CAR T-cell therapies. Different dual CAR strategies have been translated into six clinical trials on Tandem Bi-CART targeting CD19/CD20, CD19/CD22, and BCMA/CD38; four trials on cocktail/sequential infusion of two separate Si-CAR-T products on CD19/CD22 Si-CART and BCMA/CD19 Si-CART; three trials on Loop Bi-CART targeting CD19/CD22; two trials on bicistronic Bi-CART targeting CD19/CD22 ; and two trials on CD19/CD22 Bi-CART produced by co-transduction of two separate vectors. The bar chart was created using Microsoft® Excel® version 2111. Abbreviations: BCMA, B cell maturation antigen; Bi-CART, bispecific chimeric antigen receptor T cells; Bi-CAR-T, bispecific chimeric antigen receptor T-cell; CAR, chimeric antigen receptor; CAR-T, chimeric antigen receptor T cell(s); Si-CART, single-targeted chimeric antigen receptor T cells; Si-CAR-T, single-targeted chimeric antigen receptor T-cell.
No matter how effective dual CAR strategies have been in preclinical models, it may only be considered a true success when it benefits patients in the clinic. Emerging clinical data in 2021 permit a fair comparison of different dual-targeting CAR T-cell therapies possible in patients (Table 8).
When comparing the safety profiles in trials with a sample size of 10 patients, grade 3–4 CRS occurred in 8% to 13.3% of patients given Tandem Bi-CAR T cells [30,64,87], 11% in patients given Bi-CAR T cells produced by co-transduction of two vectors [14], and in none of the patients given bicistronic Bi-CAR T cells [70]. Grade 3–4 NT occurred in 2% to 8% of patients given Tandem Bi-CAR T cells [64,87], 4% in patients given Bi-CAR T cells produced by co-transduction of two vectors [14], and 5.7% in patients given bicistronic Bi-CAR T cells [70]. There was no difference in terms of safety profiles among different dual-targeting CAR T-cell therapies. Hence, it is important to compare the ultimate criteria, DOR, and survival among patients treated with different dual-targeting CAR T-cell therapies.
During the optimization of the CD19/CD22 Bi-CAR T construct, the loop construct was determined to be superior to the tandem one and, thus, moved forward to the clinical phase [33,34]. When comparing the clinical outcomes in ALL, the PFS in the trial using Loop Bi-CAR T cells [34] was longer than the LFS in the trial using Tandem Bi-CAR T cells [30], whereas OS in the former was shorter than that in the latter. It is noteworthy that the spatial structure of CD19 scFv and CD22 scFv in the Bi-CAR was different, although the transduction efficiencies were comparable between studies. Moreover, the number of patients who proceeded to HSCT after Bi-CAR T-cell therapy differed between studies. Similar outcomes were found between Bicistronic Bi-CAR T-cell threapy and Loop Bi-CAR T-cell therapy. Although the transduction efficiencies of the CD19/CD22 Bicistronic Bi-CAR T-cell product [32] were much lower than those of CD19/CD22 Loop Bi-CAR T-cell product [34] in patients with ALL, survival was better in the former. So far, the Loop Bi-CAR T-cell product has not shown superiority over Tandem Bi-CAR T-cell product and Bicistronic Bi-CAR T-cell product to benefit patients with ALL. The comparisons should be considered with caution because they are not derived from head-to-head studies.
Table 8. Comparison of optimization process, transduction efficiencies, DOR, and OS among different dual-targeting CAR T-cell therapies (n > 10; ALL and NHL).
|
Ref.: First Author |
Target |
CAR Strategy |
Optimization Process |
Final CAR Transduction Efficiency (Normal Donor vs. Patient) |
Durability |
OS (mon and %) |
|
Schneider [26], Shah [27] |
CD19/CD20 |
One Tandem Bi-CAR-T product |
2 constructs Change order of CAR19 and CAR20 Final: CD20 scFv distal to 4-1BB |
85%–89% vs. 7.4–28% |
NHL: 12 CR > 6 mon; 6 CR > 12 mon; 8 CR ongoing |
NHL: NA |
|
Tong [28] |
CD19/CD20 |
One Tandem Bi-CAR-T product |
8 constructs Change order of CAR19 and CAR20 Final: CD20 scFv distal to 4-1BB |
35% vs. 10.1%-35.1% |
NHL: 64% (12-mon PFS) |
NHL: 71% (12-mon OS) |
|
Wang [30] |
CD19/CD22 |
One Tandem Bi-CAR-T product |
Undisclosed Final: CD19 scFv distal to 4-1BB |
Undisclosed vs. 60.1 (30–75.1)% |
ALL: ~3 mon (median LFS in 13 non-HSCT pts) |
ALL: ~21 mon (median OS in all pts) |
|
Wang [16] |
CD19/CD22 |
Cocktail/Sequential infusion of two Si-CAR products |
Not required |
52.2% vs. 40.4% ± 18.4% (CAR19); 53.8% vs. 42.8% ± 19.6% (CAR22) |
ALL: 52.9% (12-mon PFS) 13.6 mon (median PFS) NHL: 9.9 mon (median PFS) 50.0% (12-mon PFS) |
ALL: 62.8% (12-mon OS) 31 mon (median OS) NHL: 18.0 mon (median OS) 55.3% (12-mon OS) |
|
Qin [33], Spiegel [34] |
CD19/CD22 |
One Loop Bi-CAR-T product |
Co-transduction vs. 4 Bivalent/Tan constructs vs. 6 Loop constructs Final: CD22 scFv distal to 4-1BB |
82% vs. 60.1% (34.6–75.2%) |
ALL: 5.8 mon (PFS) ~0% (12-mon PFS) NHL: 3.2 mon (PFS) ~25% (12-mon PFS) |
ALL: 11.8 mon (median OS in all pts) ~25% (12-mon OS) NHL: 22.5 mon (median OS) ~64% (12-mon OS) |
|
Zhang [31] |
CD19/CD22 |
One Loop Bi-CAR-T product |
Undisclosed Final: CD19 scFv distal to 4-1BB |
Undisclosed vs. 20-(~)78% |
NHL: 40.0% (12-mon PFS) 66.7% (12-mon PFS in CR at 3 mon) |
NHL: 63.3% (12-mon OS) 100% (12-mon OS in CR at 3 mon) |
|
Cordoba [32] |
CD19/CD22 |
One Bicistronic Bi-CAR-T product |
Binder humanization |
56.8% vs. 17.7% (8.6–39.3%) |
ALL: 32% (12-mon EFS) |
ALL: 60% (12-mon OS) |
Abbreviations: ALL, acute lymphoblastic leukemia; CR, complete response; DOR, duration of response; EFS, event-free survival; f/u, follow-up; LFS, leukemia-free survival; mon, month(s); NA, not available; non-HSCT, no hematopoietic stem cell transplantation; OS, overall survival; PFS, progression-free survival; pts, patients; Ref., reference; RFS, relapse-free survival.
When comparing clinical outcomes on CD19/CD22 Loop Bi-CAR T-cell therapy with different locations of CD19 scFv and CD22 scFv on CAR in NHL subjects , the PFS in the trial on CAR T cells expressing Bi-CAR with CD19 scFv distal to 4-1BB [31] was longer than the one with CD22 scFv distal to 4-1BB [34], both of which have similar OS. Of note, the transduction efficiency of the CD19/CD22 Loop CAR with CD19 scFv distal to the 4-1BB was lower than the lowest one of the CD19/CD22 Loop CAR with CD22 scFv distal to 4-1BB, indicating that optimization may still be needed. Transduction efficiencies of CD19/CD20 Tandem Bi-CAR T-cell product in patients with NHL dropped to nearly one-third of the those in vitro [28]. However, the PFS and OS rates of patients given CD19/CD20 Tandem Bi-CAR T-cell therapy were higher than those with CD19/CD22 Loop Bi-CAR T-cell therapy, despite higher transduction efficiencies observed in the latter [28,34]. Of course, caution is needed to interpret non-head-to-head studies, and the differences may be due to different targets in NHL. Overall, poor transduction efficiencies may not necessarily worsen clinical outcomes, though improving transduction efficiencies still matters in optimization.
Despite a great deal of effort directed at optimizing the Bi-CAR T-cell product, the outcomes have not been able to outperform the simple strategy of the cocktail/sequential infusion of two Si-CAR T-cell products without relentless optimization. For ALL and NHL, the cocktail/sequential infusion of CD19/CD22 Si-CAR T-cell products achieved the longest median OS [16], not only providing convincing clinical evidence of dual-targeting CAR T-cell therapy to improve survival but also dwarfing other time- and cost-consuming trials from preclinic to clinic. It is time for different research groups to collaborate and share details on optimizing Bi-CAR structure and standardize clinical trials to compare different dual-targeting therapeutic strategies in a quest for the ideal construct to produce Bi-CAR. Meanwhile, the cocktail/sequential infusion of two Si-CAR T-cell products still merits clinical application to save the lives of patients with ALL and NHL if the commercialization of other dual CAR strategies requires additional time.
In terms of experience gained from the cocktail/sequential infusion of two Si-CAR T-cell products, the timing of the second infusion warrants further exploration. During the trial on the cocktail infusion of BCMA/CD19 Si-CAR T-cell products, CD19 Si-CAR T and BCMA Si-CAR T-cell product were infused on the same day [89]. During the cocktail/sequential infusion of CD19/CD22 Si-CAR T-cell products, CD22 Si-CAR T cells were infused one day before CD19 Si-CAR T cells [16]. Comparing the cocktail infusion of BCMA/CD19 Si-CAR T-cell products [89] with BCMA/CD38 Tandem Bi-CAR T-cell product [38], the PFS of the former was much shorter than that of the latter. Again, the variance may be attributed to the difference in targets. However, it may be worthwhile to adjust the timing of the cocktail/sequential infusion of two Si-CAR T-cell products to standardize the comparison and investigate the influence of the timing of the infusion on the expansion of two different Si-CAR T-cell products in preclinical models.
5.Challenges and Perspectives
Advancing technologies have made Bi-CAR T-cell therapy readily available; however, three main limitations remain for Bi-CAR T-cell therapy: (1) Bi-CAR T-cell therapy does not address other proposed resistance mechanisms outside of target antigen loss; (2) evidence on the safety profile and in vivo activity of Bi-CAR T cells are insufficient; and (3) increased difficulty in manufacturing since the size of construct is bigger. The specific challenges within Bi-CAR T cell manufacturing are the complicated optimization process to find the suitable vectors for manufacturing, increased inconsistency in batch manufacture of viral vector, low transduction efficiency in Bi-CAR T cells, and high manufacturing failure rate due to the size of the bivalent and bicistronic vector.
The limitations of the review are as follows: (1) For earlier phase studies, PFS/OS estimations are based on small numbers of patients. It should also be taken into account that patients may receive additional treatment after CAR T-cell therapy. (2) Comparison of efficacies of different approaches must take into account wide confidence intervals of PFS/OS estimation. (3) Comparison of safety profiles must take into account differences in scoring systems for toxicity and changes in toxicity management over time.
In conclusion, dual-targeting CAR T-cell therapy has offered another hope for patients in the post era after the use of Si-CAR T-cell therapy. The clinical efficacy has been validated in trials on cocktail/sequential infusion of Si-CAR T-cell products and in a few trials of Bi-CAR T-cell therapy. However, an optimal Bi-CAR structure has not been established. The pooled safety profiles of Bi-CAR T-cell appear better than those of Si-CAR T-cell therapy, with a lower incidence of severe CRS and NT. No apparent effect of 1 + 1 > 2 in terms of DOR, OS, and PFS has been demonstrated in trials on Bi-CAR T-cell therapy, indicating that further optimization is needed. The optimization of the Bi-CAR should focus on finding the right targets for different indications, the appropriate spatial structure of two different scFvs, the suitable linker for scFvs, and the proper transduction efficiencies using patients’ T cells to enhance the efficacy and the persistence of Bi-CAR T cells in patients.
The lack of a magic bullet as Bi-CAR structure calls for collaboration of different research groups to develop solutions to benefit the global community. Models integrating clinical data with preclinical data to predict the optimal Bi-CAR may help design an ideal vector for Bi-CAR introduction. Meanwhile, more trials with Bi-CAR T-cell therapy in patients without prior exposure to Si-CAR T-cell therapy are also needed to compare the two types of CAR T-cell therapy.
Author Contributions: B.X.: Writing—original draft (lead); writing—review and editing (equal). L.Z.: Writing—review and editing (equal). J.Z.: Conceptualization (equal); writing—review and editing (equal). W.W.: Conceptualization (lead); writing—review and editing (lead). All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding.
Institutional Review Board Statement: Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Informed Consent Statement: Not applicable.
Data Availability Statement: Not applicable.
Acknowledgments: Figure 1 was created with BioRender.com. We would like to express our gratitude to Zhenxing Yang, who helped us during the writing of this review.
Conflicts of Interest: Bailu Xie and Wen Wang are employees of IASO Biotherapeutics Co., Ltd. JianFeng Zhou is an unpaid member of the Scientific and Medical Advisory Board of IASO Biotherapeutics Co., Ltd. JianFeng Zhou is among the inventors of patent applications related to the CT103A.
References
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. https://doi.org/10.1016/S0140-6736(20)31366-0.
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. J. Med. 2017, 377, 2531–2544. https://doi.org/10.1056/NEJMoa1707447.
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. Engl. J. Med. 2018, 378, 439–448. https://doi.org/10.1056/NEJMoa1709866.
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. Engl. J. Med. 2020, 382, 1331–1342. https://doi.org/10.1056/NEJMoa1914347.
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. Engl. J. Med. 2021, 384, 705–716. https://doi.org/10.1056/NEJMoa2024850.
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. https://doi.org/10.1016/S0140-6736(21)00933-8.
- Chavez, J.C.; Bachmeier, C.; Kharfan-Dabaja, M.A. CAR T-cell therapy for B-cell lymphomas: Clinical trial results of available products. Adv. Hematol. 2019, 10, 2040620719841581. https://doi.org/10.1177/2040620719841581.
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. Engl. J. Med. 2019, 380, 1726–1737. https://doi.org/10.1056/NEJMoa1817226.
- Lemoine, J.; Ruella, M.; Houot, R. Born to survive: How cancer cells resist CAR T cell therapy. Hematol. Oncol. 2021, 14, 199. https://doi.org/10.1186/s13045-021-01209-9.
- Majzner, R.G.; Mackall, C.L. Tumor Antigen Escape from CAR T-cell Therapy. Cancer Discov. 2018, 8, 1219–1226. https://doi.org/10.1158/2159-8290.CD-18-0442.
- van der Schans, J.J.; van de Donk, N.; Mutis, T. Dual Targeting to Overcome Current Challenges in Multiple Myeloma CAR T-Cell Treatment. Oncol. 2020, 10, 1362. https://doi.org/10.3389/fonc.2020.01362.
- Cronk, R.J.; Zurko, J.; Shah, N.N. Bispecific Chimeric Antigen Receptor T Cell Therapy for B Cell Malignancies and Multiple Myeloma. Cancers 2020, 12, 2523. https://doi.org/10.3390/cancers12092523.
- Kailayangiri, S.; Altvater, B.; Wiebel, M.; Jamitzky, S.; Rossig, C. Overcoming Heterogeneity of Antigen Expression for Effective CAR T Cell Targeting of Cancers. Cancers 2020, 12, 1075. https://doi.org/10.3390/cancers12051075.
- Gardner, R.; Annesley, C.; Wilson, A.; Summers, C.; Narayanaswamy, P.; Wu, V.; Lamble, A.J.; Rivers, J.; Crews, K.; Huang, L.; et al. Efficacy of SCRI-CAR19x22 T cell product in B-ALL and persistence of anti-CD22 activity. Clin. Oncol. 2020, 38, 3035. https://doi.org/10.1200/JCO.2020.38.15_suppl.3035.
- Pan, J.; Zuo, S.; Deng, B.; Xu, X.; Li, C.; Zheng, Q.; Ling, Z.; Song, W.; Xu, J.; Duan, J.; et al. Sequential CD19-22 CAR T therapy induces sustained remission in children with r/r B-ALL. Blood 2020, 135, 387–391. https://doi.org/10.1182/blood.2019003293.
- Wang, N.; Hu, X.; Cao, W.; Li, C.; Xiao, Y.; Cao, Y.; Gu, C.; Zhang, S.; Chen, L.; Cheng, J.; et al. Efficacy and safety of CAR19/22 T-cell cocktail therapy in patients with refractory/relapsed B-cell malignancies. Blood 2020, 135, 17–27. https://doi.org/10.1182/blood.2019000017.
- Pavlasova, G.; Mraz, M. The regulation and function of CD20: An "enigma" of B-cell biology and targeted therapy. Haematologica 2020, 105, 1494–1506. https://doi.org/10.3324/haematol.2019.243543.
- Ghodke, K.; Bibi, A.; Rabade, N.; Patkar, N.; Subramanian, P.G.; Kadam, P.A.; Badrinath, Y.; Ghogale, S.; Gujral, S.; Tembhare, P. CD19 negative precursor B acute lymphoblastic leukemia (B-ALL)-Immunophenotypic challenges in diagnosis and monitoring: A study of three cases. Part B Clin. Cytom. 2017, 92, 315–318. https://doi.org/10.1002/cyto.b.21373.
- Rufener, G.A.; Press, O.W.; Olsen, P.; Lee, S.Y.; Jensen, M.C.; Gopal, A.K.; Pender, B.; Budde, L.E.; Rossow, J.K.; Green, D.J.; et al. Preserved Activity of CD20-Specific Chimeric Antigen Receptor-Expressing T Cells in the Presence of Rituximab. Cancer Immunol. Res. 2016, 4, 509–519. https://doi.org/10.1158/2326-6066.CIR-15-0276.
- Han, X.; Wang, Y.; Wei, J.; Han, W. Multi-antigen-targeted chimeric antigen receptor T cells for cancer therapy. Hematol. Oncol. 2019, 12, 128. https://doi.org/10.1186/s13045-019-0813-7.
- Zah, E.; Lin, M.Y.; Silva-Benedict, A.; Jensen, M.C.; Chen, Y.Y. T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer Immunol. Res. 2016, 4, 498–508. https://doi.org/10.1158/2326-6066.CIR-15-0231.
- Roybal, K.T.; Rupp, L.J.; Morsut, L.; Walker, W.J.; McNally, K.A.; Park, J.S.; Lim, W.A. Precision Tumor Recognition by T Cells With Combinatorial Antigen-Sensing Circuits. Cell 2016, 164, 770–779. https://doi.org/10.1016/j.cell.2016.01.011.
- Srivastava, S.; Salter, A.I.; Liggitt, D.; Yechan-Gunja, S.; Sarvothama, M.; Cooper, K.; Smythe, K.S.; Dudakov, J.A.; Pierce, R.H.; Rader, C.; et al. Logic-Gated ROR1 Chimeric Antigen Receptor Expression Rescues T Cell-Mediated Toxicity to Normal Tissues and Enables Selective Tumor Targeting. Cancer Cell 2019, 35, 489–503.e488. https://doi.org/10.1016/j.ccell.2019.02.003.
- Choe, J.H.; Watchmaker, P.B.; Simic, M.S.; Gilbert, R.D.; Li, A.W.; Krasnow, N.A.; Downey, K.M.; Yu, W.; Carrera, D.A.; Celli, A.; et al. SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma. Transl. Med. 2021, 13, eabe7378. https://doi.org/10.1126/scitranslmed.abe7378.
- Hyrenius-Wittsten, A.; Su, Y.; Park, M.; Garcia, J.M.; Alavi, J.; Perry, N.; Montgomery, G.; Liu, B.; Roybal, K.T. SynNotch CAR circuits enhance solid tumor recognition and promote persistent antitumor activity in mouse models. Transl. Med. 2021, 13, eabd8836. https://doi.org/10.1126/scitranslmed.abd8836.
- Schneider, D.; Xiong, Y.; Wu, D.; Nlle, V.; Schmitz, S.; Haso, W.; Kaiser, A.; Dropulic, B.; Orentas, R.J. A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines. Immunother. Cancer 2017, 5, 42. https://doi.org/10.1186/s40425-017-0246-1.
- Shah, N.N.; Johnson, B.D.; Schneider, D.; Zhu, F.; Szabo, A.; Keever-Taylor, C.A.; Krueger, W.; Worden, A.A.; Kadan, M.J.; Yim, S.; et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: A phase 1 dose escalation and expansion trial. Med. 2020, 26, 1569–1575. https://doi.org/10.1038/s41591-020-1081-3.
- Tong, C.; Zhang, Y.; Liu, Y.; Ji, X.; Zhang, W.; Guo, Y.; Han, X.; Ti, D.; Dai, H.; Wang, C.; et al. Optimized tandem CD19/CD20 CAR-engineered T cells in refractory/relapsed B-cell lymphoma. Blood 2020, 136, 1632–1644. https://doi.org/10.1182/blood.2020005278.
- Dai, H.; Wu, Z.; Jia, H.; Tong, C.; Guo, Y.; Ti, D.; Han, X.; Liu, Y.; Zhang, W.; Wang, C.; et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. Hematol. Oncol 2020, 13, 30. https://doi.org/10.1186/s13045-020-00856-8.
- Wang, Y.; Yang, Y.; Hong, R.; Zhao, H.; Wei, G.; Wu, W.; Xu, H.; Cui, J.; Zhang, Y.; Chang, A.H.; et al. A retrospective comparison of CD19 single and CD19/CD22 bispecific targeted chimeric antigen receptor T cell therapy in patients with relapsed/refractory acute lymphoblastic leukemia. Blood Cancer J. 2020, 10, 105. https://doi.org/10.1038/s41408-020-00371-6.
- Zhang, Y.; Li, J.; Lou, X.; Chen, X.; Yu, Z.; Kang, L.; Chen, J.; Zhou, J.; Zong, X.; Yang, Z.; et al. A Prospective Investigation of Bispecific CD19/22 CAR T Cell Therapy in Patients With Relapsed or Refractory B Cell Non-Hodgkin Lymphoma. Oncol. 2021, 11, 664421. https://doi.org/10.3389/fonc.2021.664421.
- Cordoba, S.; Onuoha, S.; Thomas, S.; Pignataro, D.S.; Hough, R.; Ghorashian, S.; Vora, A.; Bonney, D.; Veys, P.; Rao, K.; et al. CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: A phase 1 trial. Med. 2021, 27, 1797–1805. https://doi.org/10.1038/s41591-021-01497-1.
- Qin, H.; Ramakrishna, S.; Nguyen, S.; Fountaine, T.J.; Ponduri, A.; Stetler-Stevenson, M.; Yuan, C.M.; Haso, W.; Shern, J.F.; Shah, N.N.; et al. Preclinical Development of Bivalent Chimeric Antigen Receptors Targeting Both CD19 and CD22. Ther. Oncolytics 2018, 11, 127–137. https://doi.org/10.1016/j.omto.2018.10.006.
- Spiegel, J.Y.; Patel, S.; Muffly, L.; Hossain, N.M.; Oak, J.; Baird, J.H.; Frank, M.J.; Shiraz, P.; Sahaf, B.; Craig, J.; et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: A phase 1 trial. Med. 2021, 27, 1419–1431. https://doi.org/10.1038/s41591-021-01436-0.
- Yang, J.; Jiang, P.; Zhang, X.; Zhu, X.; Dong, Q.; He, J.; Lin, N.; Wang, Z.; Cai, S.; Ye, X.; et al. Anti-CD19/CD22 Dual CAR-T Therapy for Refractory and Relapsed B-Cell Acute Lymphoblastic Leukemia. Blood 2019, 134, 284. https://doi.org/.
- Ruella, M.; Xu, J.; Barrett, D.M.; Fraietta, J.A.; Reich, T.J.; Ambrose, D.E.; Klichinsky, M.; Shestova, O.; Patel, P.R.; Kulikovskaya, I.; et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Med. 2018, 24, 1499–1503. https://doi.org/10.1038/s41591-018-0201-9.
- Kang, L.; Zhang, J.; Li, M.; Xu, N.; Qi, W.; Tan, J.; Lou, X.; Yu, Z.; Sun, J.; Wang, Z.; et al. Characterization of novel dual tandem CD19/BCMA chimeric antigen receptor T cells to potentially treat multiple myeloma. Res. 2020, 8, 14. https://doi.org/10.1186/s40364-020-00192-6.
- Mei, H.; Li, C.; Jiang, H.; Zhao, X.; Huang, Z.; Jin, D.; Guo, T.; Kou, H.; Liu, L.; Tang, L.; et al. A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma. Hematol. Oncol. 2021, 14, 161. https://doi.org/10.1186/s13045-021-01170-7.
- de Larrea, C.F.; Staehr, M.; Lopez, A.V.; Ng, K.Y.; Chen, Y.; Godfrey, W.D.; Purdon, T.J.; Ponomarev, V.; Wendel, H.G.; Brentjens, R.J.; et al. Defining an Optimal Dual-Targeted CAR T-cell Therapy Approach Simultaneously Targeting BCMA and GPRC5D to Prevent BCMA Escape-Driven Relapse in Multiple Myeloma. Blood Cancer Discov. 2020, 1, 146–154. https://doi.org/10.1158/2643-3230.bcd-20-0020.
- Globerson Levin, A.; Rawet Slobodkin, M.; Waks, T.; Horn, G.; Ninio-Many, L.; Deshet Unger, N.; Ohayon, Y.; Suliman, S.; Cohen, Y.; Tartakovsky, B.; et al. Treatment of Multiple Myeloma Using Chimeric Antigen Receptor T Cells with Dual Specificity. Cancer Immunol. Res. 2020, 8, 1485–1495. https://doi.org/10.1158/2326-6066.CIR-20-0118.
- Dai, Z.; Mu, W.; Zhao, Y.; Cheng, J.; Lin, H.; Ouyang, K.; Jia, X.; Liu, J.; Wei, Q.; Wang, M.; et al. T cells expressing CD5/CD7 bispecific chimeric antigen receptors with fully human heavy-chain-only domains mitigate tumor antigen escape. Signal Transduct. Target. Ther. 2022, 7, 85. https://doi.org/10.1038/s41392-022-00898-z.
- Zah, E.; Nam, E.; Bhuvan, V.; Tran, U.; Ji, B.Y.; Gosliner, S.B.; Wang, X.; Brown, C.E.; Chen, Y.Y. Systematically optimized BCMA/CS1 bispecific CAR-T cells robustly control heterogeneous multiple myeloma. Commun. 2020, 11, 2283. https://doi.org/10.1038/s41467-020-16160-5.
- Chen, K.H.; Wada, M.; Pinz, K.G.; Liu, H.; Shuai, X.; Chen, X.; Yan, L.E.; Petrov, J.C.; Salman, H.; Senzel, L.; et al. A compound chimeric antigen receptor strategy for targeting multiple myeloma. Leukemia 2018, 32, 402–412. https://doi.org/10.1038/leu.2017.302.
- Kumar, M.; Keller, B.; Makalou, N.; Sutton, R.E. Systematic determination of the packaging limit of lentiviral vectors. Gene Ther. 2001, 12, 1893–1905. https://doi.org/10.1089/104303401753153947.
- Sweeney, N.P.; Vink, C.A. The impact of lentiviral vector genome size and producer cell genomic to gag-pol mRNA ratios on packaging efficiency and titre. Ther. Methods Clin. Dev. 2021, 21, 574–584. https://doi.org/10.1016/j.omtm.2021.04.007.
- Bos, T.J.; De Bruyne, E.; Van Lint, S.; Heirman, C.; Vanderkerken, K. Large double copy vectors are functional but show a size-dependent decline in transduction efficiency. Biotechnol. 2010, 150, 37–40. https://doi.org/10.1016/j.jbiotec.2010.07.010.
- Tipanee, J.; VandenDriessche, T.; Chuah, M.K. Transposons: Moving Forward from Preclinical Studies to Clinical Trials. Gene Ther. 2017, 28, 1087–1104. https://doi.org/10.1089/hum.2017.128.
- Cheng, J.; Zhao, L.; Zhang, Y.; Qin, Y.; Guan, Y.; Zhang, T.; Liu, C.; Zhou, J. Understanding the Mechanisms of Resistance to CAR T-Cell Therapy in Malignancies. Oncol. 2019, 9, 1237. https://doi.org/10.3389/fonc.2019.01237.
- Hamieh, M.; Dobrin, A.; Cabriolu, A.; van der Stegen, S.J.C.; Giavridis, T.; Mansilla-Soto, J.; Eyquem, J.; Zhao, Z.; Whitlock, B.M.; Miele, M.M.; et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 2019, 568, 112–116. https://doi.org/10.1038/s41586-019-1054-1.
- Orlando, E.J.; Han, X.; Tribouley, C.; Wood, P.A.; Leary, R.J.; Riester, M.; Levine, J.E.; Qayed, M.; Grupp, S.A.; Boyer, M.; et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Med. 2018, 24, 1504–1506. https://doi.org/10.1038/s41591-018-0146-z.
- Sotillo, E.; Barrett, D.M.; Black, K.L.; Bagashev, A.; Oldridge, D.; Wu, G.; Sussman, R.; Lanauze, C.; Ruella, M.; Gazzara, M.R.; et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov. 2015, 5, 1282–1295. https://doi.org/10.1158/2159-8290.CD-15-1020.
- Turtle, C.J.; Hanafi, L.A.; Berger, C.; Gooley, T.A.; Cherian, S.; Hudecek, M.; Sommermeyer, D.; Melville, K.; Pender, B.; Budiarto, T.M.; et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. Clin. Investig. 2016, 126, 2123–2138. https://doi.org/10.1172/JCI85309.
- Gardner, R.; Wu, D.; Cherian, S.; Fang, M.; Hanafi, L.A.; Finney, O.; Smithers, H.; Jensen, M.C.; Riddell, S.R.; Maloney, D.G.; et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood 2016, 127, 2406–2410. https://doi.org/10.1182/blood-2015-08-665547.
- Evans, A.G.; Rothberg, P.G.; Burack, W.R.; Huntington, S.F.; Porter, D.L.; Friedberg, J.W.; Liesveld, J.L. Evolution to plasmablastic lymphoma evades CD19-directed chimeric antigen receptor T cells. J. Haematol. 2015, 171, 205–209. https://doi.org/10.1111/bjh.13562.
- Maude, S.L.; Teachey, D.T.; Rheingold, S.R.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Barker, C.S.; Callahan, C.; Noelle, V.F.; Nazimuddin, F. Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL. Clin. Oncol. 2016, 34, 3011. https://doi.org/10.1200/JCO.2016.34.15_suppl.3011.
- Lee, D.W.; Stetler-Stevenson, M.; Yuan, C.M.; Shah, N.N.; Delbrook, C.; Yates, B.; Zhang, H.; Ling Zhang, P.; James, N. Kochenderfer, M.; et al. Long-Term Outcomes Following CD19 CAR T Cell Therapy for B-ALL Are Superior in Patients Receiving a Fludarabine/Cyclophosphamide Preparative Regimen and Post-CAR Hematopoietic Stem Cell Transplantation. Blood 2016, 128, 218. https://doi.org/10.1182/blood.V128.22.218.218.
- Park, J.H.; Riviere, I.; Gonen, M.; Wang, X.; Senechal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. Engl. J. Med. 2018, 378, 449–459. https://doi.org/10.1056/NEJMoa1709919.
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jager, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Engl. J. Med. 2019, 380, 45–56. https://doi.org/10.1056/NEJMoa1804980.
- Zhang, T.; Cao, L.; Xie, J.; Shi, N.; Zhang, Z.; Luo, Z.; Yue, D.; Zhang, Z.; Wang, L.; Han, W.; et al. Efficiency of CD19 chimeric antigen receptor-modified T cells for treatment of B cell malignancies in phase I clinical trials: A meta-analysis. Oncotarget 2015, 6, 33961–33971. https://doi.org/10.18632/oncotarget.5582.
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. Engl. J. Med. 2014, 371, 1507–1517. https://doi.org/10.1056/NEJMoa1407222.
- Tang, X.; Kang, L.; Qi, W.; Cui, W.; Dai, H.; Li, Z.; Yin, J.; Qu, C.; Xu, T.; Zhu, X.; et al. Tandem CAR T Cells Targeting CD19 and CD22 Is a Safe and Highly Efficacious Treatment for Relapse/ Refractory ALL Patients. Blood 2019, 134, 1338. https://doi.org/.10.1182/blood-2019-127890.
- Shah, N.N.; Highfill, S.L.; Shalabi, H.; Yates, B.; Jin, J.; Wolters, P.L.; Ombrello, A.; Steinberg, S.M.; Martin, S.; Delbrook, C.; et al. CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: Updated Results From a Phase I Anti-CD22 CAR T-Cell Trial. Clin. Oncol. 2020, 38, 1938–1950. https://doi.org/10.1200/JCO.19.03279.
- Grupp, S.A.; Maude, S.L.; Rives, S.; Baruchel, A.; Boyer, M.W.; Bittencourt, H.; Bader, P.; Büchner, J.; Laetsch, T.W.; Stefanski, H.; et al. Updated Analysis of the Efficacy and Safety of Tisagenlecleucel in Pediatric and Young Adult Patients with Relapsed/Refractory (r/r) Acute Lymphoblastic Leukemia. Blood 2018, 132, 895. https://doi.org/10.1182/blood-2018-99-112599.
- Schultz, L.M.; Muffly, L.S.; Spiegel, J.Y.; Ramakrishna, S.; Hossain, N.; Baggott, C.; Sahaf, B.; Patel, S.; Craig, J.; Yoon, J.; et al. Phase I Trial Using CD19/CD22 Bispecific CAR T Cells in Pediatric and Adult Acute Lymphoblastic Leukemia (ALL). Blood 2019, 134, 744. https://doi.org/10.1182/blood-2019-129411.
- Locke, F.L.; Neelapu, S.S.; Bartlett, N.L.; Siddiqi, T.; Chavez, J.C.; Hosing, C.M.; Ghobadi, A.; Budde, L.E.; Bot, A.; Rossi, J.M.; et al. Phase 1 Results of ZUMA-1: A Multicenter Study of KTE-C19 Anti-CD19 CAR T Cell Therapy in Refractory Aggressive Lymphoma. Ther. 2017, 25, 285–295. https://doi.org/10.1016/j.ymthe.2016.10.020.
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019, 20, 31–42. https://doi.org/10.1016/S1470-2045(18)30864-7.
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Borchmann, P.; Jager, U.; Waller, E.K.; Holte, H.; McGuirk, J.P.; Jaglowski, S.; Andreadis, C.; et al. Sustained Disease Control for Adult Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma: An Updated Analysis of Juliet, a Global Pivotal Phase 2 Trial of Tisagenlecleucel. Blood 2018, 132, 1684. https://doi.org/10.1016/S1470-2045(21)00591-X.
- Jacobson, C.A.; Chavez, J.C.; Sehgal, A.R.; William, B.M.; Munoz, J.; Salles, G.; Munshi, P.N.; Casulo, C.; Maloney, D.G.; de Vos, S.; et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): A single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022, 23, 91–103. https://doi.org/10.1016/S1470-2045(21)00591-X.
- Zhang, W.Y.; Wang, Y.; Guo, Y.L.; Dai, H.R.; Yang, Q.M.; Zhang, Y.J.; Zhang, Y.; Chen, M.X.; Wang, C.M.; Feng, K.C.; et al. Treatment of CD20-directed Chimeric Antigen Receptor-modified T cells in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: An early phase IIa trial report. Signal Transduct. Target. Ther. 2016, 1, 16002. https://doi.org/10.1038/sigtrans.2016.2.
- Tholouli, E.; Osborne, W.; Bachier, C.; Ramakrishnan, A.; Marzolini, M.; Irvine, D.; McSweeney, P.; Bartlet, N.; Zhang, Y.; Thomas, S.; et al. Phase I Alexander study of AUTO3, the first CD19/22 dual targeting CAR.T cell, with pembrolizumab in patients with relapsed/refractory (r/r) DLBCL. Oncol. 2020, 31, S651. https://doi.org/10.1016/j.annonc.2020.08.008.
- Fry, T.J.; Shah, N.N.; Orentas, R.J.; Stetler-Stevenson, M.; Yuan, C.M.; Ramakrishna, S.; Wolters, P.; Martin, S.; Delbrook, C.; Yates, B.; et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Med. 2018, 24, 20–28. https://doi.org/10.1038/nm.4441.
- D'Agostino, M.; Raje, N. Anti-BCMA CAR T-cell therapy in multiple myeloma: Can we do better? Leukemia 2020, 34, 21–34. https://doi.org/10.1038/s41375-019-0669-4.
- Zhao, W.H.; Liu, J.; Wang, B.Y.; Chen, Y.X.; Cao, X.M.; Yang, Y.; Zhang, Y.L.; Wang, F.X.; Zhang, P.Y.; Lei, B.; et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. Hematol. Oncol. 2018, 11, 141. https://doi.org/10.1186/s13045-018-0681-6.
- Li, C.; Wang, J.; Wang, D.; Hu, G.; Yang, Y.; Zhou, X.; Meng, L.; Hong, Z.; Chen, L.; Mao, X.; et al. Efficacy and Safety of Fully Human Bcma Targeting CAR T Cell Therapy in Relapsed/Refractory Multiple Myeloma. Blood 2019, 134, 929. https://doi.org/10.1182/blood-2019-128468.
- Green, D.J. Fully Human Bcma Targeted Chimeric Antigen Receptor T Cells Administered in a Defined Composition Demonstrate Potency at Low Doses in Advanced Stage High Risk Multiple Myeloma. Blood 2018, 132, 1011.
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Singh, I.; Zudaire, E.; Yeh, T.; Allred, A.J.; Olyslager, Y.; Banerjee, A.; Goldberg, J.D.; et al. Update of CARTITUDE-1: A phase Ib/II study of JNJ-4528, a B-cell maturation antigen (BCMA)-directed CAR-T-cell therapy, in relapsed/refractory multiple myeloma. Clin. Oncol 2020, 38, 8505. https://doi.org/10.1200/JCO.2020.38.15_suppl.8505.
- Munshi, N.C.; Anderson, L.D.; Shah, J.N.; Jagannath, S.; Berdeja, J.G.; Lonial, S.; Raje, N.S.; Siegel, D.S.D.D.; Lin, Y.; Oriol, A.; et al. Idecabtagene vicleucel (ide-cel; bb2121), a BCMA-targeted CAR T-cell therapy, in patients with relapsed and refractory multiple myeloma (RRMM): Initial KarMMa results. Clin. Oncol. 2020, 38, 8503.
- Cohen, A.D.; Garfall, A.L.; Stadtmauer, E.A.; Melenhorst, J.J.; Lacey, S.F.; Lancaster, E.; Vogl, D.T.; Weiss, B.M.; Dengel, K.; Nelson, A.; et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. Clin. Investig. 2019, 129, 2210–2221. https://doi.org/10.1172/JCI126397.
- Wang, D.; Wang, J.; Hu, G.; Wang, W.; Xiao, Y.; Cai, H.; Jiang, L.; Meng, L.; Yang, Y.; Zhou, X.; et al. A phase 1 study of a novel fully human BCMA-targeting CAR (CT103A) in patients with relapsed/refractory multiple myeloma. Blood 2021, 137, 2890–2901. https://doi.org/10.1182/blood.2020008936.
- Brudno, J.N.; Maric, I.; Hartman, S.D.; Rose, J.J.; Wang, M.; Lam, N.; Stetler-Stevenson, M.; Salem, D.; Yuan, C.; Pavletic, S.; et al. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. Clin. Oncol. 2018, 36, 2267–2280. https://doi.org/10.1200/JCO.2018.77.8084.
- Ali, S.A.; Shi, V.; Maric, I.; Wang, M.; Stroncek, D.F.; Rose, J.J.; Brudno, J.N.; Stetler-Stevenson, M.; Feldman, S.A.; Hansen, B.G.; et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 2016, 128, 1688–1700. https://doi.org/10.1182/blood-2016-04-711903.
- Zhang, H.; Gao, L.; Liu, L.; Wang, J.; Wang, S.; Zhang, C.; Liu, Y.; Kong, P.; Liu, J.; He, J.; et al. A Bcma and CD19 Bispecific CAR-T for Relapsed and Refractory Multiple Myeloma. Blood 2019, 134, 3147–3147.
- Li, C.; Mei, H.; Hu, Y.; Guo, T.; Liu, L.; Jiang, H.;Tang, L.; Wu, Y.; Ai, L.; Deng, J.; Jin, D. A Bispecific CAR-T Cell Therapy Targeting Bcma and CD38 for Relapsed/Refractory Multiple Myeloma: Updated Results from a Phase 1 Dose-Climbing Trial. Blood 2019, 134, 930. https://doi.org/10.1182/blood-2019-130340.
- Madduri, D.; Usmani, S.Z.; Jagannath, S.; Singh, I.; Zudaire, E.; Yeh, T.; Allred, A.J.; Banerjee, A.; Goldberg, J.D.; Schecter, J.M.; et al. Results from CARTITUDE-1: A Phase 1b/2 Study of JNJ-4528, a CAR-T Cell Therapy Directed Against B-Cell Maturation Antigen (BCMA), in Patients with Relapsed and/or Refractory Multiple Myeloma (R/R MM). Blood 2019, 134, 577.
- Abramson, J.S.; Palomba, M.L.; Leo, I.G.; Lunning, M.A.; Jon, E.A.; Forero-Torres, A.; Wang, M.; Tina, M.A.; Allen, T.; Sutherland, C.; et al. CR rates in relapsed/refractory (R/R) aggressive B-NHL treated with the CD19-directed CAR T-cell product JCAR017 (TRANSCEND NHL 001). Clin. Oncol. 2017, 35, 7513.
- Shah, N.N.; Zhu, F.; Schneider, D.; Taylor, C.; Krueger, W.; Worden, A.; Walter, L.L.; Hamadani, M.; Fenske, T.; Johnson, B.; et al. Results of a phase I study of bispecific anti-CD19, anti-CD20 chimeric antigen receptor (CAR) modified T cells for relapsed, refractory, non-Hodgkin lymphoma. Clin. Oncol. 2019, 37, 2510.
- Zhang, Y. Safety and efficacy of optimized tandem CD19/CD20 CAR-engineered T cells in patients with relapsed/refractory non-Hodgkin lymphoma. Clin. Oncol. 2020, 38, 3034.
- Tang, F.; Lu, Y.; Ge, Y.; Shang, J.; Zhu, X. Infusion of chimeric antigen receptor T cells against dual targets of CD19 and B-cell maturation antigen for the treatment of refractory multiple myeloma. Int. Med. Res. 2020, 48, 300060519893496. https://doi.org/10.1177/0300060519893496.
- Yan, Z.; Cao, J.; Cheng, H.; Qiao, J.; Zhang, H.; Wang, Y.; Shi, M.; Lan, J.; Fei, X.; Jin, L.; et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: A single-arm, phase 2 trial. Lancet Haematol 2019, 6, e521–e529. https://doi.org/10.1016/S2352-3026(19)30115-2.
- Yang, J.; Jiang, P.; Zhang, X.;Li, J.; Wu, Y.; Xu, L.; Su, Y.; Hu, X.; Zhao, X.; Dong, Q.; et al. Successful 24-Hours Manufacture of Anti-CD19/CD22 Dual Chimeric Antigen Receptor (CAR) T Cell Therapy for B-Cell Acute Lymphoblastic Leukemia (B-ALL). Blood 2020, 136, 2–3. https://doi.org/10.1182/blood-2020-136866.
- Yang, J.; Li, J.; Zhang, X.; LV, F.; Guo, X.; Wang, Q.; Wang, L.; Chen, D.; Zhou, X.; Ren, J.; et al. A Feasibility and Safety Study of CD19 and CD22 Chimeric Antigen Receptors-Modified T Cell Cocktail for Therapy of B Cell Acute Lymphoblastic Leukemia. Blood 2018, 132, 277. https://doi.org/10.1182/blood-2018-99-114415.
- Jiang, H.; Dong, B.; Gao, L.; Liu, L.; Ge, J.; He, A.; Li, L.; Lu, J.; Chen, X.; Sersch, M.A.; et al. Long-term follow-up results of a multicenter first-in-human study of the dual BCMA/CD19 Targeted FasT CAR-T GC012F for patients with relapsed/refractory multiple myeloma. Clin. Oncol 2021, 39, 8014. https://doi.org/10.1200/JCO.2021.39.15_suppl.8014.
This entry is adapted from the peer-reviewed paper 10.3390/cancers14133230
