Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
General anesthesia has been widely utilized since the 1840s, but its underlying neural circuits remain to be completely understood. Chemogenetics and Optogenetics, the two techniques artificially modulate the activity of specific neurons and neural circuits.
- chemogenetics
- optogenetics
1. Chemogenetics
In vivo calcium imaging can be used to determine the excitatory status of specific types of neurons or neural circuits in different brain areas under general anesthesia. However, it does not provide the ability to manipulate neural endpoints or circuits. In contrast, chemogenetics and optogenetics are capable of achieving this purpose.
Chemogenetics, as a technique similar to optogenetics, was developed earlier than optogenetic technology. It is based on genetic principles and utilizes small molecular tools to modulate the excitation or inhibition of target cells. This technology works by introducing engineered ligand-activated receptors into the neurons of targeted brain regions. Receptors are designed to be activated by specific exogenous ligands that are otherwise inert [1]. Since the design of a mutant β2-adrenergic receptor by Strader in 1991 [2], engineered receptors that respond specifically to synthetic small-molecule ligands rather than natural ligands have been refined and developed. For example, receptors activated solely by synthetic ligands (RASSLs) based on the κ-opioid receptor exhibit reduced binding affinity and signaling in response to dynorphin A(1–13) and 20 other opioid peptides, while maintaining a strong affinity and signaling in response to synthetic small-molecule agonists [3].
The designer receptors exclusively activated by designer drugs (DREADDs) that are activated by specific exogenous drugs have been further developed since 2007 [4]. DREADDs, originating from human muscarinic receptors, are modified G-protein-coupled “designer” receptors. They are engineered with a low affinity for the native ligand but a high affinity for a synthetic inert “designer” ligand (e.g., clozapine-N-oxide, CNO) [4][5]. The most widely used DREADDs are hM3Dq and hM4Di, which produce excitatory and inhibitory effects, respectively, when CNO binds to the receptor. CNO binding to hM3Dq excites neurons by increasing intracellular calcium levels, whereas binding to hM4Di silences neuronal activity by reducing adenylate cyclase content [6]. In addition to the transgenic mouse lines expressing DREADDs [7], chemogenetic technology requires virus delivery to achieve specific and stable expression of hM3Dq or hM4Di in particular cells in one or more brain regions (Figure 1). Currently, DREADDs are widely applied to regulate specific neural activities and behaviors in many species, such as flies, mice, and even nonhuman primates [8][9]. DREADDs are the chemogenetic tools most widely used in the study of neural circuits under general anesthesia. Furthermore, a new κ-opioid-receptor-based inhibitory DREADD (KORD) has also been introduced. KORD is selectively activated by salvinorin B and is insensitive to endogenous opioid peptides [6]. In addition, KORD has been successfully applied to study the effects of rostromedial tegmental nucleus (RMTg) GABAergic neurons on nociception and opioid analgesia [10].
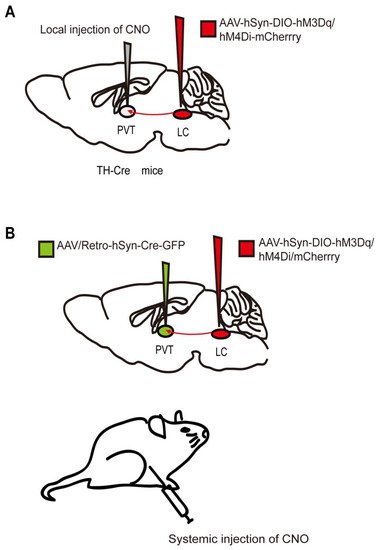
Figure 1. Two chemogenetic methods of specific neural-circuit manipulation. Taking the locus coeruleus (LC) - paraventricular thalamus (PVT) circuit as an example, (A) the AAV-hSyn-DIO-hM3Dq/hM4Di-mcherry was injected into the LC of TH-Cre transgenic mice, and CNO was injected locally in the PVT. (B) Wild-type mice were injected with the AAV-hSyn-DIO-hM3Dq/hM4Di-mcherry and AAV/Rtro-hSyn-Cre-GFP into the LC and PVT regions, respectively, followed by systematic injection of CNO.
The most attractive features of chemogenetics are that it does not need intracranial implantation and that a single dose is sufficient to induce neural activation or inhibition in multiple brain regions for several hours. In addition, it has the advantages of relatively straightforward operation and simplicity compared to in vivo calcium imaging and optogenetics, as it does not require equipment such as that needed for calcium imaging or other laser-based methods [1]. However, its disadvantages do deserve attention. First, clozapine is a metabolite of CNO in the body. It is a sedative antipsychotic drug that interferes with the experimental results, mainly when CNO is administered at high doses [11]. Therefore, the current systemic quantity of CNO commonly used in laboratories is limited to 0.6–3 mg/kg. Second, when CNO is injected intraperitoneally, it activates or inhibits target neurons and all their projections. Therefore, study conclusions are ambiguous due to the fact that upstream regions of the brain may have opposite effects on different downstream regions. Lastly, the time accuracy of the chemogenetic method is insufficient, as the effects of CNO peak between 30 to 60 min after administration and last for about 9 h [8]. As long as these shortcomings are emphasized and used reasonably, this technology still has excellent potential in the field of anesthesiology.
Like NAc, the prelimbic cortex (PrL) is one of the vital projection regions of the ventral tegmental area (VTA). VTA-NAc and VTA-PrL neural circuits are involved in both the induction and recovery periods during sevoflurane anesthesia, as evidenced by chemogenetic techniques [12][13]. By contrast, chemogenetic modulation of DRN 5-HT neurons shows that they are activated only during the recovery phase of general anesthesia [14]. These findings are inconsistent with the previous results obtained using optical-fiber photometry. This contradictory phenomenon indicates that artificially interfering with the activity of neural pathways may fail to fully mimic its normal physiological activity. LC is the primary source of norepinephrine in the brain and sends abundant outputs to many subregions of the forebrain, making it a vital arousal node [15]. Chemogenetic activation of LC in rats induces cortical arousal and a noticeable decrease in time to emergence from isoflurane, but the induction time remains unchanged [16][17]. Furthermore, the paraventricular thalamus (PVT) is a wakefulness-promoting region that receives numerous projections from the LC [18]. Researchers found that chemogenetic inhibition of LC-PVT projections significantly delayed emergence time from isoflurane anesthesia but had no impact on the induction phase [17]. Thus, although both dopaminergic and noradrenergic neurons belong to the class of monoaminergic neurons, there is a difference between these neurons in the process of consciousness transformation during general anesthesia. Dopaminergic neurons may be more integrally involved in the regulation of general anesthesia.
More interestingly, specific brain regions may have different regulatory effects based on the anesthetic used. For example, Luo et al. reported that when the parabrachial nucleus (PBN) was activated by the chemogenetic method, it only accelerated the recovery times for propofol and isoflurane [19]. When using sevoflurane anesthesia, PBN glutamatergic neurons accelerated reanimation time and prolonged induction time [20]. Moreover, orexinergic neurons of the LHA, also known as hypocretin neurons, are vital for maintaining wakefulness and have numerous projections to many arousal-promoting brain areas [21][22]. A recent study showed that the actions of LHA orexinergic neurons and LHA-PVT are quite different regarding their actions during isoflurane vs. desflurane induction [23]. These unique phenomena suggest that different anesthetics may target specific neural pathways during the induction period, based on their specific pharmacological structures and physicochemical features. Furthermore, chemogenetic regulation of lateral habenula (LHb) glutamatergic neurons similarly shows that they contribute to the recovery time but not the induction time of general anesthesia [24]. In the cholinergic system, chemogenetic activation of basal forebrain (BF) cholinergic neurons affects both the induction time and the recovery time when using either isoflurane or propofol anesthesia, thereby attenuating the efficacy of general anesthesia [25]. Similar findings have been noted using both chemogenetic and in vivo calcium imaging studies, indicating that various wake-promoting brain nuclei or neural circuits are involved in the consciousness change caused by multiple anesthetics to varying degrees.
When exploring the role of neural pathways that promote sleep in general anesthesia, Jiang et al. identified multiple anesthetic-activated neurons in the hypothalamic preoptic area, an area traditionally viewed as a regulatory sleep center. Chemogenetic activation of these neurons reliably produces slow-wave sleep and facilitates general anesthesia, and chemogenetic inhibition shortens the general anesthesia time and disrupts natural sleep [26]. Recent studies have found that chemogenetic activation of GABAergic (The γ-aminobutyric acid (GABA)) neurons in other brain regions, such as the VTA [27] and RMTg [28], promotes an anesthesia state as well, whereas chemogenetic activation of dorsal–intermediate lateral septum GABAergic neurons contributes to anesthesia emergence [29]. The fact that sleep-promoting GABAergic neurons in the aforementioned regions similarly contribute to anesthetic-state transitions further supports a common regulatory mechanism between the states of sleep and general anesthesia. However, the role of other sleep-promoting brain nuclei and neural circuits in general anesthesia induced by different anesthetics needs further exploration.
2. Optogenetics
Optogenetic technology has gradually increased in popularity due to the shortcomings of chemogenetics, such as low timing accuracy. Many researchers combine both techniques to compensate for the disadvantages of each individual technique. Optogenetics regulates neurons by activating opsins expressed on target cells with a laser at the corresponding wavelength [1][30]. The light-sensitive opsins include excitatory and inhibitory types, usually channelrhodopsin (ChR2) and halorhodopsin (NpHR) or archaerhodopsin (Arch), respectively. Mutant receptors are employed to improve the reaction efficiency of opsins to light pulses or satisfy the requirements of the study. For example, activation of ChETAH, a ChR2 mutant, causes neurons to fire at up to 200 Hz, while wild-type ChR2 activation typically results in neurons firing at 20–40 Hz [31]. Therefore, ChETAH is specifically used to regulate neurons with higher firing frequencies. Similarly, red-shifted cruxhalorhodopsin has greater photocurrents than NpHR, making it possible for noninvasive photoinhibition due to the stronger penetrating potential of red light vs. blue light [32].
As with genetically encoded calcium indicators (GECIs) and DREADDs, in addition to the most commonly used viral strategies to transfect opsins into target neurons, transgenic mice expressing ChR2 have also been developed [33]. Traditionally, optogenetic technology requires optical-fiber implantation in target sites. Once implanted, optical fibers activate ChR2 or NpHR by adjusting the pulsed light using various specific parameters (Figure 2). The parameter settings, including the duration, frequency, and intensity of the light pulse, are based on the physiological firing pattern and rate of the target neurons. Artificial real-time control of receptor activation–deactivation and the duration of the light pulse allow optogenetics to activate or inhibit neurons with millisecond timescale precision [34]. Thus, optogenetics provides the powerful ability to modulate specific neurons or neural circuits with a precise temporal resolution.
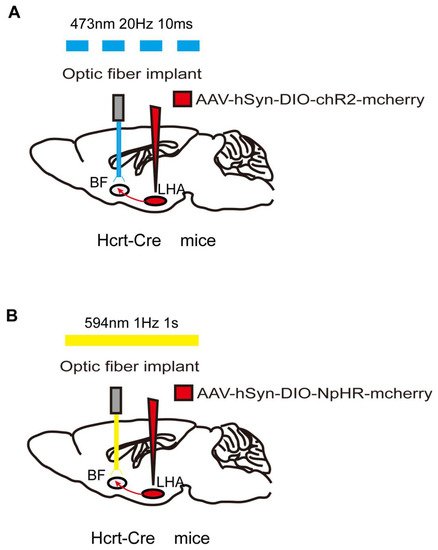
Figure 2. Optogenetic method of specific neural-circuit manipulation. Taking the LHA-BF neural circuit in Hcrt-Cre mice as an example, the opsin is typically introduced to the LHA neurons by injecting a virus containing ChR2 (A) or NpHR (B). After 3 weeks to allow for expression of the opsin, LHA-BF axon terminals can be targeted with corresponding light pulses from the optical fiber to excite (A) or inhibit (B) this pathway.
However, there are several limitations in the use of photogenetic technology and the interpretation of its results. First, brain tissue may be damaged during fiber implantation. Fortunately, this problem has long been recognized and has been gradually solved by scientists. For example, Zhang et al. developed a wireless photogenetic technique mediated by upconversion nanoparticles (UCNPs). This technology converts near-infrared light to high-energy blue light via UCNPs, activating common photosensitive proteins such as ChR2 to stimulate deep-brain regions [35]. Recently, Gong et al. designed a new step-function opsin with ultra-high light sensitivity (SOUL), allowing for transcranial stimulation of neurons in the deep-brain regions of mice. SOUL is capable of regulating neuronal spiking in the macaque cortex via optical stimulation from outside the dura [36]. This non-invasive advantage, which does not require fiber implantation, makes these new optogenetic tools much less damaging and, thus, promising for future research.
A second limitation of photogenetic technology is the possibility of false-negative results. Light energy may be partially lost when passing through optical fibers, brain tissues, etc. Additionally, viral transfection may not be effective enough, resulting in a subset of target cells lacking opsins. These factors may confound the interpretation of negative behavioral results. Moreover, prolonged light stimulation may increase the temperature of the target tissue and cause an unexpected neurophysiologic reaction. Specifically, photoinhibition requires continuous receptor stimulation to suppress the spontaneous excitation of neurons when silencing neurons [37]. Furthermore, many types of neurons typically undergo an elastic increase in firing at the end of a long period of photoinhibition, which potentially leads to confounding results [38].
Finally, immunolabeling (e.g., c-fos protein expression) or whole-cell patch-clamp techniques are often used to verify illumination-induced excitatory or inhibitory responses at the cellular level. This verification step is also necessary for chemogenetics. Additionally, histological verification is also essential for the three techniques described in this review. For example, the position of the optical fiber in optogenetics and fiber photometry and the expression of the corresponding protein in the targeted brain regions are determined by fluorescence imaging and immunolabeling.
Consistent with chemogenetic results, optogenetic activation of orexinergic terminals in the PVT induces similar changes in induction to and emergence from desflurane and isoflurane anesthesia [23]. Dong et al. further found that selective light stimulation of LHA orexinergic neurons and their projections to BF, LC, and VTA resulted in a shorter emergence time from isoflurane anesthesia in rats. In contrast, optogenetic inhibition of orexinergic terminals in the VTA delayed the time to wakefulness. These studies failed to detect a significant difference in induction time [39][40]. Whether the downstream nuclei of LHA orexinergic neurons play a role in the induction phase with other anesthetics remains to be further elucidated. As observed in chemogenetic studies, optical stimulation of VTA dopaminergic neurons [41] and VTA-NAc and VTA-PrL pathways [12] contributes to the transition of consciousness during both the induction and emergence phases of isoflurane or sevoflurane general anesthesia. Optical stimulation of LC TH axons in the PVT does not alter the induction time but does elicit emergence from 1.2% isoflurane [17]. Furthermore, it has been demonstrated that the brain’s dopaminergic system may play a more important role than its noradrenergic system in determining the effects of general anesthesia.
Using optogenetic tools, Wang et al. aimed to elucidate further the role of glutamatergic neurons of the PBN in emergence from sevoflurane anesthesia. They unexpectedly found that photostimulation of PBN neurons only caused cortical arousal and did not lead to significant changes in behaviors [20]. The authors concluded that this phenomenon was due to the limitations of optogenetic technology, such as the absorption, scattering, and distance-related attenuation of light passing through the brain tissue, and the decay of the light subsequently transmitted to the target area [42]. Optogenetics has also been employed to investigate the specific role of glutamatergic neurons in the LHA. For example, light stimulation of LHA glutamatergic neurons reduces the depth of isoflurane-inhalation anesthesia, and light activation of LHA glutamatergic projections to the LHb accelerates the recovery time from isoflurane anesthesia [43].
Optogenetics has also been used to identify sleep-promoting nuclei and neural circuits activated or suppressed during general anesthesia. Jiang et al. reported that optogenetic activation of anesthetics-activated neurons in the hypothalamus preoptic area accelerates sleep and enhances the effects of general anesthesia. In contrast, optogenetic inhibition of these neurons reduced the duration of general anesthesia [26]. Again, these results demonstrate that the hypothalamic preoptic area plays a crucial role in maintaining general anesthesia. After optogenetic activation of the LHb containing glutamatergic neurons [24] and VTA-LHA GABAergic projections, behavioral studies have shown that loss of righting reflex (LORR) significantly declines and RORR statistically increases. Optical inhibition of VTA-LHA GABAergic projections induces the opposite effects in both the EEG and behavioral outcomes [27]. The results of these optogenetic studies are similar to previous findings from in vivo imaging and chemogenetic studies, which suggest that despite anesthesia and sleep sharing some overlapping neural pathway mechanism, there are many differences that are anesthetic-specific.
This entry is adapted from the peer-reviewed paper 10.3390/biom12070898
References
- Vlasov, K.; Van Dort, C.J.; Solt, K. Optogenetics and Chemogenetics. Methods Enzymol. 2018, 603, 181–196.
- Strader, C.D.; Gaffney, T.; Sugg, E.E.; Candelore, M.R.; Keys, R.; Patchett, A.A.; Dixon, R.A. Allele-specific activation of genetically engineered receptors. J. Biol. Chem. 1991, 266, 5–8.
- Coward, P.; Wada, H.G.; Falk, M.S.; Chan, S.D.; Meng, F.; Akil, H.; Conklin, B.R. Controlling signaling with a specifically designed Gi-coupled receptor. Proc. Natl. Acad. Sci. USA 1998, 95, 352–357.
- Armbruster, B.N.; Li, X.; Pausch, M.H.; Herlitze, S.; Roth, B.L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. USA 2007, 104, 5163–5168.
- Roth, B.L. DREADDs for Neuroscientists. Neuron 2016, 89, 683–694.
- Vardy, E.; Robinson, J.E.; Li, C.; Olsen, R.H.; DiBerto, J.F.; Giguere, P.M.; Sassano, F.M.; Huang, X.P.; Zhu, H.; Urban, D.J.; et al. A New DREADD Facilitates the Multiplexed Chemogenetic Interrogation of Behavior. Neuron 2015, 86, 936–946.
- Saika, F.; Matsuzaki, S.; Kishioka, S.; Kiguchi, N. Chemogenetic Activation of CX3CR1-Expressing Spinal Microglia Using Gq-DREADD Elicits Mechanical Allodynia in Male Mice. Cells 2021, 10, 874.
- Alexander, G.M.; Rogan, S.C.; Abbas, A.I.; Armbruster, B.N.; Pei, Y.; Allen, J.A.; Nonneman, R.J.; Hartmann, J.; Moy, S.S.; Nicolelis, M.A.; et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 2009, 63, 27–39.
- Eldridge, M.A.; Lerchner, W.; Saunders, R.C.; Kaneko, H.; Krausz, K.W.; Gonzalez, F.J.; Ji, B.; Higuchi, M.; Minamimoto, T.; Richmond, B.J. Chemogenetic disconnection of monkey orbitofrontal and rhinal cortex reversibly disrupts reward value. Nat. Neurosci. 2016, 19, 37–39.
- Taylor, N.E.; Long, H.; Pei, J.; Kukutla, P.; Phero, A.; Hadaegh, F.; Abdelnabi, A.; Solt, K.; Brenner, G.J. The rostromedial tegmental nucleus: A key modulator of pain and opioid analgesia. Pain 2019, 160, 2524–2534.
- Gomez, J.L.; Bonaventura, J.; Lesniak, W.; Mathews, W.B.; Sysa-Shah, P.; Rodriguez, L.A.; Ellis, R.J.; Richie, C.T.; Harvey, B.K.; Dannals, R.F.; et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 2017, 357, 503–507.
- Gui, H.; Liu, C.; He, H.; Zhang, J.; Chen, H.; Zhang, Y. Dopaminergic Projections from the Ventral Tegmental Area to the Nucleus Accumbens Modulate Sevoflurane Anesthesia in Mice. Front. Cell Neurosci. 2021, 15, 671473.
- Song, Y.; Chu, R.; Cao, F.; Wang, Y.; Liu, Y.; Cao, J.; Guo, Y.; Mi, W.; Tong, L. Dopaminergic Neurons in the Ventral Tegmental-Prelimbic Pathway Promote the Emergence of Rats from Sevoflurane Anesthesia. Neurosci. Bull. 2022, 38, 417–428.
- Li, A.; Li, R.; Ouyang, P.; Li, H.; Wang, S.; Zhang, X.; Wang, D.; Ran, M.; Zhao, G.; Yang, Q.; et al. Dorsal raphe serotonergic neurons promote arousal from isoflurane anesthesia. CNS Neurosci. Ther. 2021, 27, 941–950.
- Berridge, C.W.; Waterhouse, B.D. The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Brain Res. Rev. 2003, 42, 33–84.
- Vazey, E.M.; Aston-Jones, G. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc. Natl. Acad. Sci. USA 2014, 111, 3859–3864.
- Ao, Y.; Yang, B.; Zhang, C.; Wu, B.; Zhang, X.; Xing, D.; Xu, H. Locus Coeruleus to Paraventricular Thalamus Projections Facilitate Emergence from Isoflurane Anesthesia in Mice. Front. Pharmacol. 2021, 12, 643172.
- Beas, B.S.; Wright, B.J.; Skirzewski, M.; Leng, Y.; Hyun, J.H.; Koita, O.; Ringelberg, N.; Kwon, H.B.; Buonanno, A.; Penzo, M.A. The locus coeruleus drives disinhibition in the midline thalamus via a dopaminergic mechanism. Nat. Neurosci. 2018, 21, 963–973.
- Luo, T.; Yu, S.; Cai, S.; Zhang, Y.; Jiao, Y.; Yu, T.; Yu, W. Parabrachial Neurons Promote Behavior and Electroencephalographic Arousal from General Anesthesia. Front. Mol. Neurosci. 2018, 11, 420.
- Wang, T.X.; Xiong, B.; Xu, W.; Wei, H.H.; Qu, W.M.; Hong, Z.Y.; Huang, Z.L. Activation of Parabrachial Nucleus Glutamatergic Neurons Accelerates Reanimation from Sevoflurane Anesthesia in Mice. Anesthesiology 2019, 130, 106–118.
- Peyron, C.; Tighe, D.K.; Van Den Pol, A.N.; De Lecea, L.; Heller, H.C.; Sutcliffe, J.G.; Kilduff, T.S. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998, 18, 9996–10015.
- Adamantidis, A.R.; Zhang, F.; Aravanis, A.M.; Deisseroth, K.; De Lecea, L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 2007, 450, 420–424.
- Zhao, S.; Wang, S.; Li, H.; Guo, J.; Li, J.; Wang, D.; Zhang, X.; Yin, L.; Li, R.; Li, A.; et al. Activation of Orexinergic Neurons Inhibits the Anesthetic Effect of Desflurane on Consciousness State via Paraventricular Thalamic Nucleus in Rats. Anesth. Analg. 2021, 133, 781–793.
- Liu, C.; Liu, J.; Zhou, L.; He, H.; Zhang, Y.; Cai, S.; Yuan, C.; Luo, T.; Zheng, J.; Yu, T.; et al. Lateral Habenula Glutamatergic Neurons Modulate Isoflurane Anesthesia in Mice. Front. Mol. Neurosci. 2021, 14, 628996.
- Luo, T.Y.; Cai, S.; Qin, Z.X.; Yang, S.C.; Shu, Y.; Liu, C.X.; Zhang, Y.; Zhang, L.; Zhou, L.; Yu, T.; et al. Basal Forebrain Cholinergic Activity Modulates Isoflurane and Propofol Anesthesia. Front. Neurosci. 2020, 14, 559077.
- Jiang-Xie, L.F.; Yin, L.; Zhao, S.; Prevosto, V.; Han, B.X.; Dzirasa, K.; Wang, F. A Common Neuroendocrine Substrate for Diverse General Anesthetics and Slee. Neuron 2019, 102, 1053–1065.e4.
- Yin, L.; Li, L.; Deng, J.; Wang, D.; Guo, Y.; Zhang, X.; Li, H.; Zhao, S.; Zhong, H.; Dong, H. Optogenetic/Chemogenetic Activation of GABAergic Neurons in the Ventral Tegmental Area Facilitates General Anesthesia via Projections to the Lateral Hypothalamus in Mice. Front. Neural. Circuits 2019, 13, 73.
- Vlasov, K.; Pei, J.; Nehs, C.J.; Guidera, J.A.; Zhang, E.R.; Kenny, J.D.; Houle, T.T.; Brenner, G.J.; Taylor, N.E.; Solt, K. Activation of GABAergic Neurons in the Rostromedial Tegmental Nucleus and Other Brainstem Regions Promotes Sedation and Facilitates Sevoflurane Anesthesia in Mice. Anesth. Analg. 2021, 132, e50–e55.
- Wang, D.; Guo, Q.; Zhou, Y.; Xu, Z.; Hu, S.W.; Kong, X.X.; Yu, Y.M.; Yang, J.X.; Zhang, H.; Ding, H.L.; et al. GABAergic Neurons in the Dorsal-Intermediate Lateral Septum Regulate Sleep-Wakefulness and Anesthesia in Mice. Anesthesiology 2021, 135, 463–481.
- Duebel, J.; Marazova, K.; Sahel, J.A. Optogenetics. Curr. Opin. Ophthalmol. 2015, 26, 226–232.
- Gunaydin, L.A.; Yizhar, O.; Berndt, A.; Sohal, V.S.; Deisseroth, K.; Hegemann, P. Ultrafast optogenetic control. Nat. Neurosci. 2010, 13, 387–392.
- Chuong, A.S.; Miri, M.L.; Busskamp, V.; Matthews, G.A.; Acker, L.C.; Sørensen, A.T.; Young, A.; Klapoetke, N.C.; Henninger, M.A.; Kodandaramaiah, S.B.; et al. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat. Neurosci. 2014, 17, 1123–1129.
- Wu, W.; Xiong, W.; Zhang, P.; Chen, L.; Fang, J.; Shields, C.; Xu, X.M.; Jin, X. Increased threshold of short-latency motor evoked potentials in transgenic mice expressing Channelrhodopsin-2. PLoS ONE 2017, 12, e0178803.
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268.
- Chen, S.; Weitemier, A.Z.; Zeng, X.; He, L.; Wang, X.; Tao, Y.; Huang, A.J.; Hashimotodani, Y.; Kano, M.; Iwasaki, H.; et al. Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics. Science 2018, 359, 679–684.
- Gong, X.; Mendoza-Halliday, D.; Ting, J.T.; Kaiser, T.; Sun, X.; Bastos, A.M.; Wimmer, R.D.; Guo, B.; Chen, Q.; Zhou, Y.; et al. An Ultra-Sensitive Step-Function Opsin for Minimally Invasive Optogenetic Stimulation in Mice and Macaques. Neuron 2020, 107, 38–51.e8.
- Owen, S.F.; Liu, M.H.; Kreitzer, A.C. Thermal constraints on in vivo optogenetic manipulations. Nat. Neurosci. 2019, 22, 1061–1065.
- Carter, M.E.; Yizhar, O.; Chikahisa, S.; Nguyen, H.; Adamantidis, A.; Nishino, S.; Deisseroth, K.; De Lecea, L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 2010, 13, 1526–1533.
- Li, J.; Li, H.; Wang, D.; Guo, Y.; Zhang, X.; Ran, M.; Yang, C.; Yang, Q.; Dong, H. Orexin activated emergence from isoflurane anaesthesia involves excitation of ventral tegmental area dopaminergic neurones in rats. Br. J. Anaesth. 2019, 123, 497–505.
- Wang, D.; Guo, Y.; Li, H.; Li, J.; Ran, M.; Guo, J.; Yin, L.; Zhao, S.; Yang, Q.; Dong, H. Selective optogenetic activation of orexinergic terminals in the basal forebrain and locus coeruleus promotes emergence from isoflurane anaesthesia in rats. Br. J. Anaesth. 2021, 126, 279–292.
- Taylor, N.E.; Van Dort, C.J.; Kenny, J.D.; Pei, J.; Guidera, J.A.; Vlasov, K.Y.; Lee, J.T.; Boyden, E.S.; Brown, E.N.; Solt, K. Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc. Natl. Acad. Sci. USA 2016, 113, 12826–12831.
- Krashes, M.J. Untangling Appetite Circuits with Optogenetics and Chemogenetics, in Appetite and Food Intake: Central Control; Harris, R.B.S., Ed.; CRC Press; Taylor & Francis Group, LLC.: Boca Raton, FL, USA, 2017; pp. 91–116.
- Zhao, S.; Li, R.; Li, H.; Wang, S.; Zhang, X.; Wang, D.; Guo, J.; Li, H.; Li, A.; Tong, T.; et al. Lateral Hypothalamic Area Glutamatergic Neurons and Their Projections to the Lateral Habenula Modulate the Anesthetic Potency of Isoflurane in Mice. Neurosci. Bull. 2021, 37, 934–946.
This entry is offline, you can click here to edit this entry!
