Glucomannan (GM) is a polysaccharide generally extracted from the tuber of Amorphophallus konjac. It consists of mannose and glucose residues linked by β-(1-4) and exhibits hydrocolloid characteristics which can be applied as thickening and gelling agents. However, it has poor water resistance and low mechanical strength when used as an excipient in solid form. Several physical and chemical modifications have been carried out to improve these drawbacks.
Chemical modification involves the substitution of functional groups in GM’s structure including esterification and etherification. It causes a decrease in its high hydrophilic film behavior and produce water-resistant films.
Physical modification involves mixing native GM with other excipients through processes involving milling, moisture, temperature, pressure, radiation, etc. It causes variations to particle size, shape, surface properties, porosity, density, and to functional properties such as swelling capacity and gelation ability
- glucomannan
- chemical modification
- physical modification
- excipient
1. Introduction
2. Structure and Physicochemical Properties of GM
GM is a natural heteropolysaccharide with a linear chain consisting of D-glucose and/or D-mannose in various proportions linked by β-1,4 glycosidic bonds. It also has multiple branching at β-1,3 glycosidic bonds to mannose units as shown in Figure 1 [30].
The molecular weight varies from 200,000 to 2,000,000 Daltons, giving it incredibly higher viscosity than any known dietary fiber such as guar or locust bean gum [31,32]. When GM sol concentration is below 0.55%, it is only slightly affected by shear rate, indicating Newtonian fluid flow characteristics. However, at higher concentrations, shear rate can affect viscosity, leading to shear thinning and indicating non-Newtonian pseudoplasticity [33]. Based on previous reports, the viscosity of konjac glucomannan solution (1.0 g/100 g) can reach ~30,000 cps [34].
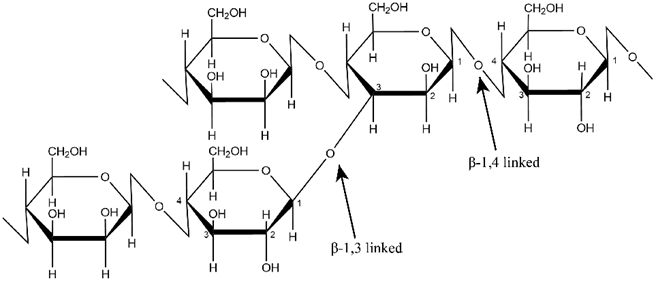
Figure 1. Structure of glucomannan.
GM is a hydrophilic polymer due to the abundance of hydroxyl and carbonyl groups in its molecular chain. The hydrogen bonds between each molecule affect its solubility; hence, the stronger the bonds, the lower the solubility in water, thereby increasing solubility; this causes high water absorption of 105.4 g/g (water/GM) [35]. Water absorption is also affected by granule size and surface morphology—a reduction in particle size will increase surface wrinkle density, which culminates in higher hydration rates [36].
The formation of gel is by hydration of water; this can be accelerated by heating and vigorous stirring. GM also forms synergistic gels in a thermally reversible reaction with other polysaccharides, such as xanthan gum [37], κ-carrageenan [38], and gum tragacanth [39], which increase the mechanical strength and decrease syneresis. This is presumably due to agglomeration or physical entanglement and dynamic hydrogen bonds with other polysaccharides [37-39].
In recent years, GM has attracted special attention from researchers and the food industry due to its bioactive, biodegradable, and hydrophilicity properties. This high-molecular-weight polymer is known as a hydrocolloid and interacts strongly with water [40]. Hydrocolloids are used in the food industry because of their thickening, gelling, stabilizing, texture-modifying, and film-forming properties.
3. Chemical Modification
Native GM forms very high viscosity solutions, where the intrinsic viscosity of 1% can reach 30,000 cps, and so it has potential as a good film-forming agent [41]. However, a very viscous external gel layer on the surface of particles immediately after dispersion prevents water penetration and drug dissolution, and thus its application is limited as a carrier for immediate drug release [42]. As a film, it has poor water resistance due to the large number of free hydroxyl and carboxyl groups distributed along the backbone, and it exhibits high moisture absorption. As a result, native GM has the weaknesses of poor water resistance and low mechanical strength [20,21].
Several structural modifications of GM have been performed to enhance its structural and functional qualities, including oxidation [32-34] and etherification by addition of acetyl [30,38,46] and carboxymethyl [2,5,13,15,47-52] moieties on hydroxyl groups of GM. Chemical modification with different degrees of substitution (DS) give different physical and mechanical properties. DS is largely affected by the amount of sodium hydroxide and by different dispersion media. Higher degrees of substitution contribute to lower viscosity and particle size, denser network structure, and better tablet strength [53].
GM is an ideal candidate for appropriate modification by chemical functionalization. Each of the glucose–mannose units have reactive hydroxyl groups, which are the major sites for chemical modification. In addition, several studies discovered that chemically modified GMs can be used for the sustained release of drugs [2,23,32]. Among various modification methods such as acetylation [46,54], carboxymethylation and oxidation [43,44,55,56], carboxymethylation is the most common and suitable for solid and film dosage [2,3,5,13,47]. The effects of carboxymethylation on GM (CMGM) are described as follows:
3.1. Increased Solubility
Chemical modification of CMGM affects solubility; carboxymethylation with NaOH catalyst substitutes chloroacetic acid with a hydroxyl group, which partially replaces hydroxyl and acetyl groups with carboxymethyl [2,47,48,57]. Incorporation of a carboxymethyl group appears as an extending chain structure that reduces hydrogen bonding between the polymer chains and increases the water-binding capacity, as shown in Figure 2 below.
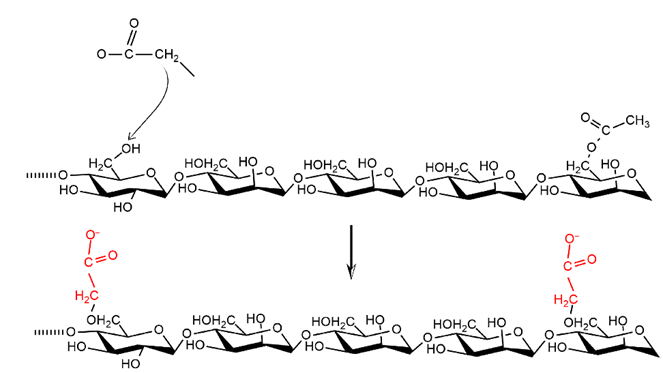
Figure 2 CMGM synthesis pathway.
Modification carboxymethyl groups breaks extensive hydrogen bonds, leading to a drastic decrease in crystallinity and an increase in solubility [58]. Modification also changes the amount of acetyl located randomly at the C-6 position of the sugar unit. The increase in solubility is due to the incorporation of water-soluble carboxylate groups during deacetylation (Figure 4). Additionally, changes in water-binding properties are caused by reduction and/or loss of crystal structure in the granules, making them mostly amorphous and more hygroscopic [49].
However, based on several studies, the smaller the degree of substitution, the lower the hydrophilicity due to the increase in the contact angle from θ = 48.1° [14] to θ = 97.3 ± 4.2° [53]. Water solubility is also lower in CMGM than in GM. This is because the particles in both are all amorphous; hence, carboxymethylation does not reduce and/or eliminate the crystalline structure inside GM granules, but, rather, alters its granular surface structure, which might affect moisture adsorption [49].
Ohya et al. reported a dicarboxy–glucomannan derivative capable of increasing the solubility of GM in water and interacting with other positively-charged polymers [59].
3.2. Reduced Viscosity
The concentration and type of polymer in coating solutions affects viscosity [60,61]. High viscosity sols produce nonuniform films due to low diffusivity, giving a “solid skin” that retards solvent evaporation and causes hydrodynamic instability. The solid skin is under mechanical tension and might break, thereby causing variations in film thickness [62]. Consequently, moderate viscosity is desirable for film formation. For utilization as a coating material, several studies suggest viscosity lower than 700 cps [63]. The viscosity of the coating solution can be increased by using polymers with high molecular weight (Mw), such as GM, which averages 200,000 to 2,000,000 Daltons [64,65], giving it the highest intrinsic viscosity compared to other polysaccharides at approximately 30,000 cps at a concentration of 1% [6].
Several methods have obtained GM with low Mw, for example, depolymerization, such as deacetylation, and carboxymethylation with strong bases to break the glycosidic bonds [66]. The viscosity of GM at 25 °C decreased significantly from 4660 cps to <500 cps after modification [2,49].
Deacetylation through carboxymethylation changes the structure from semi-flexible straight chains to elastic microspheres that decrease inherent viscosity (Figure 3) [53]. As a comparison, substitution of carboxymethylation groups in cellulose also affects viscosity. At a concentration of 1%, cellulose and carboxymethyl cellulose (CMC) have viscosities of 240 cps and <100 cps, respectively.
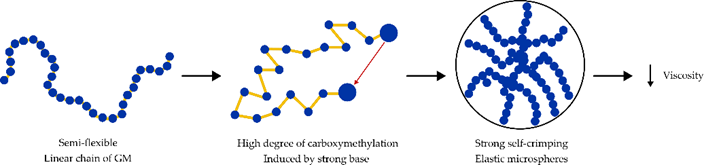
Figure 3. Effect of carboxymethylation on the structure of GM.
3.3. Increased Tensile Strength
Generally, the presence of more -COO- groups due to carboxymethylation of the CMGM backbone improves gel strength by forming more crosslinks, while a high DS also increases mechanical strength [15]. The introduced COO− group can efficiently bind more water, which can act as a plasticizer to improve elongation of the film [67]. As the DS of CMGM increases, formed pore size decreases and the tissue structure becomes denser, indicating stronger interaction at higher DS [54]. This high density also increases tablet strength [3,24], but an excessive amount of CMGM causes charge repulsion, thereby weakening its mechanical properties [47].
3.4. Improved Thermal Stability
CMGM maintains the gel network through hydrogen bonding upon heating to 95 °C for 2 h, implying excellent thermal stability [15]. Carboxymethylation increases the thermal stability of GM in a DS-dependent manner. Based on thermogravimetric analysis (TGA), GM is degraded in three stages. TGA recorded a change in mass due to moisture removal from 60–200 °C. Meanwhile, from 200–300 °C, great weight loss was recorded in GM, CMGM (DS 0.28), and (DS 0.7), with values of 64.16%, 49.73%, and 43.17%, respectively. In the final stage of decomposition at a temperature of 300–500 °C, there was a greater decrease in mass change in GM than in CMGM due to thermal degradation [2].
4. Physical Modification
Co-processing is a technique for mixing two or more excipients at the sub-particle level to synergistically enhance functionality and mask undesired properties without undergoing chemical changes [68]. This method can change fundamental characteristics such as particle size and shape, morphology, porosity, density, and surface area, which affects flowability, compressibility, compactibility, and ultimately influences the disintegration and mechanical properties of tablets [69]. Some examples of GM processed together with other excipients are shown in Table 1 for various applications, especially controlling drug release through crosslinking and/or formation of dense hydrogen bonds.
Table 1. Co-processed GM with other excipients.
|
Combination of Excipients |
Co-Processed |
Application |
Mechanism |
Ref |
|
GM and HPMC K 100 LV |
Microwave on level 5 (350 W) for 30 min |
Matrix for gastro-retentive tablets forming a porous channel that allows the polymer mixture to absorb more water and expand, followed by prolonged drug release |
Hydrogen bonds in single polymers have low energy, but the simultaneous formation of interlinked hydrogen bonds between polymer components provides significant interaction strength, resulting in a matrix that floats quickly and maintains the integrity of the polymer mixture under acidic conditions. |
[70] |
|
GM and lactose |
Wet granulation |
Filler–binder for direct compression of effervescent tablets |
GM has a high viscosity and strong adhesive properties, thus providing good tablet binding effectiveness. GM has poor solubility in water, so it is combined with lactose as a water-soluble ingredient and to improve the poor flowability of lactose. |
[71] |
|
GM, sodium alginate (SA), and graphene oxide (GO) |
Freeze dried |
Microsphere-making polymers that enhance targeted delivery of drugs or nutrients to the colon |
GM interacts with SA via hydrogen bonding and physical entanglement, and GO enhances these interactions in the microspheres. In addition, GO can greatly improve the loading efficiency of ciprofloxacin (CPFX) of microspheres, and achieve the sustained release effect of CPFX. |
[26] |
|
Oxidized GM, cassava starch, and sucrose esters |
Dry heated |
The OGM–CS combination exhibits low solubility and swellability, which makes it a possible excipient for the formulation of sustained-release drugs. However, the addition of SE significantly decreased porosity and swelling of the tablets, which inhibited immediate drug release. |
Heating OGM and CS to high temperatures causes structural damage that limits the solubility and swelling ability of the polymer. The addition of SE with HLB 5 decreased porosity and slowed drug release because the more closed structure inhibited free movement of the drug out of the matrix. In addition, more hydroxyl groups in SE form hydrogen bonds, increasing intergranular bonding. |
[57] |
|
CMGM and 2-hydroxypropyl trimethyl ammonium chloride chitosan (HACC) |
Complex coacervation and freeze dried |
The coaservation complex formed can encapsulate and control the release of the molecular model for the vaccine, namely ovalbumin (OVA). |
The anionic carboxyl group of CMGM and the cationic quaternary amine group of HACC cause intramolecular electrostatic attraction that causes the HACC and CMGM macromolecular chains to aggress and coil, forming the CMGM/HACC composite nanosphere. |
[23] |
5. Future Recommendations
GM is a polysaccharide that has promise as an excipient for solid dosage forms, especially for direct compression due to its free-flowing nature and compressibility. Some applications of chemically or physically modified GM have been reported. Chemical modification is suggested to modify the solubility, viscosity, and mechanical properties of GM, while physical modification of GM is suggested to modify swelling ability and drug release from the matrix. Although chemical and physical modifications of GM have been studied, compared to other polysaccharides such as chitosan or alginate, the studies are not wide or deep enough. The mechanisms behind the effects of modifications on pharmaceutical characteristics, such as the relationship between structure and functionality/application of modified GM, are not clearly understood. Thus, the study of mechanisms of modified GM is necessary for its development as a potential pharmaceutical excipient.
In recent years, a wide variety of innovative approaches to modify GM have been developed through non-contaminating physical modification methods (green methods) such as microwave heating, ultrasound-assisted and hydrothermal processes, and ball milling. In addition, exploration of other plants as sources of GM may also be conducted to create a wider range of functionalities, which also may expand applicability.
References
- Lajoinie, A.; Henin, E.; Kassai, B.; Terry, D. Solid oral forms availability in children: A cost saving investigation. Br. J. Clin. Pharmacol. 2014, 78, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Huang, G.Q.; Xu, T.C.; Xiao, J.X. Characterization of carboxymethylated konjac glucomannan for potential application in colon-targeted delivery. Food Hydrocoll. 2019, 94, 354–362. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Jiang, H.; Li, Y.; Pang, J. Construction of carboxymethyl konjac glucomannan/chitosan complex nanogels as potential delivery vehicles for curcumin. Food Chem. 2021, 362, 130242. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, J.; Lei, J.; Li, Y.; Chen, T.; Duan, T.; Yao, W.; Zhou, J.; Yu, Y.; Liu, Y. Silver nanoparticles incorporated konjac glucomannan-montmorillonite nacre-like composite films for antibacterial applications. Carbohydr. Polym. 2018, 197, 253–259. [Google Scholar] [CrossRef]
- Xiao, C.; Weng, L.; Zhang, L. Improvement of physical properties of crosslinked alginate and carboxymethyl konjac glucomannan blend films. J. Appl. Polym. Sci. 2002, 84, 2554–2560. [Google Scholar] [CrossRef]
- Harmayani, E.; Aprilia, V.; Marsono, Y. Characterization of glucomannan from Amorphophallus oncophyllus and its prebiotic activity in vivo. Carbohydr. Polym. 2014, 112, 475–479. [Google Scholar] [CrossRef]
- Yanuriati, A.; Marseno, D.W.; Rochmadi Harmayani, E. Characteristics of glucomannan isolated from fresh tuber of Porang (Amorphophallus muelleri Blume). Carbohydr. Polym. 2017, 156, 56–63. [Google Scholar] [CrossRef]
- Septiawan, A.R.; Darma, G.C.; Aryani, R. Preparation and Characterization of Glucomannan from Porang Bulbs (Amorphophallus muelleri Blume.) as a tablet binder. Pros. Farm. 2021, 7, 508–515. [Google Scholar] [CrossRef]
- Cui, T.; Liu, R.; Wu, T.; Sui, W.; Zhang, M. Influence of konjac glucomannan and frozen storage on rheological and tensile properties of frozen dough. Polymers 2019, 11, 794. [Google Scholar] [CrossRef]
- Du, X.; Li, J.; Chen, J.; Li, B. Effect of degree of deacetylation on physicochemical and gelation properties of konjac glucomannan. Food Res. Int. 2012, 46, 270–278. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Lindström, M.E.; Stepan, A.; Gatenholm, P. Spruce glucomannan: Preparation, structural characteristics and basic film forming ability. Nord. Pulp Pap. Res. J. 2013, 28, 323–330. [Google Scholar] [CrossRef]
- Wang, K.; Fan, J.; Liu, Y.; He, Z. Konjac glucomannan and xanthan gum as compression coat for colonic drug delivery: Experimental and theoretical evaluations. Front. Chem. Eng. China 2010, 4, 102–108. [Google Scholar] [CrossRef]
- Long, X.Y.; Luo, X.G.; Zou, N.W.; Ma, Y.H. Preparation and in vitro evaluation of Carboxymethyl konjac glucomannan coated 5-aminosalicylic acid tablets for colonic delivery. Adv. Mater. Res. 2011, 152–153, 1712–1715. [Google Scholar] [CrossRef]
- Wu, K.; Zhu, Q.; Qian, H.; Xiao, M.; Corke, H.; Nishinari, K.; Jiang, F. Controllable hydrophilicity-hydrophobicity and related properties of konjac glucomannan and ethyl cellulose composite films. Food Hydrocoll. 2018, 79, 301–309. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, M.; Li, R.; Cai, Z.; Zhang, H. Thermostable physically crosslinked cryogel from carboxymethylated konjac glucomannan fabricated by freeze-thawing. Food Hydrocoll. 2022, 122, 107103. [Google Scholar] [CrossRef]
- Ai, T.; Shang, L.; He, C.; Teng, Y.; Ren, C.; Zhou, P.; Wang, L.; Li, J.; Li, B. Development of multi-layered gastric floating tablets based on konjac glucomannan: A modified calcium supplement with enhanced bioavailability. Food Funct. 2019, 10, 6429–6437. [Google Scholar] [CrossRef]
- Zhu, G.-Q.; Zhang, Y.; Liu, J.-H. Studies on drug release from aminophylline konjac glucomannan matrix tablet. China J. Chin. Mater. Med. 2007, 32, 2236–2239. [Google Scholar]
- Liu, J.; Zhang, L.; Wang, C.; Yuan, P.; Xin, Y. Study on novel colon position pulsatile capsule and its release in vitro. China J. Chin. Mater. Med. 2010, 35, 3127–3130. [Google Scholar]
- Cuña, M.; Alonso-Sande, M.; Remunãn-López, C.; Pivel, J.P.; Alonso-Lebrero, J.L.; Alonso, M.J. Development of phosphorylated glucomannan-coated Chitosan nanoparticles as nanocarriers for protein delivery. J. Nanosci. Nanotechnol. 2006, 6, 2887–2895. [Google Scholar] [CrossRef]
- Deshpande, G.G.; Borate, H.P.; Wangikar, S.S. Fabrication and Characterization of Composite Material Connecting Rod. Techno-Societal 2021, 2, 87–95. [Google Scholar]
- Patria, D.G.; Sutrisno, A.; Sukamto, S.; Lin, J. Process optimization in the development of porang glucomannan (Amorphophallus mulleri B.) incorporated into the restructured rice using a pasta extruder: Physicochemical properties, cooking characteristics, and an estimated glycemic index. Food Sci. Technol. 2021, 42, 1–9. [Google Scholar] [CrossRef]
- Xie, W.; Du, Y.; Yuan, S.; Pang, J. Dihydromyricetin incorporated active films based on konjac glucomannan and gellan gum. Int. J. Biol. Macromol. 2021, 180, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhu, P.; Chen, N.; Ye, X.; Wang, Y.; Xiao, S. Preparation and sustainable release of modified konjac glucomannan/chitosan nanospheres. Int. J. Biol. Macromol. 2016, 91, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ye, T.; Zhou, B.; Li, J.; He, L.; Li, Y.; Liu, S.; Chen, Y.; Li, B. Fabrication of gastric floating controlled release tablet based on konjac glucomannan. Food Res. Int. 2015, 72, 47–53. [Google Scholar] [CrossRef]
- Chéret, R.; Chapleau, N.; Delbarre-Ladrat, C.; Verrez-Bagnis, V.; Lamballerie, M.D. Effects of High Pressure on Texture and Microstructure of Sea Bass (Dicentrarchus labrax L.) Fillets. Science 2005, 70, 477–483. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.; Gong, J.; Mu, R.; Li, Y.; Wu, C.; Pang, J. Fabrication of chitosan-coated konjac glucomannan/sodium alginate/graphene oxide microspheres with enhanced colon-targeted delivery. Int. J. Biol. Macromol. 2019, 131, 209–217. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Zheng, B.; Guo, Z. Effects of high pressure processing on gelation properties and molecular forces of myosin containing deacetylated konjac glucomannan. Food Chem. 2019, 291, 117–125. [Google Scholar] [CrossRef]
- Li, B.; Li, J.; Xia, J.; Kennedy, J.F.; Yie, X.; Liu, T.G. Effect of gamma irradiation on the condensed state structure and mechanical properties of konjac glucomannan/chitosan blend films. Carbohydr. Polym. 2011, 83, 44–51. [Google Scholar] [CrossRef]
- Fan, L.; Yang, J.; Wu, H.; Hu, Z.; Yi, J.; Tong, J.; Zhu, X. Preparation and characterization of quaternary ammonium chitosan hydrogel with significant antibacterial activity. Int. J. Biol. Macromol. 2015, 79, 830–836. [Google Scholar] [CrossRef]
- Shi, X.D.; Yin, J.Y.; Zhang, L.J.; Huang, X.J.; Nie, S.P. Studies on O-acetyl-glucomannans from Amorphophallus species: Comparison of physicochemical properties and primary structures. Food Hydrocoll. 2019, 89, 503–511. [Google Scholar] [CrossRef]
- Wu, W.T.; Cheng, H.C.; Chen, H.L. Ameliorative effects of konjac glucomannan on human faecal -glucuronidase activity, secondary bile acid levels and faecal water toxicity towards Caco-2 cells. Br. J. Nutr. 2011, 105, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Wu, K.; Li, C.; Liu, J.; Ni, X.; Xiao, M.; Xu, Y.; Kuang, Y.; Jiang, F. pH-Sensitive drug delivery system based on hydrophobic modified konjac glucomannan. Carbohydr. Polym. 2017, 171, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, M.; Lv, W.; Qiu, P.; Gong, Y.; Li, D. Study on Rheological Behavior of Konjac Glucomannan. Phys. Procedia 2012, 33, 25–30. [Google Scholar] [CrossRef]
- Tatirat, O.; Charoenrein, S. Physicochemical properties of konjac glucomannan extracted from konjac flour by a simple centrifugation process. LWT Food Sci. Technol. 2011, 44, 2059–2063. [Google Scholar] [CrossRef]
- Koroskenyi, B.; McCarthy, S.P. Synthesis of acetylated konjac glucomannan and effect of degree of acetylation on water absorbency. Biomacromolecules 2001, 2, 824–826. [Google Scholar] [CrossRef]
- Guo, L.; Yokoyama, W.; Chen, L.; Liu, F.; Chen, M.; Zhong, F. Characterization and physicochemical properties analysis of konjac glucomannan: Implications for structure-properties relationships. Food Hydrocoll. 2021, 120, 106818. [Google Scholar] [CrossRef]
- Alves, A.; Miguel, S.P.; Araujo, A.R.T.S.; Jes, D. Xanthan Gum–Konjac Glucomannan Blend Hydrogel for Wound Dressings. Polymers 2020, 12, 99. [Google Scholar] [CrossRef]
- Hu, Y.; Tian, J.; Zou, J.; Yuan, X.; Li, J.; Liang, H.; Zhan, F.; Li, B. Partial removal of acetyl groups in konjac glucomannan significantly improved the rheological properties and texture of konjac glucomannan and κ-carrageenan blends. Int. J. Biol. Macromol. 2019, 123, 1165–1171. [Google Scholar] [CrossRef]
- Gong, J.; Wang, L.; Wu, J.; Yuan, Y.; Mu, R.J.; Du, Y.; Wu, C.; Pang, J. The rheological and physicochemical properties of a novel thermosensitive hydrogel based on konjac glucomannan/gum tragacanth. LWT 2019, 100, 271–277. [Google Scholar] [CrossRef]
- Kurt, A.; Kahyaoglu, T. Characterization of a new biodegradable edible film made from salep glucomannan. Carbohydr. Polym. 2014, 104, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Mei, T.; Wang, Y.; Xu, W.; Li, J.; Zhou, B.; Li, B. Synergistic degradation of konjac glucomannan by alkaline and thermal method. Carbohydr. Polym. 2014, 99, 270–277. [Google Scholar] [CrossRef]
- Su, L.; Ji, W.K.; Lan, W.Z.; Dong, X.Q. Chemical modification of xanthan gum to increase dissolution rate. Carbohydr. Polym. 2003, 53, 497–499. [Google Scholar] [CrossRef]
- Yu, H.; Xiao, C. Synthesis and properties of novel hydrogels from oxidized konjac glucomannan crosslinked gelatin for in vitro drug delivery. Carbohydr. Polym. 2008, 72, 479–489. [Google Scholar] [CrossRef]
- Lu, M.; Li, Z.; Liang, H.; Shi, M.; Zhao, L.; Li, W.; Chen, Y.; Wu, J.; Wang, S.; Chen, X.; et al. Controlled release of anthocyanins from oxidized konjac glucomannan microspheres stabilized by chitosan oligosaccharides. Food Hydrocoll. 2015, 51, 476–485. [Google Scholar] [CrossRef]
- Hongbo, T.; Lan, W.; Yanping, L.; Siqing, D. Effect of acidolysis and oxidation on structure and properties of konjac glucomannan. Int. J. Biol. Macromol. 2019, 130, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wu, Q.; Luo, X.; Liu, F.; Luo, X.; He, P. Effect of degree of acetylation on thermoplastic and melt rheological properties of acetylated konjac glucomannan. Carbohydr. Polym. 2010, 82, 167–172. [Google Scholar] [CrossRef]
- Wang, M.; He, W.; Wang, S.; Song, X. Carboxymethylated glucomannan as paper strengthening agent. Carbohydr. Polym. 2015, 125, 334–339. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tsujihata, S.; Hibi, N.; Tsukamoto, Y. Preparation and rheological characterization of carboxymethyl konjac glucomannan. Food Hydrocoll. 2002, 16, 289–294. [Google Scholar] [CrossRef]
- Xiao, M.; Dai, S.; Wang, L.; Ni, X.; Yan, W.; Fang, Y.; Corke, H.; Jiang, F. Carboxymethyl modification of konjac glucomannan affects water binding properties. Carbohydr. Polym. 2015, 130, 1–8. [Google Scholar] [CrossRef]
- Xie, Y.; Yi, Z.X.; Wang, J.X.; Hou, T.G.; Jiang, Q. Carboxymethyl konjac glucomannan-crosslinked chitosan sponges for wound dressing. Int. J. Biol. Macromol. 2018, 112, 1225–1233. [Google Scholar] [CrossRef]
- Fadilah Distantina, S.; Kaavessina, M.; Wijayanti, S.T.; Andayani, R. Study on the carboxymethylation of glucomannan from porang. AIP Conf. Proc. 2018, 1931, 030005. [Google Scholar]
- Wang, L.; Xiao, M.; Dai, S.; Song, J.; Ni, X.; Fang, Y.; Corke, H.; Jiang, F. Interactions between carboxymethyl konjac glucomannan and soy protein isolate in blended films. Carbohydr. Polym. 2014, 101, 136–145. [Google Scholar] [CrossRef]
- Xiao, J.X.; Wang, L.H.; Xu, T.C.; Huang, G.Q. Complex coacervation of carboxymethyl konjac glucomannan and chitosan and coacervate characterization. Int. J. Biol. Macromol. 2019, 123, 436–445. [Google Scholar] [CrossRef]
- Wang, C.; Li, B.; Chen, T.; Mei, N.; Wang, X.; Tang, S. Preparation and bioactivity of acetylated konjac glucomannan fibrous membrane and its application for wound dressing. Carbohydr. Polym. 2020, 229, 115404. [Google Scholar] [CrossRef] [PubMed]
- Korkiatithaweechai, S.; Umsarika, P.; Praphairaksit, N.; Muangsin, N. Controlled release of diclofenac from matrix polymer of chitosan and oxidized konjac glucomannan. Mar. Drugs 2011, 9, 1649–1663. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Li, K.; Xie, C.; Liu, J. Oxidized konjac glucomannan-cassava starch and sucrose esters as novel excipients for sustained-release matrix tablets. Int. J. Biol. Macromol. 2020, 156, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Pelton, R.; Leduc, M. Mechanical properties of polyelectrolyte complex films based on polyvinylamine and carboxymethyl cellulose. Ind. Eng. Chem. Res. 2006, 45, 6665–6671. [Google Scholar] [CrossRef]
- Rashid, S.; Dutta, H. Physicochemical characterization of carboxymethyl cellulose from differently sized rice husks and application as cake additive. LWT 2022, 154, 112630. [Google Scholar] [CrossRef]
- Ohya, Y.; Ihara, K.; Murata, J.; Sugitou, T.; Ouchi, T. Preparation and biological properties of dicarboxy-glucomannan: Enzymatic degradation and stimulating activity against cultured macrophages. Carbohydr. Polym. 1994, 25, 123–130. [Google Scholar] [CrossRef]
- Sa, B.; Mukherjee, S.; Roy, S.K. Effect of polymer concentration and solution pH on viscosity affecting integrity of a polysaccharide coat of compression coated tablets. Int. J. Biol. Macromol. 2019, 125, 922–930. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, B.Z.; Wang, Q.L.; Jin, X.; Guo, X.D. Fabrication of coated polymer microneedles for transdermal drug delivery. J. Control. Release 2017, 265, 14–21. [Google Scholar] [CrossRef]
- Fowler, P.D.; Ruscher, C.; McGraw, J.D.; Forrest, J.A.; Dalnoki-Veress, K. Controlling Marangoni-induced instabilities in spin-cast polymer films: How to prepare uniform films. Eur. Phys. J. E 2016, 39, 90. [Google Scholar] [CrossRef]
- Nair, S.B.; Jyothi, A.N.; Sajeev, M.S.; Misra, R. Rheological, mechanical and moisture sorption characteristics of cassava starch-konjac glucomannan blend films. Starch-Staerke 2011, 63, 728–739. [Google Scholar] [CrossRef]
- Xu, M.; Li, D.S.; Li, B.; Wang, C.; Zhu, Y.P.; Lv, W.P.; Xie, B.J. Comparative study on molecular weight of konjac glucomannan by gel permeation chromatography-laser light scattering-refractive index and laser light-scattering methods. J. Spectrosc. 2013, 2013, 685698. [Google Scholar] [CrossRef]
- Jacon, S.A.; Rao, M.A.; Cooley, H.J.; Walter, R.H. The isolation and characterization of a water extract of konjac flour gum. Carbohydr. Polym. 1993, 20, 35–41. [Google Scholar] [CrossRef]
- He, Y.; Wang, S.; Li, J.; Liang, H.; Wei, X.; Peng, D.; Jiang, Z.; Li, B. Interaction between konjac glucomannan and tannic acid: Effect of molecular weight, pH and temperature. Food Hydrocoll. 2019, 94, 451–458. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, H.; Liu, X.; Li, Z.; Liu, B.; Wu, J.; Shi, M.; Norde, W.; Li, Y. TEMPO-oxidized Konjac glucomannan as appliance for the preparation of hard capsules. Carbohydr. Polym. 2016, 143, 262–269. [Google Scholar] [CrossRef]
- Bin, L.K.; Mohammed Helaluddin, A.B.; Islam Sarker, M.Z.; Mandal, U.K.; Gaurav, A. Effect of processing methods on xylitol-starch base co-processed adjuvant for orally disintegrating tablet application. Pak. J. Pharm. Sci. 2020, 33, 551–559. [Google Scholar]
- Narang, A.S.; Boddu, S.H. Excipient applications in formulation design and drug delivery. In Excipient Applications in Formulation Design and Drug Delivery; Springer: Cham, Switzerland, 2015; pp. 1–681. [Google Scholar]
- Hardikar, S.; Bhosale, A. Formulation and evaluation of gastro retentive tablets of clarithromycin prepared by using novel polymer blend. Bull. Fac. Pharm. Cairo Univ. 2018, 56, 147–157. [Google Scholar] [CrossRef]
- Ermawati, D.E.; Andini, B.P.; Prihapsara, F.; Farida, Y.; Rohmani, S.; Kundarto, W.; Nugraheni, E.R. Optimization of Suweg starch (Amorphophallus paeoniifolius (Dennst.) Nicolson) and lactose as co-processed excipient of Ibuprofen-PEG 6000 solid dispersion of effervescent tablet. AIP Conf. Proc. 2020, 2237, 020061. [Google Scholar]
This entry is adapted from the peer-reviewed paper 10.3390/polym14132550
