Chitosan has emerged as a biodegradable, nontoxic polymer with multiple beneficial applications in the agricultural and biomedical sectors. As nanotechnology has evolved as a promising field, researchers have incorporated chitosan-based nanomaterials in a variety of products to enhance their efficacy and biocompatibility. Moreover, due to its inherent antimicrobial and chelating properties, and the availability of modifiable functional groups, chitosan nanoparticles were also directly used in a variety of applications. In this section, the use of chitosan-based nanomaterials in agricultural and biomedical fields related to the management of abiotic stress in plants, water availability for crops, controlling foodborne pathogens, and cancer photothermal therapy is discussed. Overall, chitosan-based nanomaterials show promising characteristics for sustainable agricultural practices and effective healthcare in an eco-friendly manner.
- chitosan
- nanoparticles
- abiotic stress
- water purification
- foodborne pathogens
- cancer photothermal therapy
1. Introduction
In the realm of climate change, increasing population, and the decrease in the land that can be cultivated, agriculture and health systems are facing numerous challenges. Nanotechnology can play an essential role in addressing these issues by promoting enhanced crop production, optimum usage of the land, and the creation of advanced drugs. The small size of the nanomaterials is advantageous in crossing the biological barriers and carrying the required molecules into various locations in animals and plants. However, the nanoparticle size and concentration should be optimized based on the intended application to minimize toxic side effects. When properly used, nanoparticles engineered from chitosan and its derivatives can be indispensable in addressing issues related to feeding the increasing population and improving healthcare.
Chitosan is a linear copolymer with D–glucosamine and N–acetyl-D–glucosamine units joined via β–(1–4) glycosidic bonds (Figure 1) and has been extensively used for the production of nanoparticles by researchers, as reviewed in this article. Chitosan has been popular due to its antimicrobial, antioxidant, and chelating properties, together with its nontoxic and biocompatible nature [1]. As a cationic polymer, chitosan inherently possesses bio-adhesion, cellular transfection, anti-inflammatory, and anti-hypercholesterolemic characteristics, which can be enhanced by combining with other materials, making it an attractive candidate for biomedical and agricultural applications [2]. Chitosan is also an excellent carrier for nanoparticles due to its ability to penetrate across cellular barriers and to flow through narrow intercellular junctions in epithelial cells [3]. The availability of hydroxyl and amino groups on chitosan provides an excellent platform for complexation with other molecules/compounds and helps to transform them into more stable complexes with better pharmacokinetic properties [3]. Additionally, due to the availability of functional groups, chitosan can be modified in different ways to obtain substituted, crosslinked, carboxylated, ionic and bounded derivatives to match the various research needs [2]. The different methods utilized in synthesizing chitosan-based nanoparticles, such as emulsion crosslinking, emulsion-droplet coalescence, ionotropic gelation, reverse micellisation, and precipitation, have been described in detail in the literature [4][5].
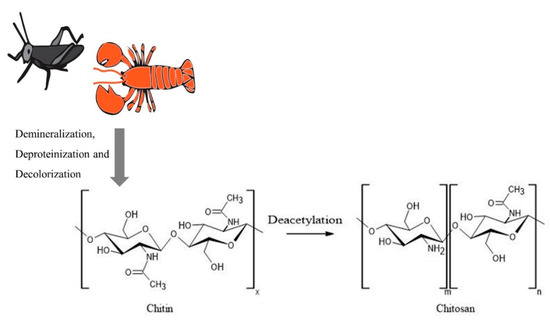
Figure 1. Process of chitosan production starting with different sources. The figure was created using ACD/ChemSketch and Adobe Illustrator 2020.
Chitosan is obtained from a variety of sources by deacetylation of chitin and contains more than 7% nitrogen and less than 40% degree of acetylation [6]. Although the primary commercial production source for chitosan is crustacean shells, their seasonal availability and the use of harsh chemicals in the production, and the generation of large amounts of alkaline waste had led to environmental issues as well as product quality variability [6]. Furthermore, the use of crustacean sources can limit the application of the polymer, specifically in the biomedical field, due to the associated shellfish allergies. Hence, researchers have looked into different sources for the production of chitosan. Shanmuganathan et al. have extensively reviewed a variety of sources for the extraction of chitosan, including various species of microorganisms, insects, and other aquatic animals [7]. Additionally, chitinous cell wall material was isolated from yeast species Rhodosporidium paludigenum and Saccharomyces cerevisiae [8]. Fungal mycelium of Allomyces arbuscula, Mucor genevensis, Tranetes versicolor, and the fruiting body of Agaricus bisporus were also utilized as more cost-effective and renewable sources for chitin compared to crustacean shells [9]. A pest attacking agricultural crop in Mexico and Central America, Schistocerca piceifrons piceifrons (Orthoptera: Acrididae) has also been used for the successful extraction of chitin and chitosan at 11.88% and 9.11% yield, respectively [10]. These sources undergo demineralization, deproteinization, and decolorization to produce chitin, and it is converted to chitosan via different chemical and enzymatic deacetylation methods as described by Shanmuganathan et al. (Figure 1).
2. Applications in Agriculture
Chitosan-based nanoparticles (CNPs) have been used in agriculture as pesticides, herbicides, insecticides, and to obtain better quality food products with a higher yield, and many of these applications of chitosan have been extensively reviewed in the literature [11]. Nano-chitosan based materials or chitosan combined with other nanoparticles were applied to preserve fresh fruits such as strawberries [12], Jujube [13], loquat [14], and longan [15] during storage. Additionally, chitosan can serve as an encapsulating agent by itself and in combination with other materials in the production of slow-release fertilizers, owing to its cationic nature, biodegradability, non-toxicity, and adsorption properties [4][16].
However, the usage of chitosan-based nanomaterials in water purification for agricultural uses and managing abiotic stress in plants are areas that had gained limited attention from the scientific community. Due to drought and rapid development of industry in some regions, people have to look for alternative water resources, such as seawater and non-conventional water, for agricultural irrigation. Non-conventional water can be wastewater emitted from domestic, municipal, and industrial sewer, and saline water from salt lakes and shale oil and gas industry. Microstructures, nanoparticles, and nanocomposites of chitosan have widely been used as an absorbent to remove various inorganic and organic pollutants as rich hydroxyl and amino groups are present in the crosslinked structure of chitosan [17][18]. Additionally, nanochitosan has been shown to be effective in combating salinity stress[19] and drought stress in plants [20]. During previous research, chitosan in bulk form was shown to alleviate the effects of salt stress for wheat [21], chickpea [22], lentils [23], and ajowan seeds [24]. Nanochitosan could possibly have a more significant effect on these crops due to its high surface to volume ratio resulting in higher penetrability and the ability to form more interactions. Also, Rabelo et al. (2019) reported that the foliar application of bulk chitosan derivatives, N-succinyl chitosan and N,O-dicarboxymethylated chitosan, had increased the tolerance of water stress in a hybrid maize species that is sensitive to drought stress [25]. These chitosan derivatives were shown to induce the antioxidant defense system, increase the production of phenolic compounds, osmoregulators, and crop yield, and promote gas exchange in leaves [25]. Hence, it is possible that nanochitosan derivatives also will have a positive effect on the growth of these plants under drought stress. Low and high-temperature stress and metal contaminated soil are two other common factors affecting Agricultural land use. Although recent research on the use of nanochitosan to alleviate the stress caused by heat and heavy metals are scarce, there are reports on the use of bulk chitosan [26][27][28]. Hence, the use of nanochitosan to overcome both high and low-temperature stress and to relieve metal toxicity in the soil are fields that can be evaluated in future research.
3. Biomedical Applications of Nanochitosan
Chitosan is preferably used in the biomedical field due to its favorable properties such as biocompatibility, cationic nature, and availability of modifiable functional groups. Some of the popular applications of chitosan nanomaterials include the preparation of bio-sensors, wound healing and wound dressing, gene delivery, and bone tissue engineering [29][30][31][32][33]. Additionally, chitosan conjugated folic acid was used for the synthesis of ZnS quantum dots, which are formulated into nanocarriers with the potential for suicide gene therapy [34], and chitosan-ZnS-FA nanoparticles were synthesized as a potential anticancer therapeutic agent [35].
Although the use of chitosan as an antimicrobial is extensively reviewed, more focused analysis on chitosan nanoparticles towards foodborne pathogens (FBPs) is scarce. According to CDC estimates, every year in the United States, around 48 million cases of foodborne illnesses can occur together with 128,000 hospitalizations and 3000 deaths [36]. Additionally, as estimated by WHO, foodborne pathogens can cause 600 million cases of foodborne illnesses and 420,000 deaths in the globe every year, where 30% of the deaths are accounted for the children under five years of age [37]. Given these facts, in recent years, there has been an increased interest among researchers to discover novel antimicrobial agents against foodborne pathogens. Chitosan-based nanoparticles have been widely ued to control FBPs, with and without combining with other materials. The analysis of the antimicrobial activity of low and high molecular weight chitosan nanoparticles (CSNPs) formulated using sodium sulfate or tripolyphosphate cross-linkers with or without sonication at different energy levels have shown to be effective against Escherichia coli O15:H7 [38]. CSNPs synthesized by ionic gelation to be used as an edible coating on grapes was reported to inhibit certain FBPs [39]. A vegetable wash prepared with CSNPs mixed with 1% citric acid has resulted in an increased reduction of the bacteria load on lettuce under simulated conditions compared to commercial formulations [40]. Mohammadi et al. (2016) reported that CSNPs showed significantly higher antimicrobial effect against E. coli compared to microparticles, and they have tested with different molecular weight chitosan formulations [41]. Additionally, CSNPs, combined with plant essential oils [42][43][44] and other naturally occurring antimicrobials such as propolis and nisin were reported in the literature [45][46]. Bulk chitosan-based materials with and without plant extracts were shown to have an antiviral effect against foodborne viruses (Murine Norovirus, MS2 phage, Feline calicivirus) [47]. Additionally, the use of chitosan-based material to treat human norovirus, the number one viral foodborne pathogen, has been tested [48]. But we didn’t come across any reports on nanochitosan against foodborne viruses. This shows a possible avenue for future research with nanochitosan against these viruses. The research has demonstrated that combined products have a higher impact compared to CSNPs by itself, indicating that the combination of different antimicrobial agents with chitosan can be more productive in controlling foodborne microbes.
Furthermore, the use of nanochitosan-based materials for cancer photothermal therapy is an emerging research field that is not broadly conversed. During recent years, cancer has become a major health crisis throughout the globe costing thousands of lives each year. According to the World Health Organization (WHO), every sixth death in the world is caused by cancer [49]. The existing treatments, such as chemotherapy and radiation therapy, can be challenging due to harmful effects exerted on adjacent healthy tissues. Chemotherapeutic agents can affect the noncancerous cells that are dividing rapidly. Additionally, the other issues with chemotherapeutics include systemic accumulation, developing drug resistance, and lower effective concentrations at the target sites, leading to undesirable outcomes in cancer patients. The high doses of radiation used in radiotherapy are known to heighten the invasive properties of cancer cells, and certain cancer types have developed resistance to the radiation. Hence, targeted thermal treatments such as photothermal therapy have gained the attention of scientists due to its advantages such as cost-effectiveness, reduced side effects, and noninvasive nature [49]. Nanoparticles containing photothermal agents introduced into tumor sites can be induced by near-infrared (NIR) light in the wavelength range at first (700–980 nm) or second (1000–1400 nm) biological window [50]. Living tissues and biological substances minimally absorb light in the NIR window leading to lower phototoxicity and have deeper tissue penetration compared to UV or visible light, making it the preferred light source for cancer therapy [51]. Various types of photothermal agents such as carbon-based compounds (graphene oxide, carbon quantum dots), inorganic nanomaterials (gold, silver, and copper nanoparticles), and NIR sensitive dyes have been extensively studied in the literature. However, their application as photothermal agents has been limited due to characteristics such as poor internalization, low stability, high toxicity, and lower biocompatibility. Chitosan and its water-soluble derivatives have been successfully used to overcome these issues due to its cationic nature, biocompatibility, and swelling properties. The chemical structure of chitosan has partial similarity to hyaluronic acid, which acts as a ligand for CD44 receptors that are highly expressed on several cancer cells. For example, a nanocarrier system formulated with chitosan has shown impressive anticancer effect against breast cancer spheroids overexpressing CD44 receptors showing its potential as a targeting moiety [52]. Hence, chitosan and chitosan derivative-based nanomaterials have been widely used for cancer photothermal therapy to obtain products with better pharmacokinetic properties [53][54][55][56][57][58][59][60][61].
4. Future Directions and Concluding Remarks
The usage of nontoxic, biodegradable polymer systems like chitosan for the progress of the agricultural and biomedical fields is beneficial for the society and the ecosystem. Among the many uses of nanochitosan in the agricultural sector, its involvement in alleviating abiotic stresses and application in water purification for agricultural purposes can maximize the land and water usage for crop production. Compared with conventional materials, renewable chitosan nanoparticles used as a bioflocculant and a heavy metal adsorbent demonstrate better or compatible performance in industrial or agricultural wastewater treatments. In the face of climate change, the well-treated non-conventional water (e.g., industrial wastewater) can be a viable option for crop irrigation to enhance food security for the increasing global population. Even though nano-chitosan has been widely studied related to its uses as a fertilizer, herbicide, insecticide, and a carrier system, additional research is needed to exploit its capabilities in abiotic stress management. Notably, in combating heat and heavy metal stress, studies with bulk chitosan have shown promising results, but there is a lack of reports on the usage of nanochitosan in these areas. Due to the chelating properties of chitosan, it can also be a useful soil conditioner to complex toxic metals in polluted soil. Additionally, it is possible to adapt nano-chitosan based heavy metal remediation methods used in other areas such as water purification and biomedical treatments to suit applications in soil treatments. Moreover, there are only a few studies on the use of chitosan derivatives to reduce abiotic stress in plants, even in the bulk form. Therefore, the use of nano-chitosan derivatives can be a novel area with a high potential to combat abiotic stress in plants that can be explored in the future.
When considering antimicrobial studies, chitosan is used together with a variety of other natural antimicrobials such as essential oils, propolis from beehives, curcumin, and peptides to control FBPs. It is also clear that the combined effect of chitosan with other antimicrobials is higher in most of the reported studies, displaying the synergistic antimicrobial effect of chitosan. However, the use of chitosan-based nanomaterials for treating viral foodborne pathogens is not well studied. Hence, this can be a future research avenue related to the antimicrobial activity of chitosan. Additionally, nanochitosan and its derivatives have been extensively used in the production of photothermal agents to treat cancer. Even though chitosan is not a photothermal agent by itself, its biocompatible and swelling properties have assisted in formulating therapeutics with required characteristics. For example, chitosan and its derivatives were able to impart greater stability, dispersibility, increased tumor retention and helped to alleviate cytotoxicity of the nanotherapeutic agents. Liu et al., 2019 reported that the incorporation of carboxymethyl chitosan on graphene oxide nanosheets had enhanced the absorption of NIR light by the compound [62]. Chitosan was used as a reducing agent for graphene oxide-based therapeutics and gold and as a precursor for the synthesis of other therapeutics [63][64][65]. Additionally, chitosan derivatives such as thiol chitosan, hydroxyethyl chitosan, and glycol chitosan have widely been used in nanoformulations to enable cellular internalization and loading of other drugs to form multifunctional therapeutics. Furthermore, pH-sensitive drug delivery systems were developed using chitosan due to its ability to undergo reversible protonation and deprotonation of amine groups inducing pH-controlled drug release in the acidic tumor microenvironment [66]. Chitosan is also known to contain inherent anticancer properties, based on its ability to induce the production of Tissue Necrotic Factor-α via monocytes, further enhancing its value in cancer therapy [67]. Owing to its nontoxic, biocompatible, and anticancer nature, the use of nano-chitosan and derivatives in noninvasive cancer therapies such as PTT show great potential for future cancer treatments.
Despite the many records related to applications of nanochitosan, still, there is more work to be done to bring these up to field trials and clinical usage. Although chitosan is known to be nontoxic by nature, when formulated into nanoparticles, dosage-dependent issues can arise due to its enhanced ability to accumulate in the water, soil, and biological systems compared to the bulk material. Additionally, other compounds incorporated with chitosan, such as metals, can pose an environmental and health risk during the usage. Therefore, more research needs to be conducted related to the toxicity of these formulations before the final stages of application. Furthermore, a better understanding of the mechanism of action of nanomaterials related to agricultural and biomedical fields can shed light on proper usage of those with minimum damage to the environment, while obtaining the highest benefits for human beings.
This entry is adapted from the peer-reviewed paper 10.3390/nano10101903
References
- Gedda, G.; Lee, C.-Y.; Lin, Y.-C.; Wu, H.-f. Green synthesis of carbon dots from prawn shells for highly selective and sensitive detection of copper ions. Sensors and Actuators B: Chemical 2016, 224, 396-403, doi:https://doi.org/10.1016/j.snb.2015.09.065.
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. International Journal of Biological Macromolecules 2020, 152, 681-702, doi:https://doi.org/10.1016/j.ijbiomac.2020.02.196.
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Noqta, O.A.; Mehrdel, B. Synthesis and coating methods of biocompatible iron oxide/gold nanoparticle and nanocomposite for biomedical applications. Chinese Journal of Physics 2020, 64, 305-325, doi:https://doi.org/10.1016/j.cjph.2019.11.014.
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. International Journal of Biological Macromolecules 2015, 77, 36-51, doi:https://doi.org/10.1016/j.ijbiomac.2015.02.039.
- Naskar, S.; Sharma, S.; Kuotsu, K. Chitosan-based nanoparticles: An overview of biomedical applications and its preparation. Journal of Drug Delivery Science and Technology 2019, 49, 66-81, doi:https://doi.org/10.1016/j.jddst.2018.10.022.
- Tayel, A.A.; Ibrahim, S.I.; Al-Saman, M.A.; Moussa, S.H. Production of fungal chitosan from date wastes and its application as a biopreservative for minced meat. International journal of biological macromolecules 2014, 69, 471-475, doi:10.1016/j.ijbiomac.2014.05.072.
- Shanmuganathan, R.; Edison, T.N.J.I.; LewisOscar, F.; Kumar, P.; Shanmugam, S.; Pugazhendhi, A. Chitosan nanopolymers: An overview of drug delivery against cancer. International Journal of Biological Macromolecules 2019, 130, 727-736, doi:https://doi.org/10.1016/j.ijbiomac.2019.02.060.
- Sun, C.; Fu, D.; Jin, L.; Chen, M.; Zheng, X.; Yu, T. Chitin isolated from yeast cell wall induces the resistance of tomato fruit to Botrytis cinerea. Carbohydrate Polymers 2018, 199, 341-352, doi:https://doi.org/10.1016/j.carbpol.2018.07.045.
- Jones, M.; Weiland, K.; Kujundzic, M.; Theiner, J.; Kählig, H.; Kontturi, E.; John, S.; Bismarck, A.; Mautner, A. Waste-Derived Low-Cost Mycelium Nanopapers with Tunable Mechanical and Surface Properties. Biomacromolecules 2019, 20, 3513-3523, doi:10.1021/acs.biomac.9b00791.
- Pérez-Ramírez, R.; Torres-Castillo, J.A.; Barrientos-Lozano, L.; Almaguer-Sierra, P.; Torres-Acosta, R.I. Schistocerca piceifrons piceifrons (Orthoptera: Acrididae) as a Source of Compounds of Biotechnological and Nutritional Interest. J Insect Sci 2019, 19, 10, doi:10.1093/jisesa/iez088.
- Kumaraswamy, R.V.; Kumari, S.; Choudhary, R.C.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Engineered chitosan based nanomaterials: Bioactivities, mechanisms and perspectives in plant protection and growth. International Journal of Biological Macromolecules 2018, 113, 494-506, doi:https://doi.org/10.1016/j.ijbiomac.2018.02.130.
- Eshghi, S.; Hashemi, M.; Mohammadi, A.; Badii, F.; Mohammadhoseini, Z.; Ahmadi, K. Effect of Nanochitosan-Based Coating With and Without Copper Loaded on Physicochemical and Bioactive Components of Fresh Strawberry Fruit (Fragaria x ananassa Duchesne) During Storage. Food and Bioprocess Technology 2014, 7, 2397-2409, doi:10.1007/s11947-014-1281-2.
- Yu, Y.; Zhang, S.; Ren, Y.; Li, H.; Zhang, X.; Di, J. Jujube preservation using chitosan film with nano-silicon dioxide. Journal of Food Engineering 2012, 113, 408-414, doi:https://doi.org/10.1016/j.jfoodeng.2012.06.021.
- Song, H.; Yuan, W.; Jin, P.; Wang, W.; Wang, X.; Yang, L.; Zhang, Y. Effects of chitosan/nano-silica on postharvest quality and antioxidant capacity of loquat fruit during cold storage. Postharvest Biology and Technology 2016, 119, 41-48, doi:https://doi.org/10.1016/j.postharvbio.2016.04.015.
- Shi, S.; Wang, W.; Liu, L.; Wu, S.; Wei, Y.; Li, W. Effect of chitosan/nano-silica coating on the physicochemical characteristics of longan fruit under ambient temperature. Journal of Food Engineering 2013, 118, 125-131, doi:https://doi.org/10.1016/j.jfoodeng.2013.03.029.
- Corradini, E.; de Moura, M.R.; Mattoso, L.H.C. A preliminary study of the incorparation of NPK fertilizer into chitosan nanoparticles. Express Polymer Letters 2010, 4, 509-515, doi:10.3144/expresspolymlett.2010.64.
- Olivera, S.; Muralidhara, H.B.; Venkatesh, K.; Guna, V.K.; Gopalakrishna, K.; Kumar, K.Y. Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: A review. Carbohydrate Polymers 2016, 153, 600-618, doi:10.1016/j.carbpol.2016.08.017.
- Divya, K.; Jisha, M. Chitosan nanoparticles preparation and applications. Environmental chemistry letters 2018, 16, 101-112.
- Dawoud, S.; Mamdouh, Z.; Elkafafi, S.; Zedan, A. Effect of Nano Chitosan on Growth, Physiological and Biochemical Parameters of Phaseolus vulgaris under Salt Stress. Journal of Plant Production 2017, 8, doi:10.21608/jpp.2017.40468.
- Behboudi, F.; Tahmasebi Sarvestani, Z.; Kassaee, M.Z.; Modares Sanavi, S.A.M.; Sorooshzadeh, A.; Ahmadi, S.B. Evaluation of Chitosan Nanoparticles Effects on Yield and Yield Components of Barley (Hordeum vulgare L.) under Late Season Drought Stress. Journal of Water and Environmental Nanotechnology 2018, 3, 22-39, doi:10.22090/jwent.2018.01.003.
- Ma, L.; Li, Y.; Yu, C.; Wang, Y.; Li, X.; Li, N.; Chen, Q.; Bu, N. Alleviation of exogenous oligochitosan on wheat seedlings growth under salt stress. Protoplasma 2012, 249, 393-399, doi:10.1007/s00709-011-0290-5.
- Mahdavi, B.; Safari, H.; Modarres Sanavy, S.A.M. Effect Of Chitosan Seed Priming On Germination, Ion Relations And Biochemical Characteristics Of Chickpea Under Salinity Stress. Plant Products Technology (Agricultural Research) 2015, 15.
- Al-Tawaha, A.R.M.; Al-ghzawi, A.L.A. Effect of chitosan coating on seed germination and salt tolerance of lentil (Lens culinaris L.). Res. on Crops 2013, 14, 489-491.
- Mahdavi, B.; Rahimi, A. Seed priming with chitosan improves the germination and growth performance of ajowan (Carum copticum) under salt stress. EurAsian Journal of BioSciences 2013, 7, doi:10.5053/ejobios.2013.7.0.9.
- Rabêlo, V.M.; Magalhães, P.C.; Bressanin, L.A.; Carvalho, D.T.; Reis, C.O.d.; Karam, D.; Doriguetto, A.C.; Santos, M.H.d.; Santos Filho, P.R.d.S.; Souza, T.C.d. The foliar application of a mixture of semisynthetic chitosan derivatives induces tolerance to water deficit in maize, improving the antioxidant system and increasing photosynthesis and grain yield. Scientific Reports 2019, 9, 8164, doi:10.1038/s41598-019-44649-7.
- Ibrahim, E.A.; Ramadan, W.A. Effect of zinc foliar spray alone and combined with humic acid or/and chitosan on growth, nutrient elements content and yield of dry bean (Phaseolus vulgaris L.) plants sown at different dates. Scientia Horticulturae 2015, 184, 101-105, doi:https://doi.org/10.1016/j.scienta.2014.11.010.
- Guan, Y.-j.; Hu, J.; Wang, X.-j.; Shao, C.-x. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J Zhejiang Univ Sci B 2009, 10, 427-433, doi:10.1631/jzus.B0820373.
- Kamari, A.; Pulford, I.D.; Hargreaves, J.S.J. Chitosan as a potential amendment to remediate metal contaminated soil — A characterisation study. Colloids and Surfaces B: Biointerfaces 2011, 82, 71-80, doi:https://doi.org/10.1016/j.colsurfb.2010.08.019.
- Ali, A.; Ahmad, M.; Abbas, G.; Akhtar, M.N.; Atif, M. UREA biosensor based on magnetic nano particles (Co3O4, Fe3O4 ) for the estimation of urea concentration in blood and urine samples. Journal of Optoelectronics and Advanced Materials 2015, 17, 1515 - 1521.
- Ishihara, M.; Kishimoto, S.; Nakamura, S.; Sato, Y.; Hattori, H. Polyelectrolyte Complexes of Natural Polymers and Their Biomedical Applications. Polymers 2019, 11, doi:10.3390/polym11040672.
- Lee, K.; Oh, M.H.; Lee, M.S.; Nam, Y.S.; Park, T.G.; Jeong, J.H. Stabilized calcium phosphate nano-aggregates using a dopa-chitosan conjugate for gene delivery. International Journal of Pharmaceutics 2013, 445, 196-202, doi:https://doi.org/10.1016/j.ijpharm.2013.01.014.
- Li, J.; Sun, H.; Sun, D.; Yao, Y.; Yao, F.; Yao, K. Biomimetic multicomponent polysaccharide/nano-hydroxyapatite composites for bone tissue engineering. Carbohydrate Polymers 2011, 85, 885-894, doi:https://doi.org/10.1016/j.carbpol.2011.04.015.
- Razavi, M.; Qiao, Y.; Thakor, A.S. Three-dimensional cryogels for biomedical applications. Journal of biomedical materials research. Part A 2019, 107, 2736-2755, doi:10.1002/jbm.a.36777.
- Jaiswal, A.; Chattopadhyay, A.; Ghosh, S.S. Functional chitosan nanocarriers for potential applications in gene therapy. Materials Letters 2012, 68, 261-264, doi:https://doi.org/10.1016/j.matlet.2011.10.082.
- Bandara, S.; Carnegie, C.-A.; Johnson, C.; Akindoju, F.; Williams, E.; Swaby, J.M.; Oki, A.; Carson, L.E. Synthesis and characterization of Zinc/Chitosan-Folic acid complex. Heliyon 2018, 4, e00737-e00737, doi:10.1016/j.heliyon.2018.e00737.
- Centers for disease control and prevention. Burden of Foodborne Illness: Findings. Availabe online: https://www.cdc.gov/foodborneburden/2011-foodborne-estimates.html (accessed on June 2020).
- World health organization. Estimating the burden of foodborne diseases. Availabe online: https://www.who.int/activities/estimating-the-burden-of-foodborne-diseases (accessed on June 2020).
- Garrido-Maestu, A.; Ma, Z.; Paik, S.-Y.-R.; Chen, N.; Ko, S.; Tong, Z.; Jeong, K.C. Engineering of chitosan-derived nanoparticles to enhance antimicrobial activity against foodborne pathogen Escherichia coli O157:H7. Carbohydrate Polymers 2018, 197, 623-630, doi:https://doi.org/10.1016/j.carbpol.2018.06.046.
- Castelo Branco Melo, N.F.; de MendonçaSoares, B.L.; Marques Diniz, K.; Ferreira Leal, C.; Canto, D.; Flores, M.A.P.; Henrique da Costa Tavares-Filho, J.; Galembeck, A.; Montenegro Stamford, T.L.; Montenegro Stamford-Arnaud, T., et al. Effects of fungal chitosan nanoparticles as eco-friendly edible coatings on the quality of postharvest table grapes. Postharvest Biology and Technology 2018, 139, 56-66, doi:https://doi.org/10.1016/j.postharvbio.2018.01.014.
- Paomephan, P.; Assavanig, A.; Chaturongakul, S.; Cady, N.C.; Bergkvist, M.; Niamsiri, N. Insight into the antibacterial property of chitosan nanoparticles against Escherichia coli and Salmonella Typhimurium and their application as vegetable wash disinfectant. Food Control 2018, 86, 294-301, doi:https://doi.org/10.1016/j.foodcont.2017.09.021.
- Mohammadi, A.; Hashemi, M.; Masoud Hosseini, S. Effect of chitosan molecular weight as micro and nanoparticles on antibacterial activity against some soft rot pathogenic bacteria. LWT - Food Science and Technology 2016, 71, 347-355, doi:https://doi.org/10.1016/j.lwt.2016.04.010.
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Development of bioactive fish gelatin/chitosan nanoparticles composite films with antimicrobial properties. Food Chemistry 2016, 194, 1266-1274, doi:https://doi.org/10.1016/j.foodchem.2015.09.004.
- Kavaz, D.; Idris, M.; Onyebuchi, C. Physiochemical characterization, antioxidative, anticancer cells proliferation and food pathogens antibacterial activity of chitosan nanoparticles loaded with Cyperus articulatus rhizome essential oils. International Journal of Biological Macromolecules 2019, 123, 837-845, doi:https://doi.org/10.1016/j.ijbiomac.2018.11.177.
- Lee, K.H.; Lee, J.-S.; Kim, E.S.; Lee, H.G. Preparation, characterization, and food application of rosemary extract-loaded antimicrobial nanoparticle dispersions. LWT 2019, 101, 138-144, doi:https://doi.org/10.1016/j.lwt.2018.10.072.
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; Ramos-García, M.d.L.; Martínez-González, M.d.C.; Hernández-Romano, J. Physicochemical characterization and antimicrobial activity of edible propolis-chitosan nanoparticle films. Progress in Organic Coatings 2019, 137, 105326, doi:https://doi.org/10.1016/j.porgcoat.2019.105326.
- Niaz, T.; Shabbir, S.; Noor, T.; Rahman, A.; Bokhari, H.; Imran, M. Potential of polymer stabilized nano-liposomes to enhance antimicrobial activity of nisin Z against foodborne pathogens. LWT 2018, 96, 98-110, doi:https://doi.org/10.1016/j.lwt.2018.05.029.
- Bosch, A.; Gkogka, E.; Le Guyader, F.S.; Loisy-Hamon, F.; Lee, A.; van Lieshout, L.; Marthi, B.; Myrmel, M.; Sansom, A.; Schultz, A.C., et al. Foodborne viruses: Detection, risk assessment, and control options in food processing. International Journal of Food Microbiology 2018, 285, 110-128, doi:https://doi.org/10.1016/j.ijfoodmicro.2018.06.001.
- DiCaprio, E.; Ma, Y.; Hughes, J.; Li, J. Epidemiology, Prevention, and Control of the Number One Foodborne Illness: Human Norovirus. Infectious Disease Clinics of North America 2013, 27, 651-674, doi:https://doi.org/10.1016/j.idc.2013.05.009.
- Murugan, C.; Sharma, V.; Murugan, R.K.; Malaimegu, G.; Sundaramurthy, A. Two-dimensional cancer theranostic nanomaterials: Synthesis, surface functionalization and applications in photothermal therapy. Journal of Controlled Release 2019, 299, 1-20, doi:https://doi.org/10.1016/j.jconrel.2019.02.015.
- Chen, Y.; Wang, L.; Shi, J. Two-dimensional non-carbonaceous materials-enabled efficient photothermal cancer therapy. Nano Today 2016, 11, 292-308, doi:https://doi.org/10.1016/j.nantod.2016.05.009.
- Saneja, A.; Kumar, R.; Arora, D.; Kumar, S.; Panda, A.K.; Jaglan, S. Recent advances in near-infrared light-responsive nanocarriers for cancer therapy. Drug Discovery Today 2018, 23, 1115-1125, doi:https://doi.org/10.1016/j.drudis.2018.02.005.
- Rao, W.; Wang, H.; Han, J.; Zhao, S.; Dumbleton, J.; Agarwal, P.; Zhang, W.; Zhao, G.; Yu, J.; Zynger, D.L., et al. Chitosan-Decorated Doxorubicin-Encapsulated Nanoparticle Targets and Eliminates Tumor Reinitiating Cancer Stem-like Cells. ACS Nano 2015, 9, 5725-5740, doi:10.1021/nn506928p.
- Xie, M.; Zhang, F.; Peng, H.; Zhang, Y.; Li, Y.; Xu, Y.; Xie, J. Layer-by-layer modification of magnetic graphene oxide by chitosan and sodium alginate with enhanced dispersibility for targeted drug delivery and photothermal therapy. Colloids and Surfaces B: Biointerfaces 2019, 176, 462-470, doi:https://doi.org/10.1016/j.colsurfb.2019.01.028.
- Wang, M.; Ruan, L.; Zheng, T.; Wang, D.; Zhou, M.; Lu, H.; Gao, J.; Chen, J.; Hu, Y. A surface convertible nanoplatform with enhanced mitochondrial targeting for tumor photothermal therapy. Colloids and Surfaces B: Biointerfaces 2020, 189, 110854, doi:https://doi.org/10.1016/j.colsurfb.2020.110854.
- Guo, Y.; Chen, Y.; Han, P.; Liu, Y.; Li, W.; Zhu, F.; Fu, K.; Chu, M. Biocompatible chitosan-carbon nanocage hybrids for sustained drug release and highly efficient laser and microwave co-irradiation induced cancer therapy. Acta Biomaterialia 2020, 103, 237-246, doi:https://doi.org/10.1016/j.actbio.2019.12.010.
- Boca, S.C.; Potara, M.; Gabudean, A.-M.; Juhem, A.; Baldeck, P.L.; Astilean, S. Chitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly effective photothermal transducers for in vitro cancer cell therapy. Cancer Letters 2011, 311, 131-140, doi:https://doi.org/10.1016/j.canlet.2011.06.022.
- Yang, Z.; Liu, T.; Xie, Y.; Sun, Z.; Liu, H.; Lin, J.; Liu, C.; Mao, Z.-W.; Nie, S. Chitosan layered gold nanorods as synergistic therapeutics for photothermal ablation and gene silencing in triple-negative breast cancer. Acta Biomaterialia 2015, 25, 194-204, doi:https://doi.org/10.1016/j.actbio.2015.07.026.
- Li, R.; Du, Y.; Guo, W.; Su, Y.; Meng, Y.; Shan, Z.; Feng, Y.; Meng, S. Methotrexate coated AZA-BODIPY nanoparticles for chemotherapy, photothermal and photodynamic synergistic therapy. Dyes and Pigments 2020, https://doi.org/10.1016/j.dyepig.2020.108351, 108351, doi:https://doi.org/10.1016/j.dyepig.2020.108351.
- Xu, W.; Wang, J.; Qian, J.; Hou, G.; Wang, Y.; Ji, L.; Suo, A. NIR/pH dual-responsive polysaccharide-encapsulated gold nanorods for enhanced chemo-photothermal therapy of breast cancer. Materials Science and Engineering: C 2019, 103, 109854, doi:https://doi.org/10.1016/j.msec.2019.109854.
- Hou, G.; Qian, J.; Xu, W.; Sun, T.; Wang, Y.; Wang, J.; Ji, L.; Suo, A. A novel pH-sensitive targeting polysaccharide-gold nanorod conjugate for combined photothermal-chemotherapy of breast cancer. Carbohydrate Polymers 2019, 212, 334-344, doi:https://doi.org/10.1016/j.carbpol.2019.02.045.
- Huang, X.; Xu, C.; Li, Y.; Cheng, H.; Wang, X.; Sun, R. Quaternized chitosan-stabilized copper sulfide nanoparticles for cancer therapy. Materials Science and Engineering: C 2019, 96, 129-137, doi:https://doi.org/10.1016/j.msec.2018.10.062.
- Liu, W.; Zhang, X.; Zhou, L.; Shang, L.; Su, Z. Reduced graphene oxide (rGO) hybridized hydrogel as a near-infrared (NIR)/pH dual-responsive platform for combined chemo-photothermal therapy. Journal of Colloid and Interface Science 2019, 536, 160-170, doi:https://doi.org/10.1016/j.jcis.2018.10.050.
- Zaharie-Butucel, D.; Potara, M.; Suarasan, S.; Licarete, E.; Astilean, S. Efficient combined near-infrared-triggered therapy: Phototherapy over chemotherapy in chitosan-reduced graphene oxide-IR820 dye-doxorubicin nanoplatforms. Journal of Colloid and Interface Science 2019, 552, 218-229, doi:https://doi.org/10.1016/j.jcis.2019.05.050.
- Zhang, M.; Wang, W.; Zhou, N.; Yuan, P.; Su, Y.; Shao, M.; Chi, C.; Pan, F. Near-infrared light triggered photo-therapy, in combination with chemotherapy using magnetofluorescent carbon quantum dots for effective cancer treating. Carbon 2017, 118, 752-764, doi:https://doi.org/10.1016/j.carbon.2017.03.085.
- Shanavas, A.; Rengan, A.K.; Chauhan, D.; George, L.; Vats, M.; Kaur, N.; Yadav, P.; Mathur, P.; Chakraborty, S.; Tejaswini, A., et al. Glycol chitosan assisted in situ reduction of gold on polymeric template for anti-cancer theranostics. International Journal of Biological Macromolecules 2018, 110, 392-398, doi:https://doi.org/10.1016/j.ijbiomac.2017.11.127.
- Sheng, Y.; Dai, W.; Gao, J.; Li, H.; Tan, W.; Wang, J.; Deng, L.; Kong, Y. pH-sensitive drug delivery based on chitosan wrapped graphene quantum dots with enhanced fluorescent stability. Materials Science and Engineering: C 2020, 112, 110888, doi:https://doi.org/10.1016/j.msec.2020.110888.
- Unsoy, G.; Khodadust, R.; Yalcin, S.; Mutlu, P.; Gunduz, U. Synthesis of Doxorubicin loaded magnetic chitosan nanoparticles for pH responsive targeted drug delivery. European Journal of Pharmaceutical Sciences 2014, 62, 243-250, doi:https://doi.org/10.1016/j.ejps.2014.05.021.
