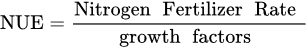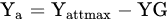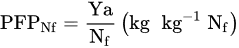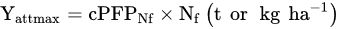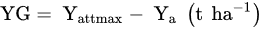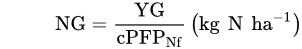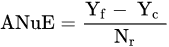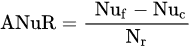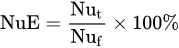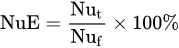
where: NuE—Nutrient uptake Efficiency, kg kg−1; Nut—the uptake of a tested nutrient, kg or g ha−1; Nuf—the rate of a nutrient applied as fertilizer, kg, g ha−1.
The efficiency of N, P, and K using this method show much higher values or even a surplus of nutrients [
10]. The low efficiency of nutrients using the differential methods, but high yield indirectly indicates that the main source of nutrients for crops grown in one growing season is soil [
11].
The main problem is the assessment of the production role of nitrogen, which plants take in in two distinct inorganic forms, i.e., as nitrate (NO
3−1) and ammonium (NH
4+) [
12]. Nitrates affect plant growth in many ways, inducing plant morphology, physiology through hormones and finally metabolism through their influence on the production of organic acids [
13,
14,
15]. Plants fed with nitrates, compared to ammonium, show a high growth rate, which results in higher yields [
16]. The above-identified aspects of the impact of N on plants are fully supported by field experiments and agricultural practice [
17,
18]. The yields of winter wheat grown on the control plot (non-fertilized) and on the plots fertilized with K, P in the same way since 1957, did not show large differences. The average yield for these three objects of 4.38 ± 0.14 t ha
−1, can be considered as high. The primary reason for such a high yield, despite the lack of N fertilization, was alfalfa as a forecrop. The use of 90 kg N ha
−1 increased the yield by 1.94 t ha
−1. The same level of yields was also recorded for the NP and NK plots. The lack of response to the P or K application clearly emphasizes the importance of these two nutrients for plant growth and yield. This conclusion was fully confirmed by the yield achieved on the NPK plot. Even more important is the fact that N use efficiency (NUE) increased by 10–13%, compared to incomplete fertilization treatments. The observed interaction was even more important for P use efficiency (PUE), which in the NPK plot increased by 9% and by 73% compared to NP and P treatments, respectively. The same trend was observed for potassium. The importance of the N × PK interaction on the productivity of N
f is observed for all crops, regardless of the world region [
17,
19,
20]. The complex effect of N on plant growth and yielding clearly indicates the superior function of N in crop production. It can, therefore, be concluded that the production efficiency of nutrients, applied as mineral fertilizers, can be mainly evaluated through their impact on NUE. Thus, the search for indicators of productivity or efficiency for other nutrients is pointless. This is well presented in the analysis of the causes of the NG.
1.3. Factors Affecting Fertilizer Use Efficiency
Fertilizer use efficiency is the result of a series of interactions between plant genotype and environment, including both abiotic and biotic factors. Full recognition of these factors is the basis for proper fertilization of plants in farming practice, aimed at maximizing the FUE values. The soil is both the growth environment for plants and their main reservoir of water and nutrients. Hence, the impact of soil factors on nutrient uptake and FUE should be considered at the level of several groups of phenomena and processes.
In the first group (A) all of the factors, both abiotic and biotic, that lead to the release of nutrients from their solid phase in the soil to their solution phase should be analyzed. The next group of factors (B) is concerned with the processes of transporting nutrients from the soil to the root surface. The third group (C) of factors influencing FUE concerns plant responses manifested by changes in architecture and root growth rate. This group of factors, also related to plant activity, should consider the composition of the root exudates in the plant root—mycorrhizal system. For the assessment of the effectiveness of fertilizer application, the processes taking place in the plant itself, related to transport, assimilation in the aboveground mass (D), as well as remobilization of components and their transfer from the vegetative parts to the generative crop (E), are also important.
2. Soil Factors Affecting FUE
2.1. Soil Texture
The most important soil physical properties include: soil texture, density, structure, porosity, consistence, temperature, air and color. Among them, soil texture is the basic physical feature that determines not only the other physical properties of the soil, but also the chemical ones [
84]. The percentage and mineralogical composition of the smallest mineral fractions in the parent rock determines the primary soil potential to supply plants with nutrients, which is the function of weathering and transforming primary minerals [
85]. In addition, the content of mineral colloids is positively correlated with soil organic matter (SOM), which in turn is a source of organic colloids, which have a great impact on the water retention of the soil, cation exchange capacity, erosion processes, as well as soil microbial activity [
86]. SOM sequestration is achieved through various mechanisms which include the formation of clay-humic complexes, sorption of organic matter on clay particles, fixation of organic carbon in the crystal lattices of clays and the formation of organo-metallic compounds such as Ca, Fe and Al humates through humification processes [
87,
88]. In general, the greater the SOM concentration, the greater the sorption capacity of the soil, and potential for water retention in soil [
89] and nutrients [
90]. Numerous studies show that soils with a high proportion of clay particles have a higher content of nutrients than soils with a low content of nutrients, not only in terms of general forms, but also plant-available forms [
91,
92]. At the same time, the clay content affects the fixation and de-fixation processes of some nutrients, especially K
+ [
91]. On the one hand, excessive fixation reduces the pool of mobile K
+ ions in the soil and reduces the use of potassium from fertilizers, especially in dry soil conditions. On the other hand, it prevents the leaching of potassium from the soil [
93]. Moreover, adsorption and non-exchangeable ammonium nitrogen (NH
4+) fixation in soil is highly dependent on clay mineral composition [
94]. Another problem with soil texture is water infiltration and the leaching of nitrates (NO
3−) resulting from ammonium nitrification. Coarser-textured soils are more susceptible to soil N loss following the leaching of NO
3−, and thus have potentially lower FUE values [
64]. Furthermore, soil texture largely affects fertilizer and soil P transformations in soils. In coarser-textured soils the content of labile P fractions after adding phosphorus fertilizers is higher than in clay and loam soils. Therefore, in these soils there is a high risk of P transfer from soil to water systems [
95].
2.2. Water Content
One of the most important factors controlling nutrient uptake and utilization by plants is the water content of the soil. First of all, water determines the processes of nutrient release from the soil solid phase to the solution phase [
96,
97]. Water deficiency in soil negatively affects microbiological activity and the processes of mineralization/biological fixation [
98]. Water is also essential for dissolving and releasing nutrients from mineral fertilizers, including controlled release fertilizer [
99]. However, from the point of view of the process of uptake of nutrients by plants, two phenomena deserve special mention: mass flow and diffusion [
100]. Water deficiency in the soil reduces the intensity of both processes, and thus leads to a reduction in the amount of nutrients flowing to the root surfaces [
101]. In this aspect, the degree of plant reaction to water stress depends on the element and its function. According to Oliveira et al. [
102], in maize the proportion of mass flow contribution to Ca, Mg, N, S and K transport was as follows: 100, 63, 56, 45 and 10%, respectively. This series clearly shows that the supply of plants with Ca and Mg may be severely limited in drought conditions, despite their relatively high concentration in the soil compared to other macronutrients [
103]. Taking into account the diffusion processes, a water shortage in the soil will primarily limit the mobility of phosphate ions and micronutrients. Moreover, it will lead to the intensification of precipitation processes and the crystallization of amorphous compounds of phosphorus with other cations, depending on the pH [
104]. As the water deficit in the soil increases, the proportion of pores filled with air increases, mechanical resistance increases, and the rate of root growth decreases. Under conditions of high soil oxygenation, the potential of the soil to supply plants with some micronutrients is reduced (Fe, Mn), whose higher oxidation state forms are less plant-available than the reduced forms [
105]. The second group factors effecting NUE directly relates to the plant response (growth) and its ability to convert in biomass the assimilated/remobilized nutrients, especially nitrogen [
106]. Water has a direct effect on root growth. In order to meet the demand for water, the roots constantly explore the soil, building a very complex, branched architecture [
107]. An increase in the number of hairs and diameter root tips has been observed in plants under drought conditions. Root hairs greatly increase root-soil contact and the surface area available for adsorbing water and nutrients [
108]. However, dense and deep root systems are not always good under all hydrological conditions, for example they poorly capture water from the topsoil under low rainfall conditions [
109]. In drought conditions, the above-ground mass is reduced more than the underground mass, which in the case of a long-lasting drought may limit the inflow of assimilations and stop root growth, with all the negative effects of this phenomenon [
110]. Lupini et al. [
111] reported that water stress in durum wheat reduces the values of NUE, NUpE, and NUtE indices, regardless of the genotype. However, it should be remembered that excess water is just as harmful to plants as is its deficiency. One of the reasons for this is the reduction in the oxygen content in the soil needed for the respiration of plants and microorganisms [
98]. In addition, large amounts of iron or manganese are released, which in excess may be toxic or interfere with the absorption of other nutrients. This phenomenon is particularly harmful in the cultivation of rice paddy on acidic soils [
112].
2.3. Soil Compaction
Another important physical factor influencing nutrient uptake from soil, as well as their utilization from fertilizers, is soil compaction. Compaction affects plant growth by reducing the content of soil air and plant-available water, and the consequent restricted root growth results in the plant being unable to obtain an adequate amount of nutrients. Soil compaction can be assessed by measuring the following soil properties: bulk density, porosity and mechanical impedance [
113]. Mechanical impedance is defined as a physical barrier to developing roots as a result of excessive bulk density. In general, root growth rates decrease sharply for soil mechanical impedance values between 0.8 and 3 MPa. On the other hand, when assessing soil compaction by soil bulk density, most authors give the value of 1.47–1.85 g cm
−3 as critical for crops, depending on the percentage of clay [
114,
115]. The turgor in the cells in the elongation part of the roots determines their ability to overcome the mechanical resistance of the soil [
116]. The greater it is, the greater the probability of root growth into the zone of compacted soil [
117]. At the same time, root elongation is facilitated by root secretions and abraded side cells of the roots, which reduce the effect of the friction force [
118]. When the mechanical resistance is too high, changes are observed at the physiological level (accumulation of solutes, reduction in the growth rate, new cell production) as well as anatomical (increase in the root diameter and the share of mechanical tissue in the direction of growth) [
119,
120]. The entire root system develops into less resistant parts of the soil, often forming a shallow system with the roots parallel to the soil surface [
121]. According to Ramalingam et al. [
122] the root length density at 30–60 cm soil depth decreased with hard compaction (to 70% of control) and increased with moderate compaction (to 135%). At the same time, the number of roots with a deep angle (i.e., 45° to 90° from the horizontal) correlated with the root length density and its proportion was lower in compacted soil. Considering the root architecture, the studies carried out so far have shown that deeper root growth is more important for N uptake than increased root density [
123]. In this respect, it is necessary to remove the soil compaction in the subsoil. On arable land, the use of heavy machinery increases the risk of soil compaction especially in the subsoil [
124]. Changes in the root architecture mean that the plant is unable to fully use nutrients, especially those whose main reservoirs are in deeper layers of soil [
125]. Regardless of the soil depth, when the soil is characterized by excessive bulk density and/or mechanical impedance, the roots develop mainly in macro-pores [
126]. This results in a poor supply of nutrients in plants under soil drought conditions, as the macro-pores in soil water retention only contribute to a small extent [
98]. Another important issue with soil compaction is the loss of nitrogen from the soil through the emission of its gaseous forms into the atmosphere. As a result of soil compaction and the oxygen deficiency caused by this process, the activity of denitrifying bacteria increases and the production of N
2O and N
2 increases [
127]. The emission of these gases to the atmosphere is favored by the low values of the parameters that define gas diffusivity in compacted soils [
128]. According to Ruser et al. [
129], high N
2O emissions in compacted soils occurred at a water-filled pore space > 70%. N
2 production took place only at the highest soil moisture level (>90% water-filled pore space) but it was considerably less than the N
2O-N emission in the most compacted areas in a potato field. Soil compaction also increases the volatilization of ammonia, as compared to uncompacted soils [
130]. However, for this gas, the emissions are mainly determined by other soil physical and chemical characteristics [
131].
2.4. Soil Temperature
Temperature has a substantial effect on some soil properties as well as root growth. Important processes depend on the temperature of the soil, such as: soil structure, aggregate stability, soil moisture content and aeration, soil pH, cation exchange capacity (CEC), soil microbial activities and organic matter decomposition [
132]. A soil temperature between 2–38 °C increases the decomposition of organic matter by stimulating microbial activities and increasing the solubility of chemical compounds [
133]. As a result of decomposition, the resources of N, P, S and other nutrients available to plants increase [
134]. From the point of view of the nutritional status of plants, an extremely important temperature-dependent process is the availability of P to plants. Soils with low temperature have low availability of P because the release of P from organic material is limited [
135]. Soil temperature also influences the P diffusion coefficient in the soil. Yilvainio and Pettovuori [
136] observed that water-soluble P increased with soil temperature from 50 to 250 °C due to the increase in the movement of P in soil controlled by diffusion. Soil temperature also affects nutrient uptake by changing soil water viscosity and root nutrient transport. At low soil temperature, nutrient uptake by plants is reduced as a result of high soil water viscosity and low activity of root nutrient transport [
137]. In general, low temperature decreases both root elongation and branching. However, low temperatures inhibit shoot growth more than root, leading to a high root/shoot dry matter ratio [
138]. Vessel lignification can be delayed and axial hydraulic conductivity is higher in roots grown at low temperatures compared to high temperatures [
139]. Thus, tomato, for example, showed that low soil temperature results in reduced root growth, tissue nutrient concentrations and, as a consequence, the amount of the component taken from the soil [
140]. The unfavorable effect of higher temperature is marked in various ways. Too high a temperature may lower the CEC, and at the same time cause an increase in the concentration of hydrogen protons (increase in soil acidification) due to the high rate of soil organic matter decomposition. The plant’s response to temperature changes depends not only on the plant species, but also on the content of nutrients in the soil. According to Xia et al. [
141], negative effects of excessive temperature on P content and uptake occur especially in P-poor soils. The authors also found that an overly high root zone temperature reduced root vitality and plant phosphorus content, which in turn affected plant growth and light energy utilization efficiency.
2.5. Soil Reaction
Among a number of chemical parameters describing the chemical properties of soils, the use of nutrients from fertilizers is very much influenced by its pH [
142]. This feature directly relates to the concentration of active H
+ protons in aqueous solutions, and indirectly it is a measure of the acidity or alkalinity of a soil. The influence of soil pH on the nutrient uptake of plants results from many different phenomena and processes. The most important ones include: effecting the content of plant-available forms of nutrients in soil; capacity and proportions between cations in CEC; activity of trace elements and heavy metals; soil microbial activity, biological N
2 fixation; emissions of ammonia and other gases from the soil [
143,
144]. Both too acidic and alkaline soils have a negative effect on nutrient uptake. However, the phenomena occurring in acidic and alkaline soils differ significantly in terms of processes contributing to their degradation. A significant problem of acidified soils is an increase in exchangeable aluminum (Al
3+) [
145]. The content of this form of aluminum monomers rapidly increases in soils below pH 5.0–5.5 [
146]. An excessive amount of Al
3+ ions in the soil negatively affects the nutrient uptake processes and plant growth [
147]. Numerous studies show that even at the stage of nutrient uptake, unfavorable phenomena take place, such as the competition of Al
3+ ions with other ions for attachment sites in the apoplast, in carriers, attachment to the ATPase of cytoplasmic membranes and disruptions in the operation of the proton pump [
148,
149]. An excessive content of Al
3+ ions in the soil significantly reduces the uptake of Mg
2+ ions. This is due to the similar size of the hydrated ions [
150]. One of the most important consequences of the presence of exchangeable aluminum in the soil is the disturbance of the growth and development of the root cap, and consequently the shortening of the root length and unfavorable changes in its structure [
151]. For most crops, even a small concentration of exchangeable aluminum (in nanomoles) in the root cells is a toxic factor for the metabolic, physiological, genetic and biochemical processes taking place in the plant [
152]. The reduction in the root system negatively affects the use of nitrogen in fertilizers and increases the risk of nitrate being washed out from the soil [
153]. Moreover, nitrate nitrogen, which is not taken up by plants, is reduced to gaseous compounds, including N
2O [
154]. In highly acidic soils, apart from exchangeable aluminum, excessive amounts of manganese (Mn
2+) and iron (Fe
2+) can also appear, which can further disrupt the proper growth and development of plants [
155].
2.6. Soil Salinity
In arid or semi-arid climates, the problem is not soil acidification, but alkalization and salinity [
156]. Under low rainfall conditions and a high evaporation rate, Na
+ ions, as well as various soluble salts, accumulate in the soil. Their excessive accumulation contributes to the significant advantage of OH
− ions over H
+ and, consequently, to an increase in soil pH to the level of 9–10 [
157]. Soil alkalinity can also be increased by the addition of water containing dissolved bicarbonates, especially when irrigating with high-bicarbonate water [
158]. The low osmotic potential of water in saline soils adversely affects water absorption by plants and nutrient uptake [
159]. Salinity of soil significantly decreases P uptake by plants because phosphate ions precipitate with Ca ions contained in saline soils [
160]. However, the alkalinity of soils is most often associated with the Na concentration [
161]. Alkaline soils are characterized by unfavorable physical conditions, low content of plant-available forms of microelements and phosphorus, components determining nitrogen metabolism in the plant. During nutrient uptake processes, Na
+ ions compete for carriers with other nutrients in cationic form, in particular with K
+ ions [
162]. This is a negative phenomenon because Na, dissimilar to K, negatively affects the activity of plant enzymes [
163]. The reduced uptake of K
+ ions also means the insufficient or slower transport of NO
3− from the roots to the above-ground parts, and thus poor efficiency of N from fertilizers [
164]. Furthermore, an excess of Cl
− ions in the soil has a negative effect on NO
3− uptake. However, as recent studies show, optimal NO
3− vs. Cl
− ratios become a useful tool to increase crop yield and quality, agricultural sustainability and reduce the negative ecological impact of NO
3− on the environment and on human health [
165]. Under saline soil conditions, plants change their root architecture, which also has negative consequences for nutrient uptake [
166].
2.7. Soil Organic Matter
The content of soil organic matter (SOC) in soil is one of the most important features influencing soil fertility [
167]. Changes in SOC are associated mainly with changes in macronutrient contents, such as N, P and sulfur (S) which are chemically bound to carbon (C) in organic compounds [
168]. Therefore, in systems where SOC content is declining, soil fertility declines over time and soils become increasingly dependent on the use of mineral fertilizers, especially nitrogen [
169]. A total loss of organic N directly translates into a weaker potential of soils to release mineral forms that are taken up by plants. At the same time, under such conditions, the demand for N from fertilizers increases. Numerous experiences show that the most effective use of N from fertilizers is observed in the small dose range [
9,
170]. Conventional tillage with plowing can reduce SOC stocks by 30–60% [
168]. Changes in NUE resulting indirectly from the increase in the degree of SOC degradation are confirmed by research of Luis et al. [
171]. The authors calculated that over many years the efficiency of nitrogen fertilization application decreased from 68% in 1961 to 47% in 2010. This means that the use of N from fertilizers deteriorated and N losses to the environment increased by 21%.
In general, the transformation of native soil to agricultural uses leads to a decline in SOC levels [
172]. However, agricultural land uses do not always result in losses of SOC. The rate and direction of changes in the C content in soils depend on the soil use system, irrigation, crops, and the level of organic matter return to the soil [
173,
174]. Failure to plow or use various simplified systems leads to the accumulation of SOC, especially in topsoil [
175]. Reduced tillage in comparison with ploughing increased SOC stocks in the surface layer (0–10/15 cm) by 20.8% or 3.8 t ha
−1, depleted SOC stocks in the intermediate soil layers to 50 cm soil depth with a maximum depletion of 6.6% or 1.6 t ha
−1 in 15/20–30 cm and increased SOC stocks in the deepest (70–100 cm) soil layer by 14.4% or 2.5 t ha
−1 [
176]. However, the use of natural and organic fertilizers is of greater practical importance in maintaining an appropriate SOC [
177]. Szajdak et al. [
178] reported that a yearly application of 30 t ha
−1 of manure to light soil over 38 years doubled the SOC content. The increase in plant biomass as a result of the use of NPK fertilizers leads to an increase in the influx of C to the soil. Nevertheless, accumulation of C in soil is not favored by an excess of N in the soil from high fertilizer application rates and/or low plant uptake can cause an increase in the mineralization of organic carbon which, in turn, leads to an increased loss of C from soils [
179].
2.8. Nutrient Shortage
Factors responsible for nutrient deficiency in crops can be divided into two main groups: (i) causing an absolute deficiency of nutrients in soil, resulting from low nutrient contents in the parent soil material, low level of SOC, nutrient losses from the soil, e.g., Mg leaching, long-term unbalanced crop fertilization practice neglecting nutrient depletion in soils through crop nutrient removal; (ii) causing an induced deficiency, resulting from factors that disturb the flow of nutrients to the root such as: improper moisture and temperature of soil, ion competition, factors responsible for root system size, etc. [
12]. The natural source of most nutrients in the soil are primary and secondary minerals. As a result of weathering, often stimulated by the activity of living organisms, potentially available nutrients are released into the environment. Their reactions in soil and fate in the environment depend on the type of element. Some nutrients are strongly absorbed in the soil, others are easily lost (by leaching or emission). The first group includes K. The ions of this element can be absorbed in the soil in an exchangeable and non-exchangeable form [
180]. The second type of adsorption prevents the elution of K
+ ions from soil, but on the other hand this leads to a reduction in the potential to supply plants with K. This phenomenon is responsible for the poor efficiency of K from fertilizers on soils rich in mineral colloids. The strength of the non-exchangeable K ion fixation increases in dry years, which further aggravates the symptoms of water stress [
181]. Non-exchangeable adsorption may also apply to other cations, e.g., Mg. However, in relation to Mg, the degree of soil moisture has a greater practical importance, as this element is assimilated by plants as a result of a mechanism known as mass flow. Contrary to K, Mg is less readily absorbed [
103]. This is one of the reasons for the relatively easy leaching of Mg from the soil. An absolute deficiency of K and Mg leads to a poor efficiency of N, as both elements greatly affect the metabolism and transport of N in plants [
16]. Studies conducted on sugar beet show that Mg applied to the soil significantly increases the agronomic efficiency of N, but in the range of low doses of N. This indicates that an excess of nutrients in the soil may not lead to better FUE/NUE values. Phosphorus and micronutrient deficiencies in the soil are often the result of an inappropriate pH range in the soil. In an acidic reaction, the adsorption of P on iron and aluminum compounds increases, while in an alkaline pH, insoluble calcium phosphates precipitate in the soil [
104]. An inappropriate soil pH also influences, directly or indirectly, the content of plant-available forms of K, Mg and Ca [
144]. Thus, in order to restore the optimal conditions for the uptake of nutrients, it is necessary to regulate and/or constantly control the soil pH. If this does not help, then one option is to enrich the soil with nutrients to eliminate their absolute deficiency, or to support the plants by foliar fertilization.
3. FUE—A Message for Agricultural Practice
The stagnation in the increase in the crop yields is well-documented [
247,
248]. Despite considerable progress in breeding and the continual release of new varieties, the real improvement in NUE is small [
249]. The challenge for the farmer to exploit the yield potential of the grown variety is:
-
Determine the maximum attainable yield (Yattmax). This is the basis for choosing the most suitable variety for the actual climatic and soil conditions of the farm;
-
Identify soil conditions that constrain:
-
Divide the whole field area into units of homogenous productivity;
-
Identify Nitrogen Hotspots both on the farm and on the specific field;
-
Observe the viability of plants at stages preceding the cardinal phases of yield formation;
-
Schedule the correction of the plant nutritional status during the season to exploit its yield potential.
The effective control of the set of factors indicated above is crucial to optimizing NUE. The general formula can be written as: