Electronic cigarettes (e-cigarettes) continue to be considered an alternate model of curbing smoking addiction. The presence of various electronic vaping products used as solvent carriers, flavorings, addictive substances, and their byproducts may lead to vaping product use-associated lung injury (EVALI). The continuing pandemic of coronavirus disease 2019 (COVID-19) has initiated a global health emergency with the ongoing crisis related to vaccination, therapy, and diagnosis. Patients with a history of using e-cigarettes could have overlapping clinical symptoms of COVID-19, thereby leading to incorrect or delayed diagnosis and consequently, wrong treatment regimen. The diagnosis of EVALI during the COVID-19 pandemic is a challenge for treatment in these patients, and it is important to bring awareness to young adults and adolescents against the adverse effect of e-cigarettes containing addictive substances during the COVID-19 pandemic.
- electronic cigarette
- COVID-19
- lung injury
- coronavirus
- Electronic Cigarette (E-cigarette) Components and Their Health Effects
The Center for Disease Control and Prevention (CDC) along with the Food and Drug Administration (FDA) and health departments are continuously monitoring the vaping product use-associated lung injury (EVALI). E-cigarettes contain the solvent carriers such as vegetable glycerin and propylene glycol along with the volatile organic compounds, metals, polycyclic aromatic hydrocarbons, flavorings, and addictive substances such nicotine, tetrahydrocannabinol (THC) extract and cannabidiol-containing compounds [1]. The heating of vegetable glycerin and propylene glycol is reported to release the inflammatory infiltrates, thus resulting in the production of cytokines, oxidants, and inflammatory gene expression, thereby leading to lung infection [2]. Several studies have shown that vitamin E acetate and THC are the causal agents of EVALI [3,4]. However, e-cigarette devices have a wide variety of electronic liquids that can be aerosolized; therefore, it is hard to confirm specific harmful chemicals as a source of lung injury [5].
Over the last few years, there has been a change in the design of e-cigarettes, but it did not lessen any harmful components. There is a correlation between high plasma nicotine levels with the advanced technology and design of e-cigarettes when compared to the first generation of e-cigarettes [6]. There is a 30% increase in developing lung disease in users of e-cigarettes when compared to the population who do not use e-cigarettes [7]. Therefore, electronic vaping products, such as e-cigarettes, could contribute to the onset of respiratory diseases [7].
The health effects of using e-cigarettes and other electronic vaping products could lead to alteration in the platelet function, damage to the pulmonary structures, enlargement of the alveolar airspace, and the disappearance of peripheral vasculature [8]. Electronic vaping products increase the inflammatory profile of respiratory pathogens by increasing the platelet-activating factor receptor expression thereby leading to the susceptibility to pneumonia [9]. The injury to the lungs is primarily because of compromised lung function and shows signs of fever and shortness of breath. In summary, the inhalation of chemicals in the aerosols could cause inflammation in the lungs, as evident by the inflammatory disorders of lung pneumonia, such as lipoid pneumonia, hypersensitivity pneumonitis, and eosinophilic pneumonia [10-12].
- Diagnosis and Treatment dilemma in COVID-19 pandemic
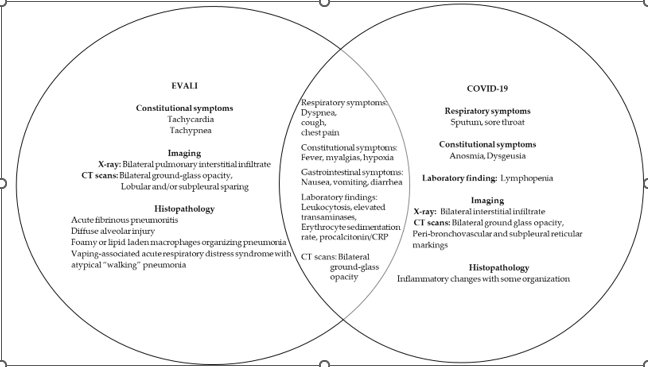
The pandemic coronavirus disease (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) exhibits symptoms like EVALI, which creates a problem in patients with a history of vaping, thereby leading to the delay in the diagnosis and treatment. EVALI, being the acute lung injury, exhibits characteristic symptoms, such as fever, cough, shortness of breath, dyspnea, vomiting, diarrhea, headache, and fatigue with a pathological diagnosis of acute fibrinous pneumonitis, diffuse alveolar damage, and pneumonia [9]. There is a considerable amount of data showing the diagnostic challenges in differentiating the EVALI symptoms during the COVID-19 pandemic. The anchoring bias due to the patients’ symptoms similarity with COVID-19 symptoms was a challenge in the early diagnosis of EVALI. There are case reports from patients’ using e-cigarettes that were treated for COVID-19 despite testing negative for COVID-19 [13-15]. The symptoms and pathological diagnosis in these patients were like EVALI due to the use of e-cigarettes by the patient. There was a challenge in diagnosing rarer etiologies of respiratory distress during the COVID-19 pandemic, leading to the delay in the diagnosis of EVALI. [15]. The symptoms associated with using the e-cigarette may include shortness of breath, chest pain, cough, fever, myalgias, headache, and gastrointestinal symptoms, with the clinical symptoms including inflammatory markers, such as c-reactive protein (CRP), erythrocyte sedimentation rate, and procalcitonin, coagulation markers, diffuse hazy or consolidative opacities and ground-glass opacities, many of which are similar to COVID-19 patients [13,16].
Figure 1 highlight the overlapping respiratory features of EVALI and COVID-19 disease. The overlapping symptoms of EVALI and COVID-19 are dyspnea, cough, chest pain, fever, myalgias, and hypoxia. The gastrointestinal overlapping symptoms of EVALI and COVID-19 are nausea, vomiting, and diarrhea. The clinical findings common in both diseases include leukocytosis, elevated transaminases, erythrocyte sedimentation rate, procalcitonin, and CRP. The ground-glass opacities are another common feature in the patients of EVALI and COVID-19, thus making the diagnosis challenging in patients with a history of using e-cigarettes during the COVID-19 pandemic.
- Young adult and adolescent e-cigarette users’ health concerns during the ongoing COVID-19 pandemic
The recent popularity of e-cigarettes containing addictive substances has led to some serious health concerns. The youth and adolescents consuming e-cigarettes are five times more likely to be susceptible to COVID-19 [10]. The angiotensin-converting enzyme-2 (ACE-2) has been identified as the receptor for the SARS-CoV-2 viral entry [17,18], and e-cigarettes could contribute to the upregulation of ACE-2 owing to the high content of nicotine, thereby aggravating the COVID-19 complications [19,20]. E-cigarette use induces oxidative stress and the inflammatory response, thus leading to complications in the COVID-19-related symptoms. In vivo studies have shown that e-cigarette vapors led to an increase in airway inflammation, impairment in lung function, neutrophil activation, and upregulation of the ACE-2 expression in the lungs may be due to the presence of nicotine in the vapor [21-23]. However, the mechanism for non-nicotine-containing e-cigarette vapor induction in ACE-2 expression in the lungs is not well defined in humans and needs further studies.
- Dysbiosis of microbiota in the e-cigarette users and COVID-19 disease
The microbiota in different organ systems, including the oral cavity, gut, and lungs, is related to the host’s immune tolerance of the viruses and the severity of the viral infection [24,25]. Any alteration in the microbiota species and metabolites may alter the course of respiratory viral infections, thus affecting the function of the lungs [26]. The reduced microbiome diversity, significant change in the microbiome in the nasopharyngeal and upper respiratory tract, high dysbiosis, and complications in the lungs is associated with a loss of microbial genes and metabolic pathways and have been reported in the hospitalized COVID-19 patients [27-32]. The use of e-cigarettes compromises airway microbiota and its antiviral responses. The usage of e-cigarettes leads to oral inflammation and increased secretion of cytokines, which indicates the dysbiosis of specific bacteria. The exposure to aerosol in e-cigarettes triggers the release of cytokines and antimicrobial peptides following inflammation that can alter the oral microbiome [33]. COVID-19 patients using e-cigarettes might have an increased effect on the dysbiosis of the oral, gut, and lung microbiome, thus affecting the function of the lungs. There is a need for research studies on patients with a history of vaping and the extent of dysbiosis of the microbiome in the respiratory system during the ongoing COVID-19 pandemic.
References:
- Sharma, P.; Philpot, L.M.; Rosedahl, J.K.; Jose, T.T.; Ebbert, J.O. Electronic Vaping Product Use among Young Adults Who Receive Care at a Major Medical Institution. Subst Use Misuse 2021, 56, 224-237, doi:10.1080/10826084.2020.1853777.
- Lechasseur, A.; Jubinville, E.; Routhier, J.; Berube, J.C.; Hamel-Auger, M.; Talbot, M.; Lamothe, J.; Aubin, S.; Pare, M.E.; Beaulieu, M.J.; et al. Exposure to electronic cigarette vapors affects pulmonary and systemic expression of circadian molecular clock genes. Physiol Rep 2017, 5, doi:10.14814/phy2.13440.
- Blount, B.C.; Karwowski, M.P.; Shields, P.G.; Morel-Espinosa, M.; Valentin-Blasini, L.; Gardner, M.; Braselton, M.; Brosius, C.R.; Caron, K.T.; Chambers, D.; et al. Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N Engl J Med 2020, 382, 697-705, doi:10.1056/NEJMoa1916433.
- Crotty Alexander, L.E.; Ware, L.B.; Calfee, C.S.; Callahan, S.J.; Eissenberg, T.; Farver, C.; Goniewicz, M.L.; Jaspers, I.; Kheradmand, F.; King, T.E.; et al. E-Cigarette or Vaping Product Use-associated Lung Injury: Developing a Research Agenda. An NIH Workshop Report. Am J Respir Crit Care Med 2020, 202, 795-802, doi:10.1164/rccm.201912-2332WS.
- Ahmed, A.R.; Etchey, B.; Ahmed, M. Explosions, Burn Injuries and Adverse Health Effects of Electronic Nicotine Delivery Systems: A Review of Current Regulations and Future Perspectives. J Pharm Pharm Sci 2021, 24, 462-474, doi:10.18433/jpps32242.
- Farsalinos, K.E.; Spyrou, A.; Tsimopoulou, K.; Stefopoulos, C.; Romagna, G.; Voudris, V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep 2014, 4, 4133, doi:10.1038/srep04133.
- Bhatta, D.N.; Glantz, S.A. Association of E-Cigarette Use With Respiratory Disease Among Adults: A Longitudinal Analysis. Am J Prev Med 2020, 58, 182-190, doi:10.1016/j.amepre.2019.07.028.
- Kasahara, Y.; Tuder, R.M.; Cool, C.D.; Lynch, D.A.; Flores, S.C.; Voelkel, N.F. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med 2001, 163, 737-744, doi:10.1164/ajrccm.163.3.2002117.
- Sharma, P.; Sheikh, T.; Williams, C. Electronic Vaping Product Use Among Adolescents in the Era of the COVID-19 Pandemic: An Updated Scientific Review for Clinicians. WMJ 2021, 120, 205-208.
- Arter, Z.L.; Wiggins, A.; Hudspath, C.; Kisling, A.; Hostler, D.C.; Hostler, J.M. Acute eosinophilic pneumonia following electronic cigarette use. Respir Med Case Rep 2019, 27, 100825, doi:10.1016/j.rmcr.2019.100825.
- Sommerfeld, C.G.; Weiner, D.J.; Nowalk, A.; Larkin, A. Hypersensitivity Pneumonitis and Acute Respiratory Distress Syndrome From E-Cigarette Use. Pediatrics 2018, 141, doi:10.1542/peds.2016-3927.
- Viswam, D.; Trotter, S.; Burge, P.S.; Walters, G.I. Respiratory failure caused by lipoid pneumonia from vaping e-cigarettes. BMJ Case Rep 2018, 2018, doi:10.1136/bcr-2018-224350.
- Ganne, N.; Palraj, R.; Husted, E.; Shah, I. E-cigarette or vaping product use-associated lung injury (EVALI) masquerading as COVID-19. BMJ Case Rep 2021, 14, doi:10.1136/bcr-2021-243885.
- Pitlick, M.M.; Lang, D.K.; Meehan, A.M.; McCoy, C.P. EVALI: A Mimicker of COVID-19. Mayo Clin Proc Innov Qual Outcomes 2021, 5, 682-687, doi:10.1016/j.mayocpiqo.2021.03.002.
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061-1069, doi:10.1001/jama.2020.1585.
- Smith, J.C.; Sausville, E.L.; Girish, V.; Yuan, M.L.; Vasudevan, A.; John, K.M.; Sheltzer, J.M. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. Dev Cell 2020, 53, 514-529 e513, doi:10.1016/j.devcel.2020.05.012.
- Ansari-Gilani, K.; Petraszko, A.M.; Gilkeson, R.C. COVID-19 pneumonia versus EVALI, distinguishing the overlapping CT features in the COVID-19 era. Heart Lung 2020, 49, 885-886, doi:10.1016/j.hrtlng.2020.06.008.
- Galo, J.; Celli, D.; Gross, D.; Holt, G.; Campos, M. A presentation of E-Cigarette vaping associated lung injury (EVALI) caused by THC-Containing electronic smoking device. Respir Med Case Rep 2020, 31, 101154, doi:10.1016/j.rmcr.2020.101154.
- McAlinden, K.D.; Eapen, M.S.; Lu, W.; Sharma, P.; Sohal, S.S. The rise of electronic nicotine delivery systems and the emergence of electronic-cigarette-driven disease. Am J Physiol Lung Cell Mol Physiol 2020, 319, L585-L595, doi:10.1152/ajplung.00160.2020.
- Jensen, K.; Nizamutdinov, D.; Guerrier, M.; Afroze, S.; Dostal, D.; Glaser, S. General mechanisms of nicotine-induced fibrogenesis. FASEB J 2012, 26, 4778-4787, doi:10.1096/fj.12-206458.
- Naidu, V.; Zeki, A.A.; Sharma, P. Sex differences in the induction of angiotensin converting enzyme 2 (ACE-2) in mouse lungs after e-cigarette vapor exposure and its relevance to COVID-19. J Investig Med 2021, 69, 954-961, doi:10.1136/jim-2020-001768.
- Masso-Silva, J.A.; Moshensky, A.; Shin, J.; Olay, J.; Nilaad, S.; Advani, I.; Bojanowski, C.M.; Crotty, S.; Li, W.T.; Ongkeko, W.M.; et al. Chronic E-Cigarette Aerosol Inhalation Alters the Immune State of the Lungs and Increases ACE2 Expression, Raising Concern for Altered Response and Susceptibility to SARS-CoV-2. Front Physiol 2021, 12, 649604, doi:10.3389/fphys.2021.649604.
- Sivaraman, V.; Parker, D.; Zhang, R.; Jones, M.M.; Onyenwoke, R.U. Vaping Exacerbates Coronavirus-Related Pulmonary Infection in a Murine Model. Front Physiol 2021, 12, 634839, doi:10.3389/fphys.2021.634839.
- Dominguez-Diaz, C.; Garcia-Orozco, A.; Riera-Leal, A.; Padilla-Arellano, J.R.; Fafutis-Morris, M. Microbiota and Its Role on Viral Evasion: Is It With Us or Against Us? Front Cell Infect Microbiol 2019, 9, 256, doi:10.3389/fcimb.2019.00256.
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol 2019, 12, 843-850, doi:10.1038/s41385-019-0160-6.
- Wypych, T.P.; Wickramasinghe, L.C.; Marsland, B.J. The influence of the microbiome on respiratory health. Nat Immunol 2019, 20, 1279-1290, doi:10.1038/s41590-019-0451-9.
- Mostafa, H.H.; Fissel, J.A.; Fanelli, B.; Bergman, Y.; Gniazdowski, V.; Dadlani, M.; Carroll, K.C.; Colwell, R.R.; Simner, P.J. Metagenomic Next-Generation Sequencing of Nasopharyngeal Specimens Collected from Confirmed and Suspect COVID-19 Patients. mBio 2020, 11, doi:10.1128/mBio.01969-20.
- Zhou, H.; Li, C.; Hu, T.; Liu, T.; Ni, N.; Chen, W.; Zhao, H.; Ruan, S.; Li, J.; Wu, H.; et al. Total infectomes of 162 SARS-CoV-2 cases using meta-transcriptomic sequencing. J Infect 2021, 82, e44-e48, doi:10.1016/j.jinf.2020.12.004.
- Xu, R.; Lu, R.; Zhang, T.; Wu, Q.; Cai, W.; Han, X.; Wan, Z.; Jin, X.; Zhang, Z.; Zhang, C. Temporal association between human upper respiratory and gut bacterial microbiomes during the course of COVID-19 in adults. Commun Biol 2021, 4, 240, doi:10.1038/s42003-021-01796-w.
- Villapol, S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Transl Res 2020, 226, 57-69, doi:10.1016/j.trsl.2020.08.004.
- Stavropoulou, E.; Kantartzi, K.; Tsigalou, C.; Konstantinidis, T.; Voidarou, C.; Konstantinidis, T.; Bezirtzoglou, E. Unraveling the Interconnection Patterns Across Lung Microbiome, Respiratory Diseases, and COVID-19. Front Cell Infect Microbiol 2020, 10, 619075, doi:10.3389/fcimb.2020.619075.
- Haiminen, N.; Utro, F.; Seabolt, E.; Parida, L. Functional profiling of COVID-19 respiratory tract microbiomes. Sci Rep 2021, 11, 6433, doi:10.1038/s41598-021-85750-0.
- Holliday, R.; Chaffee, B.W.; Jakubovics, N.S.; Kist, R.; Preshaw, P.M. Electronic Cigarettes and Oral Health. J Dent Res 2021, 100, 906-913, doi:10.1177/00220345211002116.
This entry is adapted from the peer-reviewed paper 10.3390/pharma1020006
