Monkeypox virus (MPXV) is a double-stranded DNA virus belonging to the Orthopox genus in the family Poxviridae. It has a zoonotic origin and MPXV infected wild rodents and primates have been found in central Africa. In humans, symptoms of MPXV infection include fever, head and muscle ache, lymphadenopathy and a characteristic rash that develops into papules, vesicles and pustules which eventually scab over and heal. Monkeypox is less often fatal (case fatality rates range from less than 1% to 3.6% depending on geographic localisation, viral clade and age group) than smallpox (up to 30%) caused by a related Variola virus. MPXV used to be endemic in sub-Saharan Africa, infecting wild animals and occasionally causing zoonotic outbreaks. Exotic animal trade and international travel combined with the increasing susceptibility of the human population due to lack of vaccination facilitated the spread of MPXV to new areas. Since April 2022, over 15.000 of MPX cases have been detected in >60 non-endemic countries around the world, predominantly among men who have sex with men, making it the largest described MPXV outbreak known to date.
1. Monkeypox Discovery and Outbreaks
Monkeypox virus (MPXV) was identified in 1958 during outbreaks of a pox-like disease in macaque monkeys kept at a research facility in Denmark, hence the disease name “monkeypox” (MPX) [
1]. The first human case was discovered in 1970 in the Democratic Republic of the Congo (DRC) during intensified smallpox surveillance [
2]. The patient, a 9-months-old baby, developed tell-tale signs of MPX including fever and a pox-like rash which developed into haemorrhagic lesions that crusted over and healed over the next 2 weeks. Despite the initial recovery, the patient succumbed to secondary infections and died in the hospital. Within the next decade, additional MPX cases, mainly among children (83% of all cases), have been identified in the Democratic Republic of the Congo (DRC, formerly Zaire from 1971 to 1997) as well as four additional Central and West African countries: Liberia, Sierra Leone, Nigeria and Ivory Coast [
3]. In the 1970s and 80s, most of the reported cases occurred in DRC, with an estimated 11% case fatality rate (CFR) among those who had not received a smallpox vaccination. The CFR was the highest among children under 4 years (15%) [
4]. Extended surveillance led to the identification of additional MPX-endemic regions in Benin, Cameroon, the Central African Republic, Gabon, Ghana, Sierra Leone, as well as South Sudan and confirmed that most of the cases occurred in the DRC [
5]. The first outbreak of MPXV in humans outside of Africa was documented in 2003 in the United States and linked to an exotic pet import from Ghana. In total, 71 MPX cases but no deaths were reported, all in patients who had been exposed to infected prairie dogs [
6]. In recent years, a few small clusters and single MPX cases were identified in the UK (2018 and 2019), Israel (2018), Singapore (2019) and the US (2021), all linked to travel to Nigeria, which has experienced re-emergence of MPX and reported over 500 suspected cases since 2017 [
7]. On the 4 May 2022, a patient with a recent travel history to Nigeria and an unexplained rash presented to a UK hospital. Polymerase chain reaction (PCR) on a vesicular swab confirmed a MPX diagnosis [
5]. Within the next months, thousands more cases were identified in over 50 countries on 6 continents, with major clusters in England, Germany, Spain, France and Portugal [
8] (
Figure 1).
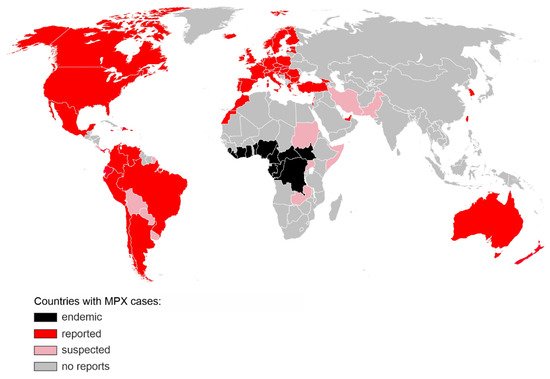
Figure 1. Countries with confirmed (red) or suspected (pink) MPX cases during the 2022 non-endemic outbreak. Regions, where MPX was endemic prior to 2022, are shown in black. The map includes cases reported until 12 July 2022 [
8]. The base layer map was obtained from
https://commons.wikimedia.org/wiki/File:BlankMap-World.svg (accessed on 1 July 2022).
This is the largest and most dispersed non-endemic MPXV outbreak known to date. Unlike previous outbreaks, there is no clear link between the infected people or a shared virus exposure source, such as travel to an endemic region or handling infected animals. While the transmission dynamics and route of infection are still uncertain, the virus appears to spread through close physical contact, and the vast majority of the affected are young or middle-aged men who have sex with men (MSM) and had recent sexual contact with new or multiple partners [
5]. Diagnosed patients are being advised to isolate and their close contacts traced. As of 12 July 2022, no MPX-associated fatalities have been reported in the non-endemic areas. In the light of a rapid increase in cases, and unprecedented scale of human-to-human transmission, WHO increased the risk level to global public health of MPX from low to moderate, with a high risk level in the European Region which accounts for over 80% of all new MPXV infections [
5].
2. Virus Classification and Phylogeny
MPXV is a double-stranded DNA virus belonging to the Orthopox genus in the family Poxviridae. Its closest known relatives include Variola (VARV) and Vaccinia viruses (VACV) (Figure 2).
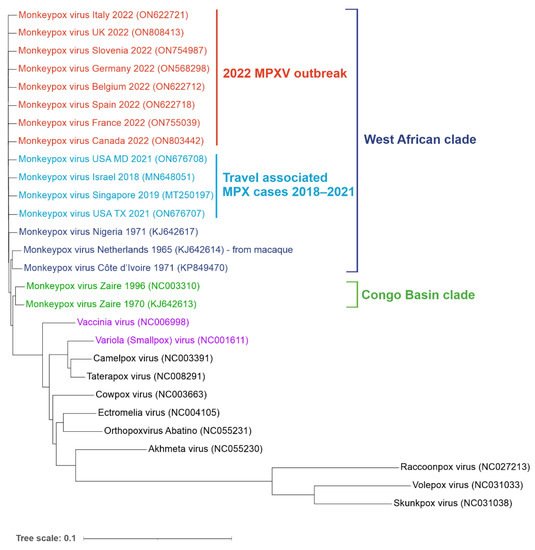
Figure 2. Phylogenetic tree of orthopoxvirus genomes. Reference genomes and MPXV genomes from the non-endemic 2022 outbreak (shown in red) as well as travel-associated cases detected in recent years (light blue). West African (dark blue) and Congo Basin (green) clades are indicated. Vaccinia (VACV) and Variola (VARV) viruses are shown in purple. Aligned sequences were obtained from the NCBI virus database. Genbank accession numbers are shown in the brackets. The tree was generated using ngphylogeny.fr and visualized using iTOL online tools (itol.embl.de/) (both accessed on 1 July 2022).
The genomes of MPXV and VARV share a high level of sequence similarity (96%); however, phylogenetic studies suggest that they did not evolve from one another. VARV is more closely related to the Camelpox virus and the Taterapox virus found in gerbils (98% genome similarity) and both VARV and MPXV may have originated from poxviruses infecting rodents and/or ruminants [
9,
10,
11,
12]. MPXV strains are grouped into two clades: Congo Basin (CB) and West African (WA). The more pathogenic CB clade (CFR up to 11%) is found mainly in the DRC and surrounding counties and was responsible for the first documented human case of MPX in 1970 [
2]. The ongoing 2022 outbreak, as well as the previous smaller outbreaks in Texas (2003), the United Kingdom (2018 and 2021), Singapore (2019) and Israel (2018–2019), were all caused by the WA clade characterized by lower CFR (estimated between less than 1% to 3.6%) [
13,
14,
15,
16,
17]. Phylogenetic analysis of sequenced MPXV genomes from the current outbreak revealed two distinct virus lineages. Almost all strains from the 2022 non-endemic outbreak form a single subclade (B.1) and most likely have a single origin [
18]. This lineage is most related to a single case in a traveller from Nigeria to Maryland in 2021. The second lineage (A.2) currently includes only two strains identified in the US, which differ by 80 nucleotide changes relative to the other 2022 MPXV sequences, suggesting an independent virus introduction event [
19].
3. Animal Reservoir
MPXV has been detected in various species, and it is still not clear which of them serves as the main animal reservoir. For example, the virus has been isolated from African squirrels and MPXV reactive antibodies or viral DNA have been detected in multiple wild rodent and shrew species as well as pigs [
20,
21,
22,
23,
24,
25]. The largest documented series of zoonotic MPXV transmissions occurred in 2003 in the US, when 71 people became infected after handling prairie dogs. These pets had been housed together with infected dormice, rope squirrels and a Gambian giant rat imported from Ghana [
26]. There is also evidence of non-human primate infections in the wild. For example, MPXV has been isolated from a dead sooty mangabey monkey [
27], and poxvirus-reactive antibodies have been detected in 2% of Zambian baboons as well as Cercopithecus and Colobus monkeys [
28]. A MPXV outbreak has been reported in a population of chimpanzees, with symptoms resembling those of humans [
29]. In addition, a case of MPXV infection in a baby bitten by a wild chimpanzee was described [
30]. Apart from contact with live animals, preparation or consumption of wild game or bushmeat also causes a risk of contracting MPXV. However, it is frequently impossible to establish the exact zoonotic source and route of transmission in endemic regions due to concurrent exposure to multiple wild species [
5,
31]. Thus, unlike VARV which is confined to humans, multiple mammalian species could serve as natural animal reservoirs of MPXV, which poses a significant challenge to MPXV control and containment efforts.
4. Virus Genome and Morphology
The MPXV genome encompasses ~197,000 bp and includes hairpin termini as well as >190 non-overlapping open reading frames (ORFs) [
10,
32]. The highly conserved central coding region of the genome is flanked by variable ends that contain inverted terminal repeats. At least 90 ORFs are known to be essential for poxvirus replication and morphogenesis. Many of the additional so-called non-essential ORFs play a role in the differences in poxvirus host tropism, immunomodulation and pathogenesis, with many ORFs still waiting to be functionally characterized [
33]. MPXV virions are barrel- or oval-shaped particles, with an average size of ~280 nm × 220 nm [
16]. Mature poxvirus particles have a characteristic dumbbell-shaped nucleoprotein core containing a large double-stranded linear DNA genome [
6]. Similarly to VACV, MPXV virions contain over 30 structural and membrane viral proteins as well as virus-encoded DNA-dependent RNA polymerase and associated transcriptional enzymes [
34,
35] (
Figure 3).
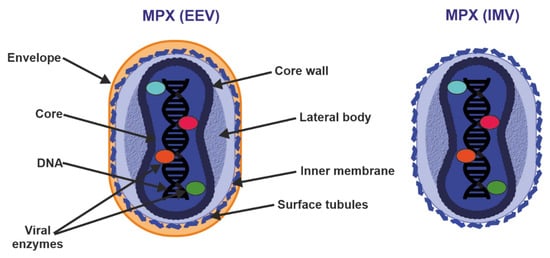
Figure 3. Structure of enveloped extracellular virions (EEV) and intracellular mature virions (IMV). See text for further details.
Poxvirus particles have two mature forms, both of which can mediate infection: extracellular enveloped virus (EEV) (thought to be responsible for early dissemination) and the intracellular mature virus (IMV) released during cell lysis [
36,
37]. The major structural difference between IMV and EEV is that IMVs lack the additional outermost membrane layer. However, the levels of incorporated viral proteins also differ between the two types of virions [
35,
36].
5. Transmission and Replication Cycle
Handling infected rodents appears to be a common source of zoonotic MPXV transmission, and human-to-human spread can occur through close contact with lesions, body fluids, respiratory droplets and contaminated objects [
5]. Studies using macaques that were exposed to aerosolized MPXV showed that the pathogen initially infects lower airway epithelial cells and spreads to lymph nodes, followed by systemic dissemination through monocytic cells. MPX lesions may subsequently form in lymph nodes, thymus, spleen, skin, oral mucosa, gastrointestinal tract and reproductive system [
38]. In vitro studies suggest that the MPXV can infect most mammalian cells [
39,
40]. Widely abundant glycosaminoglycans such as chondroitin and heparin sulfates, as well as laminin, play a role in cellular attachment of other poxviruses [
41,
42,
43] (
Figure 4).
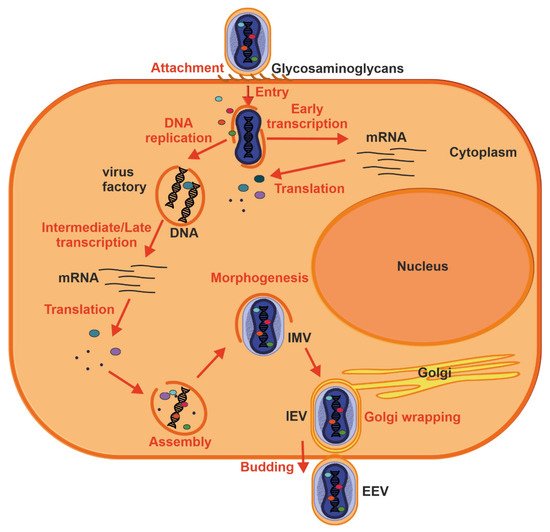
Figure 4. Replication cycle of a poxvirus. Key events are outlined: attachment (1), entry (2), early viral gene transcription and translation (3), DNA replication (4), intermediate and late transcription and translation (5), assembly (6), morphogenesis (7), envelopment by intracellular membranes (8) and budding (10). See text for further details.
Proteins involved in glycosaminoglycan biosynthesis were also recently identified in a genome-wide screen for factors facilitating MPXV infection [
44]. Thus, MPX virion attachment is most likely mediated by external virion proteins and cellular glycosaminoglycans on the surface of the target cell or by components of the extracellular matrix. Following attachment, poxviruses enter the host cells by a low pH endosomal pathway or direct fusion with the plasma membrane at neutral pH, which releases the viral core in the cytoplasm. Fusion of IMV and EEVs with the cell is dependent on a complex of ~12 non-glycosylated, viral membrane proteins [
45]. Following entry, viral transcription is initiated by the virus-encoded multi-subunit DNA-dependent RNA polymerase followed by the translation of early, intermediate and late proteins on host ribosomes [
46]. Poxvirus DNA synthesis occurs in cytoplasmic structures, often referred to as “factories”, which gradually transition from compact DNA-containing structures wrapped by ER membrane to crescent-shaped structures where virion assembly occurs [
16,
47]. While the majority of mature virions remain inside of the cell (IMV), some are transported via microtubules and become enveloped by two ER or Golgi-derived membranes. These enveloped virions can initiate actin polymerization, which propels the particle on an actin tail toward an adjacent cell, or exit the cell by fusion with the cytoplasmic membrane and become EEV [
47].
This entry is adapted from the peer-reviewed paper 10.3390/ijms23147866