Grain legumes play a significant role in smallholder farming systems in Africa because of their contribution to nutrition and income security and their role in fixing nitrogen. Biological Nitrogen Fixation (BNF) serves a critical role in improving soil fertility for legumes. Dinitrogen can be reduced to ammonium (NH3) through the Haber–Bosch process or via biological nitrogen fixation (BNF) utilizing some soil bacteria or archaea (diazotrophs). Rhizobia belong to Alphaproteobacteria and Betaproteobacteria, a group of Gram-negative bacteria that forms nodules on roots (sometimes stems) of leguminous plants to fix nitrogen in a symbiotic relationship with their host plants. The rhizobia–legume symbiosis is the most studied plant–microbial mutualism because of the importance of nitrogen fixation for almost all agricultural systems.
1. Nodulation and Nitrogen Fixation Processes
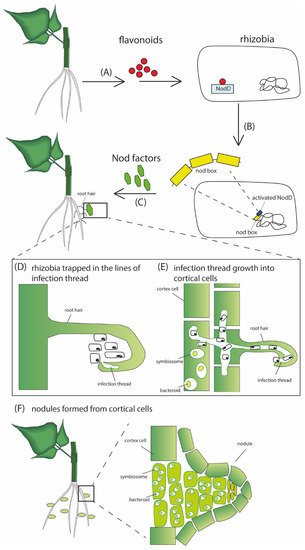
Nodulation is a host-specific process, with each rhizobia having a specific range of hosts [
19]. It is a multistep process involving the host plant and its symbionts in the rhizosphere. Under severe nitrogen starvation, legume roots release flavonoid-containing exudates in the rhizosphere, interacting with nodulation protein D (NodD) in rhizobia [
20]. Activated NodD induces the expression of
nod genes through its binding to the
nod operon. This causes the synthesis and release of nod factors, lipochitooligosaccharide(LCO)-based signaling molecules whose interaction with specific genes in the root hairs leads to cortical cell divisions forming root nodule primordia and simultaneously initiates an infection process to deliver the bacteria into the nodule cells. The infection of most legumes involves developing plant-made infection threads through root hair branching, deformation, and curling [
21]. There are two nodule types: indeterminate (e.g., in
Medicago, pea, and clover) and determinate (e.g., in common beans and soybeans). Indeterminate nodules are thought to originate from cell divisions in the inner cortex and usually possess a persistent apical meristem. They are cylindrical, with a developmental gradient from the apex to the base of the nodule, which may divide into different nodule zones [
22]. However, determinate nodules result from cell divisions in the middle/outer cortex of the root, lack a persistent meristem, and are spherical. Cell divisions of a determinate nodule stop at early developmental stages. Therefore, mature nodule develops through cell enlargement; as such, the infected cells develop more or less synchronously to the nitrogen-fixing stage [
23].
The infection threads harboring the dividing bacteria grow through an epidermal cell layer into the nodule primordial cells (
Figure 1). The bacteria are then released and internalized in an endocytosis-like process by the cortical cells. In nodule cells, individual bacteria are enclosed by a membrane of plant origin, forming an organelle-like structure called the symbiosome. The bacteria further proliferate and differentiate into nitrogen-fixing bacteroids [
23] that actively synthesize nitrogenase enzymes. Nitrogenase is extremely sensitive to oxygen; thus, the nodule must maintain low oxygen concentrations while maintaining oxygen supply for bacterial metabolism [
24]. Infected cells synthesize leghemoglobin that is thought to buffer free oxygen in the nanomolar range, avoiding the inactivation of oxygen-labile nitrogenase while maintaining high oxygen flux for respiration [
25]. Abolishing leghemoglobin synthesis in
Lotus japonicus caused an increase in nodule free oxygen, a decrease in the ATP/ADP ratio, loss of rhizobial nitrogenase protein, and an absence of nitrogen fixation [
26].
Figure 1. A schematic representation of the nodulation process. (A) Host plant releases flavonoids into the rhizosphere that are perceived by specific rhizobia. The flavonoids induce transcription of the genes for biosynthesis of the rhizobial Nod factors, which the plant perceives to allow symbiotic infection of the root. After transcription, the activated NodD binds to the nod box promoter (B), inducing the transcription and synthesis of Nod factors (C). The Nod factors induce the development of the infection thread that traps rhizobia within the curled surfaces (D). The infection thread grows through epidermal cells into the cortical cells, where rhizobia are released and internalized by the cortical cells (E). Further proliferation and differentiation of both bacteria and infected cortical cells results in nodule formation (F).
The symbiosome membrane acts as the interface between eukaryotic and prokaryotic symbionts, and thus it possesses transporters for nutrient exchange between the symbiotic partners [
27]. The host plant provides carbon sources for bacteroid activities in the form of dicarboxylates, malate, and succinate [
28]. Phosphoenolpyruvate carboxylase and malate dehydrogenase convert the carbon flux from glycolysis to form malate, which can be taken directly by bacteroids [
29]. Ammonia exported from the bacteroids diffuses into the cytosol of the infected host cells, where it is converted to glutamine (Gln) and glutamate (Glu) by the enzymes Gln synthetase and Glu synthase. In indeterminate nodules, Glu and Gln are further converted to aspartate (Asp) and Asn by Asp aminotransferase and Asn synthetase [
30]. In indeterminate nodules, Gln further enters the purine synthesis pathway and is converted to ureides (McLaughlin et al., 1987) as the final product exported to the host plant.
In addition to root hair entry and through infection thread observed in most rhizobia, another mechanism known as crack entry occurs in
Bradyrhizobium. The infection by crack entry necessitates penetration of the bacterial microsymbiont through epidermal breaches located at sites where lateral roots (root epidermis) or adventitious roots (stem epidermis) protrude and so provide penetration sites at the fissure [
31,
32]. After entry, the cells occupy the space between epidermal and cortical cells. Beneath the axillary root hairs, basal cells become enlarged. Such enlarged basal cells are the first to become infected by some of the invading rhizobia cells, while others continue to spread intercellularly [
33]. After intercellular spreading of the rhizobial cells, the cell walls of particular plant cells that will eventually internalize the bacteria are structurally altered and appear partially degraded, as in a cellulolytic process. Ultimately, the plasma membrane is partly exposed to the bacteria in such invaginations, and the bacteria are internalized in host cells in an undefined matrix [
33]. The encapsulated cells multiply rapidly within the infected plant cell. In the beginning, they are enclosed in the same membrane envelope, but the envelope is later divided so that each cell becomes enclosed separately [
34]. The invaded plant cell also divides rapidly, thereby distributing the endophytes such as other cell organelles. The nodule tissue eventually originates from one or few infected plant cells.
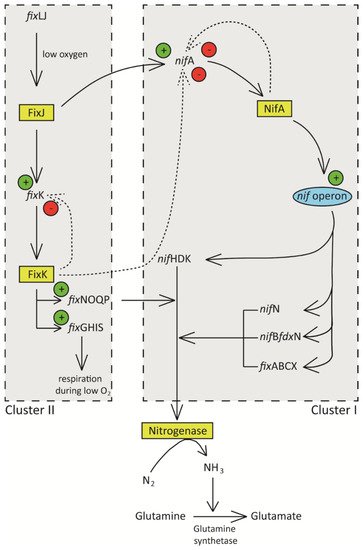
Nitrogen fixation is an energy-intensive process that requires strict control, e.g., it requires 40 moles of ATP to reduce a single molecule of dinitrogen to ammonia [
53,
54]. In rhizobia, the regulation of nitrogen fixation is mainly controlled by the amount of free oxygen in the nodule, unlike free-living bacteria, whose nitrogen fixation is regulated by the amount of fixed nitrogen in the rhizosphere [
41,
55]. NifA and FixK constitute two central cascades that regulate nitrogen fixation genes [
56]. NifA targets nitrogenase complex encoding and accessory genes (
nifHDKE,
nifN,
fixABCX,
nifA,
nifB, and
frdX), while FixK is involved in the transcriptional regulation of
fixNOQP and
fixGHIS genes [
41].
Figure 2 below is the current model that depicts the regulation of nitrogen fixation rhizobia.
Figure 2. Schematic diagram showing regulation of nitrogen fixation in rhizobia. Low oxygen concentration in the nodules induces transcription of fixJ, whose product induces the expression of nifA and fixK. NifA activates the nif operon resulting in the expression of nif genes that ultimately leads to the synthesis of nitrogenase enzyme and its accessory components. FixK induces the expression of fix operon whose products are involved in redox reactions necessary for the nitrogen fixation process. High FixK and NifA result in repression of nif and fix operons.
2. Distribution of Rhizobia in African Soil
Leguminosae comprises more than 19,000 species [
57] that have independently evolved in different places. For example,
Phaseolus was developed in Andean and Mesoamerican regions [
58,
59],
Medicago,
Trifolium, and
Pisum in the Middle East [
60], soybean in Asia [
61],
Galega oficinalis and G. orientalis in the Caucasus [
62], and Bambara groundnuts as well as cowpea in Africa [
63]. Several older legumes, such as acacia, have evolved world-wide [
64]. However, legumes are currently distributed throughout the world. The principal symbionts of particular legumes seem to have developed locally, coinciding with the legumes’ distribution (
Table 1) [
65].
Table 1. Rhizobia isolated from African soils.
| Strain |
Legume |
Reference |
| R. phaseoli |
P. vulagaris |
[17,66] |
| R. paranaense, |
P. vulagaris |
[66] |
| R. sophoriradicis |
P. vulagaris |
[66] |
| R. leucaenae |
P. vulagaris |
[66] |
| R. aegyptiacum |
P. vulagaris |
[66] |
| R. tropicii |
P. vulagaris, Sesbania sesban |
[67,68,69] |
| R. etli |
P. vulagaris |
[67,68] |
| R. leguminosarum |
Sesbania sesban, V. faba |
[69,70] |
| M. amorphae |
S. sesban, C. arietinum |
[69,71] |
| M. plurifarium |
S. sesban |
[69] |
| R. huautlense |
S. sesban, C. arietinum |
[69,71] |
| M. plurifarium |
S. sesban, C. arietinum |
[69,71] |
| B. elkanii |
Vigna subterranea, Glycine max, A. hypogaea |
[72,73,74] |
| B. japonicum |
V. subterranea, Glycine max, A. hypogaea |
[72,73] |
| M. ciceri |
Cicer arietinum |
[71] |
| M. mediterraneum |
C. arietinum |
[71,75] |
| M. loti |
C. arietinum |
[71] |
| M. opportunistum |
C. arietinum |
[71] |
| M. haukuii |
C. arietinum |
[71] |
| M. tianshanense |
C. arietinum |
[71] |
| M. cicero |
C. arietinum |
[75] |
| B. yuanmingense |
A. hypogaea |
[73] |
| B. canariense |
A. hypogaea |
[73] |
| B. liaoningense |
A. hypogaea |
[73] |
| R. pisi |
V. faba |
[70] |
| R. anhuiense |
V. faba |
[70] |
| R. laguerreae |
V. faba |
[70] |
| R. binae |
V. faba |
[70] |
| R. bangladeshense |
V. faba |
[70] |
| R. lentis |
V. faba |
[70] |
| R. aethiopicum |
V. faba |
[70] |
| R. aegypticum |
V. faba |
[70] |
3. Factors Affecting the Distribution of Rhizobia in Africa
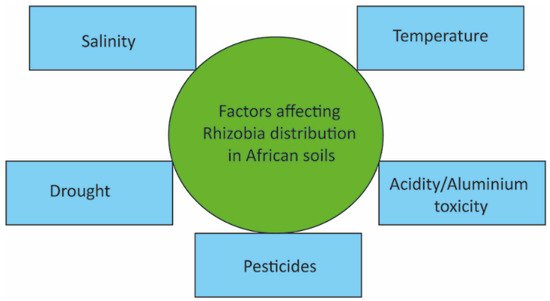
According to Puozaa et al. [
86], the physicochemical properties of soil serve a critical role in the diversity of microorganisms present in a particular habitat. Environmental factors significantly affecting rhizobia distribution and diversity in Africa include temperature, pH, salinity, drought, and pesticides (
Figure 3) [
87].
Figure 3. Factors affecting the distribution of rhizobia in African soils.
Temperature is a significant factor in legume–rhizobia interactions and determines the strains’ distribution and nodulation ability [
88]. For optimal performance, each combination of rhizobia and legumes requires specific conditions. The ideal temperature for rhizobia growth is 25–30 °C, although some can tolerate up to 42 °C in arid and semiarid areas. In some regions of Africa, the temperature can go up to 60 °C. High temperature reduces nitrogen fixation [
89] mainly through reduced nitrogenase activity [
90,
91]. Decreased glutamate synthase and glutamine synthetase activities lower nitrogen assimilation in heat-stressed plants [
92]. Genetic modifications such as loss of plasmids [
93] and genome rearrangement [
94] have been reported for some rhizobia strains exposed to temperature stress. Furthermore, high temperatures reduce the release of
nod gene induction signals (specific flavonoids) [
95] and, consequently, the nodulation process [
96] in common beans. Although some rhizobia strains fix nitrogen at temperatures as low as 4 °C [
97], nodule formation is severely impaired because fewer flavonoids are secreted [
88].
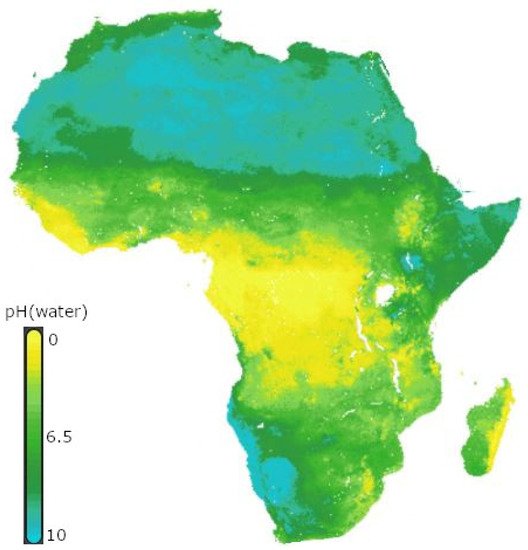
Biological nitrogen fixation is effective in neutral or slightly acidic soils [
98]. However, most African soils are acidic because of industrial pollution and poor agricultural practices, such as the overutilization of inorganic fertilizers (
Figure 4) [
8,
100]. In general, acidic soils negatively affect crop production [
67,
101,
102] and the proliferation of soil bacteria. When the pH is below 5.5, the soil is considered acidic, and the solubilization of toxic metal ions is promoted, severely increasing metal ion toxicity [
88]. Acidic conditions and the presence of heavy metals are detrimental to rhizobia growth, symbiosis, and nitrogen fixation. Reduced rhizobia growth restricts their distribution, propagation, nodulating ability, and survival [
89].
Figure 4. Soil pH in Africa Africa [
124].
Al toxicity in rhizobia might be caused by the direct binding of polymeric Al ions to the cell membrane [
106]. However, when such polymeric compounds were removed from the media before inoculation, Al toxicity to rhizobia was not eliminated [
103]. Another explanation for the toxicity of Al could be that the ions bind to the phosphate moieties of the DNA helix after penetrating the cell membrane. This increases its stability and prevents or slows down its replication [
107]. Though significant in rhizobia research, this theory was also questioned because rhizobia, like any other bacteria, maintains its internal pH values slightly alkaline (pH 7.2–7.5) and, therefore, intracellular Al is expected to precipitate out [
108].
Soil salinity is another global problem that negatively impacts agricultural productivity and sustainability. Soil salinity occurs in arid and semiarid regions where rainfall is insufficient to meet the water requirements of the crops and microbial populations. In Africa, about 80 × 10
6 hectares are either saline, sodic, or saline–sodic, of which the Sahel in West Africa is the most affected region [
110]. Furthermore, flooding, seepage, over-irrigation, silting, and a rising water table are the main reasons for the increased salinization in Africa [
111]. Salinity has harmful effects on both plant hosts and bacteria and restricts the survival of rhizobia, colonization, and nodulation activity. In a recent study, plants growing at 2, 4, 6, and 8 mS/cm salt experienced a mean reduction in nodulation ability between 60 to 93% [
112].
4. Potential and Challenges to Commercialization of Rhizobia-Based Inoculants
The eradication of food insecurity, poverty, and hunger is a crucial agenda identified in the United Nations Sustainable Development goals (SDGs). About 700 million people do not have access to sufficient food, most of them from Latin America, Asia, and Africa [
163]. The cost of commercial fertilizer is way beyond the affordability of poor African farmers. More so, overuse of fertilizer has been identified as a causative agent of soil and water pollution. The ongoing COVID-19 pandemic has exacerbated the situation. Therefore, there is a need to embrace clean and affordable agricultural practices, such as plant growth-promoting microorganisms, to enhance plant productivity with reduced environmental effects.
The use of rhizobia in BNF is a viable strategy to enhance crop yields through nitrogen fixation [
164,
165] and ensures that the appropriate strains of rhizobia for the specific legume exist in the soil. The popularity of BNF technology has recently improved due to its ability to reduce overreliance on inorganic fertilizers [
166,
167]. Apart from fixing nitrogen, rhizobia inhibit disease-causing phytopathogens by chelating iron away or production of antibacterial agents in the rhizosphere [
168,
169], solubilizing phosphate [
170], and supplying growth-promoting hormones such as IAA to host plants [
17]. Inoculating legumes with rhizobia is the most used technique in BNF [
4,
171]. Native rhizobia inoculated on different legumes, including peanut, common bean, soybean, cowpea, lentils, and green grams, have improved their productivity [
16,
18,
172,
173,
174,
175].
The selection of elite rhizobia strains as commercial inoculants is based on various considerations. They should effectively compete with native rhizobia for nodule occupancy, and the strains must have a high nitrogen fixation ability for the intended host under greenhouse and field conditions [
176,
177]. Moreover, they should possess characteristics such as stress tolerance, satisfactory growth, and genetic stability when the inoculum is being manufactured [
178]). Moreover, Checcucci et al. [
176] noted that the strain should possess limited year-to-year persistence in unplanted soil so that newly improved strains can be introduced without concern about competition. Elite rhizobia selected as seed and soil inoculants in field trials have shown increased crop yields in Africa [
13,
176]. Other reports [
18,
179,
180] of increased use of microbial inoculants in smallholder farming systems in Africa are encouraging. Therefore, the use of appropriate inoculants in legumes offers an opportunity to improve legumes’ productivity. However, the technology has not been well established in Africa, yet African farmers can immensely benefit from its affordability [
181].
Compared with the global biofertilizer market in countries such as the United States, Canada, Argentina, Europe, China, and India, the potential benefits of biofertilizers have been largely untapped in Africa due to a myriad of challenges. One of the challenges facing the commercialization of rhizobia for BNF is the effect of indigenous rhizobia and other bacteria in the soil. It has been noted that when other factors are constant, lower indigenous rhizobia levels lead to a higher response of the inoculums. At the same time, it becomes difficult to improve nitrogen fixation if native populations exceed 10
2 cells per gram of soil [
4]. Therefore, it is important first to determine the rhizobia population in the soil, followed by their characterization.
Another critical challenge is inefficient production technology for commercial strains [
178,
200] tailored to the local conditions, especially product shelf life, given the high temperature that is unsuitable for prolonged strain survival in some African regions, and this may lead to substandard/poor quality inoculants found in a number of countries [
201,
202]. In this regard, instead of including only foreign isolates in the inoculants, studies should be performed to identify elite isolates from African soils with the capacity to withstand these harsh conditions.