1. Brief Introduction of Percutaneous Closure of PFO by Traditional Devices
In 1975, King and Mills performed for the first time a percutaneous atrial septal defect closure in humans [
7] by using an automatically opening “umbrella-like” device, further refined to the clamshell double-umbrella device, which had the advantage of being recapturable or removable up to the end of the procedure [
7]. Due to the occurrence of late fractures of the metal arms [
8], the device was withdrawn from the market. In the mid-1990s, the first Amplatzer Septal Occluder (Abbott, Chicago, IL, USA) was designed as a self-expandable, fully retrievable double disc device made of nitinol wire [
9]. Nitinol is a metal alloy of nickel and titanium characterized by thermal memory. This property allows devices to be compressed at a small diameter into a delivery system. When the device is deployed, it comes back to its original configuration.
Then, the Amplatzer PFO Occluder was specifically designed for PFO closure [
10], becoming the most widely investigated and used device for percutaneous PFO closure and atrial septal closure in general [
11,
12,
13].
In recent decades, several dedicated devices and closure systems have been developed for the catheter-based therapy of PFO [
4,
5,
11,
12,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24]. The most used are the double disc devices (the traditional ones).
2. Double Disc Devices
The Amplatzer PFO Occluder and the Gore Cardioform Septal Occluder (W.L. Gore and Associates; Flagstaff, AZ, USA) are the most used devices in clinical practice so far [
2] (
Figure 1).
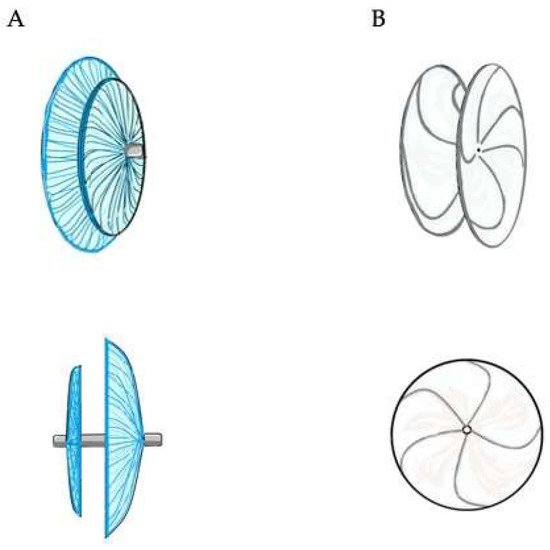
Figure 1. Double disc devices. The Amplatzer PFO Occluder (panel (A) on the left) is a self-expandable device consisting of two atrial discs, of which the right is larger than the left one, linked together by a short connecting waist. It is made from a nitinol wire mesh and a thin polyester fabric, securely sewn to each disc by a polyester thread, in order to increase its closing ability. The Gore Cardioform Septal Occluder (panel (B) on the right) consists of two atrial discs made from a nitinol wire frame with a platinum filled core. The two discs are covered with a thin, soft and conformable, expanded polytetrafluoroethylene (ePTFE) membrane.
The Amplatzer PFO Occluder, made from nitinol and polyester, is a self-expanding, non-self-centering, double disc device with a smaller left and a larger right atrial disc (except for the sizes 18 mm and 30 mm, in which the diameter is the same for both discs) and a central thin stem. The Amplatzer Multi-Fenestrated Septal Occluder—Cribriform (Abbott, Chicago, IL, USA) is also often implanted for PFO closure, and it differs from the Amplatzer PFO Occluder just for the presence of two equal size discs. Procedural success rates are close to 100%, and effective closure rates are as high as 95% at 6 months [
12]. Thrombus formation on the device is exceedingly rare, as is clinically relevant new-onset atrial fibrillation.
The Gore Cardioform Septal Occluder is a non-self-centering, double disc device made out of a nitinol frame, covered by expanded polytetrafluoroethylene (ePTFE). Despite high success rates, a lower complete closure rate has been reported with this device [
2]. On the other hand, it has been reported that the Cardioform device has the highest complete closure rate, based on a transcranial Doppler bubble study assessment for residual shunting [
25]. However, no cases of cardiac erosion with this device are reported in the literature.
Amplatzer Septal Occluders (Abbott, Chicago, IL, USA) and Gore Cardioform ASD Occluder (W.L. Gore and Associates; Flagstaff, AZ, USA) are self-centering, double discs devices characterized by a central large waist which allows perfect device-centering. They are designed for atrial septal defect closure; however, they may be useful for closing PFO with specific anatomies.
A successful double disc devices implantation rate amongst all randomized clinical trials (RCTs) was achieved of 95.6%, with no or minimal residual shunt. These results support the fact that percutaneous PFO closure is a feasible and safe procedure, with no increase in serious adverse events. The most frequent late complications are device thrombosis, the incidence of which is 1.0–2.0%, and device embolism, which occurs at a rate of 0.9–1.3% [
26]. A recent meta-analysis showed no significant differences between the device closure group and medical therapy group as regards the risk of serious adverse events and major bleedings [
27,
28]. Residual shunts, which are associated with increased rates of recurrent events, might be treated successfully with a second device [
29,
30,
31]. New-onset atrial fibrillation (AF) and flutter are the most frequent arrhythmic complications, with an incidence of about 3.2% [
32], possibly due to catheter manipulation, wire crossing into the left atrium, or stretching of the atrial wall with the device. However, AF commonly occurs within 45 days after device implantation, and in 76% of cases is a transient phenomenon without recurrence [
32]. According to a recent retrospective cohort study on 1533 Ontarian patients, the incidence of AF after PFO closure was relatively low (6.26%) over a follow-up of about 8 years [
33].
Currently, the following devices can also be used: Figulla Flex II PFO Occluder (Occlutech GmbH, Jena, Germany), Figulla Flex II UNI Occluder (Occlutech GmbH, Jena, Germany), Figulla Flex II ASD Occluder (Occlutech GmbH, Jena, Germany), CeraFlex PFO Occluder (Lifetech Scientific Corporation, Shenzhen, China), Ultrasept PFO Occluder (Cardia Inc., Eagan, MN, USA), Hyperion PFO Occluder—II (Comed B.V., Bolsward, The Netherlands), Nit-Occlud PFO (PFM Medical, Cologne, Germany), Amender PFO Occluder (Kewei Rising Medical Co., Ltd., Guangdong, China) and MemoPart PFO Occluder (Lepu Medical Technology Co., Beijing, China).
3. Procedural Steps
PFO closure is routinely performed as a day-case procedure, usually under TEE guidance.
A venous vascular access is obtained to perform the procedure. The femoral vein is the preferred vascular access; however, in rare cases, the femoral vein cannot be used due to bilateral thrombosis or specific anatomical variants (i.e., inferior vena cava interruption with azygos-continuation). In the latter cases, the internal jugular vein, the axillary/subclavian vein or the suprahepatic veins may be used for the procedure [
34,
35,
36].
PFO is usually crossed with a 5–6 Fr multipurpose catheter and a standard 0.035-inch guidewire from the right atrium. From the inferior vena cava, the Eustachian valve directs the guidewire into the PFO tunnel. However, in some cases, a stiff tunnel prevents the smooth crossing of the guidewire. In such cases, it might be used as a hydrophilic guidewire to cross the tunnel or a smaller catheter (4 Fr multipurpose or JR) may be wedged into the tunnel with subsequent crossing after the initial thenting of the septum primum. Subsequently, a stiff guidewire can be placed in a left pulmonary vein (preferably the upper one to have a better alignment with interatrial septum). Often, an evaluation of the septum primum raising and the size of the consequent left-to-right shunt is enough to choose the device to use. However, balloon sizing of the tunnel may allow PFO stretching with a better device selection [
37]. Usually, a tunnel stretch with a diameter ≤12 mm is addressed to a non-self-centering device with a maximum disc diameter of 25 mm, while a larger stretch diameter is addressed to bigger devices.
A long sheath is pushed beyond the PFO tunnel, the left-disc of the device is opened in the left atrium, pulled-back to the interatrial septum and then the right disc is opened in the right atrium and pushed to the PFO entry. Before releasing the device, it is crucial to ensure that:
- -
-
The device has well hugged the septum primum, particularly the septum secundum;
- -
-
There is no interference with the atrioventricular valves or the other intracardiac structures
- -
-
There are no patent accessory fenestrations
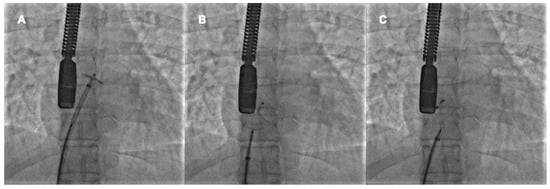
When the device is well-deployed, the cranial left anterior oblique fluorscopic view shows a typical pattern of the prosthesis with the upper edges of the discs well separated and the lower ones very close, the so-called “Pacman sign”. In fact, the thick septum secundum divided the two discs at the superior vena cava outlet, whereas the thin septum primum allows greater disc compacting at the inferior vena cava outlet [
38]. The device’s stability may be checked with a push-and-pull maneuver it is released (
Figure 2).
Figure 2. Percutaneous PFO closure by Amplatzer PFO Occluder 25 mm. The left disc is opened in the left atrium and pulled back to the interatrial septum (A). The right disc is opened in the right atrium and pushed towards the interatrial septum (B). After the push-and-pull manoeuvre, the device is released (C).
4. Role of TEE
4.1. TEE before PFO Closure: Characterization of the PFO and Decision Making
TEE provides a detailed visualization of the interatrial septum and other relevant structures, and it can show the shunt across the septum by using contrast (c-TEE). However, the accuracy and sensitivity of c-TEE in diagnosing PFO are inferior to other techniques, probably due to the inability to perform an adequate Valsalva maneuver during examination [
39,
40,
41]. The imaging method with the highest sensitivity in detecting the right-to-left interatrial shunt is contrast-enhanced transcranial Doppler (c-TCD), which is the first-line investigation in PFO diagnostic workup. TEE, rather than a diagnostic role, plays a key role in defining the anatomy of the interatrial septum, aiming at stratifying the risk and assessing the suitability for device closure.
TEE imaging of the interatrial septum begins with the transverse plane at 0° [
42]. The interatrial septum should be swept from the superior vena cava down through the fossa ovalis up to the inferior vena cava and the coronary sinus at a high-, mid-, and low-esophageal level, respectively. Fossa ovalis, which is the region where the “septum primum” is exposed on the right atrium, is easy to recognize at mid-esophageal level, since it is thinner than the “septum secundum”. From 0°, a slow continuous sweep of the interatrial septum should be made with increments of 10–20° [
43]. At 90° the interatrial septum is assessed along its longitudinal (supero-inferior) plane, always advancing the probe from the superior vena cava to the mid-esophageal level towards the inferior vena cava. The 45° view at the mid-esophageal level displays the relationship of the interatrial septum and fossa ovalis with the aortic valve and aortic root (
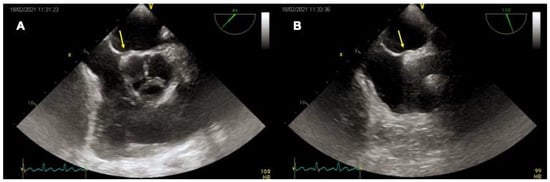
Figure 3). The opening of the PFO in the left atrium can be seen in each of the above-mentioned views from the high- or mid-esophageal levels. For each plane, it may be useful to visualize the entire interatrial septum with both 2-dimentional (2D) gray-scale imaging and 2D color Doppler imaging to avoid missing other atrial defects (e.g., small fenestrations in the fossa ovalis or other larger defects). Reducing the color Doppler scale (20–30 cm/s) can be helpful to optimize blood flow visualization across the PFO. Then, intravenous agitated saline contrast should be administered to confirm that the passage occurs across the PFO (bubble test). In the presence of a PFO, a right-to-left shunt should be observed early after a correctly performed Valsalva maneuver (within 3–5 beats from the release). The effectiveness of the Valsalva maneuver is evidenced echocardiographically by the leftward bulging of the “septum primum”, denoting that the right atrial pressure is higher than the left atrial. A real-time three-dimensional (3D) analysis may complement the 2D TEE, allowing a clearer understanding of PFO morphology and its relationship with surrounding structures. In particular, real time 3D TEE offers improved spatial resolution for the features of complex PFOs (e.g., aneurismatic interatrial septum, multiple fenestrations). The best 3D imaging of the interatrial septum should be performed in “3D zoom” mode, acquiring the bicaval view (90–110°), where the atrial septum is perpendicular to the ultrasound beam.
Figure 3. TEE imaging of the interatrial septum. Mid-esophageal 45° (A) and 90° (B) views of a tunnel-like PFO (yellow arrows).
Table 1 contains the anatomical features of the interatrial septum that should be assessed by TEE for a patient’s risk stratification and interventional treatment. Atrial septal aneurysm, moderate-to-severe shunt, atrial septal hypermobility, and large PFO size were found to be strongly associated with a causal role of PFO in left circulation thromboembolism. [
4,
14,
44,
45,
46,
47]. Hence, their presence is currently an indication for percutaneous PFO closure. Prominent Eustachian valve, Chiari network, and long PFO tunnel are other despite the lower level of scientific evidence [
48,
49].
Table 1. Interatrial septum variables to be assessed by transesophageal echocardiography for decision making.
-
PFO location
- -
-
Length of the PFO tunnel
- -
-
Width of the PFO tunnel opening
- -
-
Patency of foramen ovale at rest and after Valsalva manoeuvre by color Doppler and by
- -
-
injecting contrast
- -
-
Distance between PFO and aortic root, superior and inferior vena cava, right
- -
-
upper pulmonary vein, atrio-ventricular valves, and posterior atrial wall (rims)
- -
-
Atrial septal mobility
- -
-
Concomitant atrial septal aneurysm
- -
-
Septal length
- -
-
Thickness of the septum secundum
|
- -
-
Additional atrial septal defects
- -
-
Pulmonary venous anatomy
- -
-
Left atrial appendage orientation
- -
-
Eustachian valve and/or Chiari network
|
PFO: patent foramen ovale.
4.2. TEE during PFO Closure: Intra-Procedural Guidance
During the percutaneous procedure of PFO closure, TEE is used in combination with fluoroscopy, since it displays long segments of catheters and wires and their relationship with surrounding structures. TEE guidance is needed to cross the PFO, position the closure device, verify if its location is proper, and ensure its stability and efficacy [
43]. Closure device stability is evaluated by the so-called “wiggle maneuver”. During the “wiggle maneuver” the occluder, which is deployed but still connected to the delivery cable, is pushed and pulled before release to make sure that neither the right nor the left disk slips into the contralateral chamber [
50]. TEE imaging also allows the post-closure monitoring of adjacent structures, in particular mitral valve competency and coronary sinus patency, and the detection of complications immediately following device deployment, including left atrial appendage perforation, intracardiac thrombus formation, or early device embolization.
During the procedure, the “3D zoom” mode can be used to visualize the position of guide wires, catheters, and devices in real time. The PFO closure device position and its spatial relationship with adjacent native structures can be displayed at any time and from each side by 3D TEE.
4.3. TEE after PFO Closure: Post-Procedural Follow-Up
A TEE 6 months after PFO device implantation is recommended to confirm the correct device localization and exclude late complications. C-TEE allows us to visualize residual right-to-left shunts around or within the device. Shunts following the PFO closure procedure generally disappear or decrease overtime after device endothelialization. However, in some patients they persist and can be seen many months after the procedure. Serial TEEs are useful for monitoring the degree of passage and for identifying large and persistent shunts, which may benefit from re-intervention. TEE is more sensitive than transthoracic echocardiography in detecting thrombus formation on device surface, a complication mostly observed within the first month after device implantation [
51,
52]. TEE is also the tool of choice to diagnose the rare but serious infective endocarditis, following PFO closure device implantation, revealing the presence of vegetations on the left or the atrial side of the device [
53]. The erosion of adjacent cardiac structures, including right or left atrial roof and aortic root, and the device embolization, are other possible late complications following percutaneous device closure of PFO, which can be detected by TEE.
4.4. Intracardiac Echocardiography
Intracardiac echocardiography (ICE) is an alternative to TEE during PFO closure, which can directly diagnose PFO and continuously guide the procedure [
54]. It is typically performed by an interventional cardiologist using a 7–10 F catheter that is introduced via a second femoral venous sheath [
55]. ICE has high image resolution, allowing an accurate assessment of PFO size, position, and edges at different angles [
56]. Two orthogonal views (the transverse section at the aortic valve level and the longitudinal section of the four-chamber plane) are generally acquired to measure the defect and guide the deployment of the device. ICE can also be used to detect residual shunting immediately after percutaneous PFO closure, and to monitor acute complications, such as thrombus formation and pericardial effusion [
56]. ICE has the advantage of avoiding the need for general anesthesia and intubation, and their associated risks. Moreover, it reduces the duration of radiation exposure (X-ray is only used before the catheter arrives at the right atrium), which is crucial for children and pregnant women. However, ICE use is limited by the cost of a single-use probe, the need for specific training, and the risk of complications related to the femoral puncture.
This entry is adapted from the peer-reviewed paper 10.3390/jcm11144001