Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Polymer Science
Poly(lactic acid) (PLA) is an important polymer that is based on renewable biomass resources. Because of environmental issues, more renewable sources for polymers synthesis have been sought for industrial purposes.
- poly(lactic acid)-PLA
- azeotropic dehydration
- copolymers
- polymerization based on D,L-lactic acid
- composites of PDLLA
- stereo polymerization of D,L-lactic acid
1. Poly(lactic acid) (PLA) Polymers
PLA is one of the main bio-based polymers sold worldwide. It is considered a hydrolytically degradable, compostable, biocompatible and bioabsorbable polymer, which makes it attractive for applications in the biomedical area (tissue engineering, drug delivery system, sutures, etc.), in agriculture, ecology, packaging, among others [66,67]. It is a thermoplastic polyester that has a processing temperature in the range of 170 to 230 °C, and can be processed by extrusion, spinning, biaxial stretching and blown injection, thus covering a wide and dynamic field of applications, including food packaging, textile, and non-textile fabrics (such as curtains and wet wipes, respectively), toys, among others [29,41,68]. When mixed with other polymers or in the form of composites, some properties of PLA can be modified and improved, such as flexibility, impact resistance and heat stability, allowing its application as flexible films and engineering plastics [41]. Nevertheless, despite being often classified as a biodegradable plastic, PLA, especially the high molar mass (>100 kDa), is hardly biodegradable under ambient conditions, requiring very specific composting conditions (e.g., temperature above Tg (glass transition temperature), specific microorganism, adequate humidity, and aerobic environment for its complete decomposition into CO2 and H2O) [25,67].
2. A Brief Outline
Poly(lactic acid) or polylactide (PLA) is a bio-based aliphatic polyester produced from renewable resources such as wheat, straw, corn, cellulose, starch, sorghum etc., [29,36,42]. In the early 1800s, Pelouze used a lactic acid distillation process to remove water (polycondensation), thus obtaining a low molar mass PLA [69]. Later, in 1932, Wallace Carothers, a DuPont scientist, synthesized PLA by heating lactide under a vacuum, in a process known as ROP (Ring-Opening Polymerization) [57,69,70,71]. The PLA obtained so far had characteristics that limited its potential for application, such as low molar mass and instability in a humid atmosphere [71]. In 1954, a lactide purification method was developed by DuPont and made it possible to obtain high molar mass (HMW) PLA [70,72]. However, it was from 1966 onwards, with the studies by Kulkarni et al., about the biodegradation and non-toxicity of PLA [71,73] that it and its copolymers started to be applied in the biomedical field as sutures, prostheses, matrices for drug delivery systems, scaffolds among others [31,42,69,71,72].
The advancement and dissemination of PLA production and processing technology have caused its field of applications to expand significantly in recent decades, especially after the creation, in 2002, of the NatureWorks (USA) industrial large-scale PLA production plant, which currently operates with a production capacity of 150,000 metric tons/year [41,69,74,75]. The joint venture Total and Corbion (Amsterdam, The Netherlands) and Hisun (Wuhan, China) also produce PLA on a large scale, with a production capacity of 75,000 and 10,000 metric tons/year, respectively [41,42,69,75,76]. Other manufacturers produce PLA on a smaller scale and have been listed in the references [32,41,77].
3. Synthetic Routes
High molar mass PLA can be obtained from three main synthetic routes: direct condensation polymerization, azeotropic dehydration condensation and lactide ring-opening polymerization (ROP) [55].
Direct polycondensation takes place from the dehydration of lactic acid with simultaneous esterification of the monomers and the release of water for each acid unit added by condensation [78]. Water readily reacts with the formed oligomers, shifting the equilibrium towards the reactants and making it difficult to obtain a high molar mass polymer [41,45,59,79]. Removing condensed water from the reaction medium is quite complicated, as the increase in the concentration of oligomers leads to an increase in the viscosity of the medium [41,42,45]. This requires the use of high temperatures, in the range of 150–200 °C, pressure below 5 torr and a long reaction time in the presence of a chain coupling agent [29,31,32,55,70,80,81], or in some cases, an azeotropic solvent [32]. Other alternatives for the synthesis of high molar mass PLA by polycondensation such as solid-state polycondensation (SSP) were discussed by Masutani [32].
In condensation by azeotropic dehydration, first the LA is distilled under a vacuum for about 3 h to remove most of the condensation water. Then, the azeotropic solvent (diphenyl ether) and the catalyst are added to the LA solution, with the solvent being refluxed and returning to the reaction flask after passing through a molecular sieve, without the need for chain extenders or adjuvants to obtain high molar mass PLA [29,31,32,42,55,70]. The main disadvantages of this synthesis lie in the use of diols and diacids as solvents, catalyst residue and low yields [29,78].
The most widespread and industrially employed route in PLA synthesis is lactide ring-opening polymerization (ROP). ROP is a propagation process of cyclic monomers initiated by different ions [78] and generally occurs in a two-step process. The first step consists of obtaining lactide with high optical purity; the second consists of the lactide polymerization promoted by an initiator or catalyst [31,55,70,82]. The catalysts commonly used in this synthesis are metallic catalysts, such as Zn and Sn oxides, zinc and tin chlorides or tin octoate [78,82,83,84].
Compared to direct polycondensation, ROP can be performed under milder conditions, such as a reaction temperature of 130 °C and a shorter reaction time [32,78,85]. ROP can be classified in terms of the reaction mechanism as: anionic polymerization, cationic polymerization, and coordination-insertion mechanism [32,55,78,80,85,86]. The most popular catalyst used in this synthesis is tin(II) bis-2-ethylhexanoic acid (tin octoate), due to its solubility in molten lactide, low product racemization, high conversion and catalytic activity, and for providing PLA of high molar mass (≥1000 kDa) [72,80,84]. Another relevant aspect of ROP is that it makes it possible to control the microstructure of the polymer, including the order of insertion of monomers in the polymer chain based on their stereochemistry, as well as the combination of reaction time, temperature, type, and concentration of catalyst [80,82,86].
4. Structural Variety and PLA Properties
PLA presents a great structural diversity based on its enantiomeric constitution. The enantiopure monomers of L-lactic acid and D-lactic acid (or their lactide analogues) lead to the formation of poly(L-lactic acid) (PLLA) and poly(D-lactic acid) (PDLA), respectively. These homopolymers are semi-crystalline and have the same thermal properties, such as melting temperature (Tm) ranging between 170–180 °C, glass transition temperature (Tg) around 55–60 °C and crystallinity around 35% [29,31,32,82,84,87,88]. PLLA with a percentage of L-isomer above 90% in its composition tends to be semi-crystalline, while PLA with a content lower than this tends to be amorphous [89].
Polymerization of the racemic mixture of lactic acid, rac-lactide (rac-LA) and meso-lactide (m-LA) results in poly(D,L-lactic acid) or PDLLA, a random copolymer of D and L-lactic units, with an irregular and completely amorphous structure [71,75,90]. PDLLA has no Tm but has a Tg around 60 °C [32,87]. Due to its amorphous nature, PDLLA shows a faster degradation rate than stereoregular PLA, making it preferred for applications as a drug delivery vehicle and as a low-strength scaffolding material for tissue engineering [75,91]. PDLLA may show some crystallinity when synthesized by stereocontrolled ROP, through the action of a catalyst/initiator [32,80]. Aluminum alkoxide catalysts, Schiff bases and other single-site complexes have been used in the synthesis of stereoregular PDLLA [80].
In 1987, Ikada et al., reported, for the first time, that a blend of PLLA and PDLA in equal proportions (1:1) produced stereocomplex crystals, which had different properties from pure homopolymers [32,92]. PLA stereocomplexes (sc) have a melting temperature (Tm) about 50 °C higher than that of homopolymers, varying around 230 °C [93,94]. However, these sc-PLA can be formed concomitantly with PLA homocrystals (hc), as the kinetics of homocrystal formation is favored in high molar mass PLLA/PDLA mixtures (weight-average molecular weight, Mw > 40 kDa) [95], thus limiting the exclusive production of sc-PLA. One way to circumvent this problem is the production of stereoblock PLA (sb-PLA), which is a copolymer containing isotactic sequences of PLLA and PDLA [96]. The sc-PLA and sb-PLA can be obtained from the stereoselective ROP (rac- or m-LA) using a chiral catalyst [97,98], as can be seen in Scheme 3, which has been described in the literature [32]. In addition to stereoblock synthesis, other alternatives to improve PLA stereocomplex are melt blending, the addition of nucleating agents and polymer blending [93]. Several review articles have been published on the synthesis, structure, crystallization behavior, other properties, and applications of these sc-PLA [99,100,101,102,103].
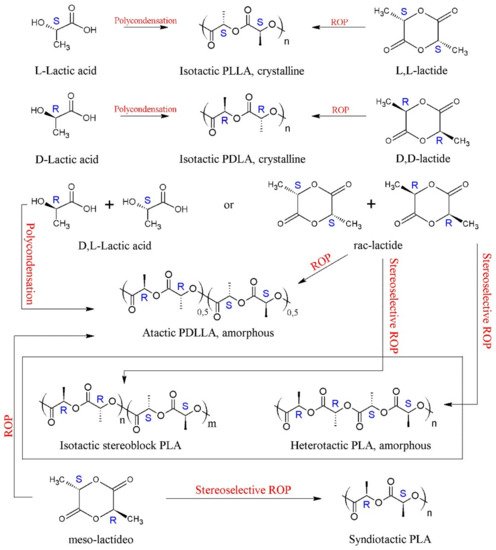
Scheme 3. The variety of PLA microstructures and synthetic routes.
5. PLA Modifications: Blends, Copolymers and Composites
Some common desired properties of PLA, such as rigidity, permeability, crystallinity and thermal stability, and hydrophobicity/hydrophilicity can be improved by processes such as copolymerization, blending and production of polymer composites [36]. The copolymerization process consists of the simultaneous polymerization of two or more monomers that interact via chemical reactions and produce PLA copolymers. These copolymers can be sequenced alternately, in blocks or grafts, but this considerably compromises their biocompatibility. However, biocompatibility can be improved by the copolymerization of lactic acid with other hydrophilic monomers or polymers. For example, block copolymers of lactic acid and polyethylene glycol (PEG) are hydrophilic and some of them are even water-soluble [100].
The blending process consists of two or more different polymers mechanically mixed and connected through physical interactions [104], producing blends. That is the case of PLLA/PDLA, PLLA/PDLLA, PLA/starch blends, PLA/PHB (polyhydroxybutyrate) and others. In the literature [88,105,106] several possibilities of PLA blends were revised, considering systems that include mixtures with hydrophobic and hydrophilic polymers, other polyesters and so on. A recent review article by Hamad et al., discussed the production of PLA modified by polymer blending techniques to achieve suitable properties for some applications, such as medical, packaging, battery and semiconducting [107]. They pointed out that biodegradable polymers have been mostly utilized due to their environmental advantages and high toughness compared with pure PLA, but other polymers still show low cost, better mechanical properties, high thermal stability, and processability. For instance, they verified in the literature that PLA/TPS (PLA-thermoplastic starch) blend could be useful in general packaging applications by means of compatibilizers such as PLA-g-MA (PLA-g-maleic anhydride), GMA-g-PEO (glycidyl methacrylate-g-poly(ethylene oxide)), TPS-g-MA (thermoplastic starch-g-maleic anhydride), PLA-g-TPS (PLA-g-thermoplastic starch). The PLA blend improved the biodegradation rate, but this process promoted weakness and low elastic modulus. Some PLA blends also have a higher flexibility than pure PLA, which make this material more appropriate for the fabrication of 3D scaffolds. Furthermore, PLA blends can be used to produce hierarchically porous materials for biomedical applications, since micropores can enhance the tissue ingrowth and the smaller pores (submicrometer scale) can provide the cell differentiation. Given this scenario, the authors proposed that research on PLA blends should preferably focus on their application. In addition, significant effort is required to improve the biodegradability of PLA-containing systems, after the material has fulfilled its specific role in the application.
Polymer composites processes, in turn, are multiphase systems formed by two or more components, generally polymeric and one non-polymeric [104,108]. When at least one of the phases of this composite has nanometric dimensions, it is called a nanocomposite [104]. Unlike blends, the constituents of a composite interact with each other through strong chemical and physical reactions [108]. A literature review by Murariu and Dubois highlights recent and current developments, results, and trends in the field of PLA-based composites [109]. In addition, a comprehensive review of PLA composites reinforced with synthetic and natural fibers has been published by Ashothaman et al. [110]. In this review, the authors mainly address some methods of manufacturing polymeric composites (especially using compression molding and injection molding methods) and reinforcement of PLA composites with different fibers, between natural fibers (treated or not) and synthetic fibers. They pointed out that PLA composites reinforced with synthetic fibers are more easily manufactured, since both are hydrophobic and have good compatibility, while natural fibers, due to their hydrophilic nature, have low adhesion or incompatibility with the polymer. PLA composites reinforced with bioactive glass fibers and magnesium, PLA biocomposites with cellulosic fibers treated by microwave and enzymatic treatment, PLA-based biocomposite reinforced with flex fiber with treated surface, PLA reinforced with maple wood flour, PLA reinforced with hydroxyapatite, among others were mentioned. The authors concluded, based on the cited studies, that it is possible to improve the mechanical strength, stiffness and crystalline behavior of PLA composites reinforced with these fibers.
This entry is adapted from the peer-reviewed paper 10.3390/polym14122317
This entry is offline, you can click here to edit this entry!
