1. Modelling Ischaemic Stroke In Vitro
1.1. Inducing Ischaemia-like Conditions In Vitro
In vitro models of ischaemic stroke typically mimic the conditions of the ischaemic penumbra—the target tissue for therapeutic intervention—where cells are functionally silent but initially viable. To study ischaemic stroke in vitro, ischaemia-like conditions can be achieved by different approaches. The most common and most physiologically relevant way to induce ischaemia-like conditions is by so-called ‘oxygen-glucose deprivation’ or OGD. In this approach, cell or tissue cultures are placed in a hypoxic or anaerobic chamber, containing a N
2/CO
2 atmosphere, where the O
2/CO
2 equilibrated medium becomes replaced by the glucose-free N
2/CO
2 equilibrated medium at the start of incubation [
13,
14,
15,
16,
17,
18,
19]. The cultures are maintained for a duration of 30 min up to 24 h in the chamber, depending on the specific cell type used and the desired degree of ischaemic damage. Typically, a longer duration of oxygen and glucose deprivation is needed to cause cell injury or death in vitro than in vivo. Compared to ischaemia in vivo, adenosine triphosphate (ATP) depletion is less severe and the release of glutamate is delayed [
7]. OGD is often terminated by glucose addition and reoxygenation and is cultured under ‘normal’ conditions for up to 24 h prior to downstream analyses. This allows modelling of in vivo reperfusion, known to further aggravate ischaemic injury [
20].
Besides OGD, hypoxia can be induced through either chemical or enzymatic inhibition of cellular metabolism. The chemical method relies on inhibition of the mitochondrial electron transport chain and has been regularly applied to cell cultures to study ischaemic stroke. For instance, sodium azide and antimycin are commonly used chemical-hypoxia inducers in these studies [
21,
22,
23,
24]. Less common is the enzymatic induction of hypoxia, which relies on manipulating the glucose oxidase and catalase (GOX/CAT) system [
25,
26,
27]. Though less physiologically relevant, these chemical and enzymatic approaches can result in hypoxic/ischaemic injury in a shorter time frame than conventional OGD [
28].
Due to implementation of novel technologies in in vitro stroke model development, recently, researchers were able to recapitulate another factor besides oxygen and glucose depletion, namely the interrupted blood flow, by employing microfluidic systems [
23,
29]. This appears to be another factor affecting the downstream ischaemic cascade by reducing the integrity of the BBB and thereby allowing it to mimic in vivo stroke even more closely.
Moreover, specific aspects of the ischaemic cascade can be modelled. For example, excitotoxicity models have been developed by exposing cultures to glutamate or glutamate receptor agonists such as N-methyl-D-aspartate (NMDA) [
30]. The increase in the levels of intracellular free calcium is also an important effector of secondary injury subsequent to an ischaemic insult and has been simulated in in vitro models by thapsigargin treatment [
31].
1.2. Most Common Cellular Platforms in In Vitro Stroke Research
The main cellular platform used for in vitro stroke research consists of monocultures of rodent primary neurons. In general, the use of monocultures is preferred when studying cell-specific responses to OGD and/or to evaluate the action of neuroprotective compounds on specific cell types. Among a lot of other applications, primary rat neurons have been used to evaluate the protective effect of the basic fibroblast growth factor [
32], intermittent hypothermia [
33] and oxytocin against damage induced by an ischaemic insult [
18], as well as to elucidate the mechanisms underlying neuronal autophagy in ischaemic stroke [
34]. Moreover, rat primary neurons have been used to study the effect of hypoxia on the neuronal activity by plating them on multi-electrode arrays during exposure of the culture to different durations of hypoxia [
35].
Another widely used platform to model ischaemia-like damage are organotypic brain slice cultures, typically from rodent origin. In these cultures, brain slices are obtained from young animals (postnatal day P3 to P10) and allowed to further develop and mature in vitro [
36,
37]. The advantage of this culture type is that it largely preserves tissue structure maintaining neuronal activities and synapse circuitry [
38]. Moreover, since multiple cell types are present, this model additionally allows one to study cell–cell interactions [
38]. Due to these unique features, this system is closer to an animal model than cell culture. Organotypic brain slice cultures have been valuable in the study of pathogenic mechanisms leading to ischaemia-induced neuronal cell death, in particular with the excitotoxic mechanism. For instance, the involvement of glutamate—accumulating extracellularly after an ischaemic insult—and glutamate receptors and transporters in the excitotoxic-induced damage have been extensively studied using the brain slice model [
39,
40], reviewed in detail by Noraberg et al. [
41]. Related or not to this glutamate-induced damaging mechanism, brain slice models have been applied to study calcium overload, mitochondrial dysfunction and oxidative stress, as well as to evaluate neuroprotective drugs [
41]. Furthermore, in contrast to nearly all other in vitro systems where OGD media is applied over the entire culture, brain slice cultures could also be used as a platform to mimic focal ischaemia. A protocol by Richard et al. describes a focal ischaemia model by focally applying OGD medium to a small portion of the brain slice while bathing the remainder of the slice with normal oxygenated media [
42].
2. Factors Defining the Predictive Value of In Vitro Ischaemic Stroke Models
2.1. Origin of Cells or Tissue Used for In Vitro Models of Ischaemic Stroke
As described above, in vivo and in vitro rodent-based models are standards used in stroke research. Their use has led to our increased understanding of the ischaemic cascade of human stroke as the main aspects of stroke hold true across all mammals. However, as rodents and humans are separated by 80 million years of evolution [
10], species-specific anatomical, cellular and molecular differences exist between humans and rodents potentially affecting the outcome of neuroprotective strategies.
At the anatomical level, differences between human and rodents are evident, with humans having large gyrencephalic brains with a high proportion of white matter, whereas rodents have small smooth brains with relatively little white matter [
10]. Associated to this difference in brain anatomy, the number of outer radial glia cells in rodent brains is small, while in primates this cell type is more abundant and possesses a higher self-renewal capacity [
43,
44]. Furthermore, species-specific differences have been reported on the expression levels and function of several BBB-transporters [
45,
46]. Likewise, comparison of the distributions of predominant glial glutamate transporters revealed significant differences between species [
47]. This variation may translate into differences in pathophysiological stroke mechanisms or available targets between species. Specifically related to stroke, it has been demonstrated that the duration of excitotoxity after the ischaemic insult differs between mice and humans, with a longer duration for humans [
48]. Moreover, at the immunological level, important differences exist between rodents and humans. A pioneering study of Seok et al. compared genomic responses to different acute inflammatory stresses (including endotoxemia, burns and trauma) between humans and mice, and found that the responses elicited in humans are not reproduced in the mouse models [
49]. Moreover, there is increasing evidence that there are important differences between human and murine microglia [
50]. Moreover, in the context of ischaemic stroke, dissimilarities are becoming apparent [
51]. A study by Du et al. demonstrated that the baseline expression of cytokines/chemokines and response after OGD and reoxygenation in primary neurons, astrocytes and microglia differed significantly between rodents and humans [
52]. For instance, while human primary neurons showed a downregulation in many of the determined chemokines (CX3CL1, CXCL12, CCL2, CCL3, and CXCL10) after OGD and reoxygenation, mouse neurons showed a mixed response with the up- and downregulation of the same chemokines. These findings exemplify the importance of using human-based in vitro models in fundamental as well as translational stroke research, next to traditional in vivo models. The introduction of human-based in vitro models in the preclinical phase of drug discovery and development would allow target identification and proof-of-principle demonstration that attacking these targets elicits appropriate cellular responses in a human context before entering the clinic, increasing chances of success for the agents to be effective in clinical setting [
10]. Nevertheless, the use of human-based in vitro systems is rare in the field of ischaemic stroke. The few human-based systems that have been used to date consist mainly of transformed cell lines and primary human brain slice preparations, each associated with their own limitations.
2.2. Multicellular Co-Culture Models for In Vitro Ischaemic Stroke Research
As mentioned earlier, the majority of in vitro stroke research is conducted using monocultures of neurons. Apart from neurons, a monoculture of rodent primary astrocytes has been used to determine the protective roles of pinin and stem-cell derived exosomes after ischaemic stroke [
84,
85]. Furthermore, when focusing on the BBB-disruption facet under ischaemic conditions, the use of pure cultures of brain endothelial cells has been regularly reported [
86,
87,
88,
89,
90,
91,
92]. Monoculture systems are particularly useful to investigate mechanisms restricted to specific cell types or to determine the contribution of specific cell types to different pathophysiological mechanisms. However, several reasons substantiate the use of co-culture models to obtain models better resembling the human brain. First, the human brain consists of an intricate cellular network, including neurons, astrocytes, oligodendrocytes, microglia, pericytes and endothelial cells. Therefore, every cell type added to the in vitro system increases the complexity, approaching more the in vivo complexity of the human brain. Second, co-cultures enable cellular interactions that occur in vivo and as such the presence of different cell types and interactions can influence RNA transcription, protein production and functionality of certain cell types.
The importance of cell–cell interactions occurring under physiological conditions become evident from different publications. For example, astrocytes provide metabolic substrates to neurons (i.e., energy supply to neurons) and are actively involved in the formation and refinement of neuronal networks. Indeed, they are demonstrated to integrate and modulate neuronal excitability and synaptic transmission [
93,
94,
95]. These functions of astrocytes could also be observed in in vitro astrocyte-neuron co-culture models. Astrocytes, from rodent and human origin, co-cultured with human PSC-derived neurons improves the functional maturation of those neurons, as demonstrated by an increased percentage of active neurons, bursting frequency and synchronisation of neuronal calcium oscillations when compared to the neuronal monocultures [
96,
97,
98,
99]. Moreover, mutual interactions between microglia and neurons in the healthy brain exist, where neurons (e.g., through CX3CR1-CX3CL1 or CD200-CD200R interactions), or neural environment in general, keep microglia in a non-activated state, thereby favouring their homeostatic functions maintaining neuronal health and regulating proper function of neuronal networks [
70,
100,
101,
102]. Furthermore, co-cultures of brain endothelial cells with other CNS cells, such as astrocytes and pericytes, contribute to BBB integrity and function among others by stimulating tight junction formation and expression of polarised transporters in endothelial cells [
28,
83,
103,
104,
105,
106].
Also under pathological ischaemic conditions, cellular interactions are important in regulating cell behaviour and contribute to the mechanisms leading to brain injury or recovery. For example, co-cultures of microglia/macrophages with neurons or brain slices have been developed and employed in the field of stroke research to investigate the inflammatory response secondary to an ischaemic insult. After an ischaemic insult, brain-resident microglia and blood-derived macrophages can acquire a pro-inflammatory neurotoxic phenotype, further exacerbating brain damage. To study the cross-talk between hypoxic neurons and macrophages, Desestret et al. subjected an organotypic hippocampal slice to OGD for 30 min and subsequently added macrophages for 2 days [
107]. Other studies used co-cultures of rat primary microglia with primary neurons or a combination of primary neurons and astrocytes to elucidate the effect of neuronal ischaemia on microglia polarisation and, conversely, the effect of microglia phenotype on the fate of healthy or ischaemic neurons [
13,
108,
109]. These studies confirmed that the pro-inflammatory activation of microglia by damage-associated molecular patterns released from damaged neurons after OGD further exacerbates neuronal death. Likewise, a neutrophil-neuronal co-culture was recently developed to investigate mechanisms of neutrophil-dependent neurotoxicity [
110]. The last-mentioned study found that cell–cell contact was required for the process of neutrophil-induced neuronal injury. Next to neuro-immune interactions, neurovascular and gliovascular interactions occurring during cerebral ischaemia have also been identified. From a study comparing brain endothelial cells in monoculture versus co-cultures of brain endothelial cells with neurons or astrocytes, it became apparent that neurons and astrocytes, exposed to either OGD, aglycemia or hypoxia, affect different endothelial properties, including its barrier and lymphocyte adhesion properties, endothelial cell adhesion molecule expression and in vitro angiogenic potential [
111]. For instance, the interaction of brain endothelial cells with neurons or astrocytes under OGD and subsequent reoxygenation, results in attenuation of BBB permeability and in recovery of the barrier. This compensatory mechanism of astrocytes for maintaining BBB function after ischaemic stroke has been confirmed in another study, identifying a role for astrocyte-derived pentraxin 3 [
112]. However, the excessive production of cytokines, chemokines and proteases in the ischaemic infarct might undermine the adaptive nature of the BBB, leading to increased permeability [
111]. Identification of these interactions is important as changes in BBB permeability can affect cerebral oedema, post ischaemic brain angiogenesis (associated with survival of stroke patients) and leukocyte interactions that aggravate ischaemia reperfused stroke brain damage [
111].
2.3. Dimensionality of Cell Culture Models for In Vitro Ischaemic Stroke Research
Most of the knowledge derived from in vitro stroke studies is based on neural cells grown as monolayers. This traditional simplified culture system has been of undisputable significance for biomedical research, including stroke, especially considering their relatively low cost and reproducibility when compared to animal models [
115]. Moreover, decades of research using these monolayer cultures has led to the optimisation and standardisation of many downstream applications tailored for 2D cultures, including the easy visualisation by means of microscopic imaging. Nevertheless, 2D cultures are unable to mimic the complicated microenvironment cells experience in tissue. Unlike cells cultured in 2D, in the in vivo brain, cells are able to generate 3D projections and establish multiple interactions with other cells and cell types and the extracellular matrix (ECM) [
115,
116], eventually affecting their morphology, survival, proliferation, differentiation, gene expression and even function (e.g., electrophysiological network properties) [
31,
115]. Therefore, 3D models of the brain are considered more realistic models of the human brain than conventional 2D models, better mimicking its complexity and possibly ischaemia-induced responses [
115].
A first model that allows ischaemic stroke studies to be conducted in a more relevant 3D microenvironment is posed by ex vivo acute and organotypic brain slices. As described earlier, this model is able to largely retain the tissue structures, where multiple cell types retain most of cells’ in vivo properties and spatial organisation and intricate network organisation and function. However, next to different considerations, such as the limited culture time or maturation of acute and organotypic brain slices, respectively [
36,
37], the scarcity of human-derived brain slices restrict research to the used rodent-based (organotypic) brain slices, less faithfully predicting human pathophysiological mechanisms.
Second, the advent of iPSC-technology has boosted the development of 3D models of the brain, such as brain spheroids or organoids [
117]. Neural organoids are self-assembled PSC-derived 3D in vitro cultures that recapitulate the developmental processes and cytoarchitecture of the developing human brain [
117,
118,
119]. Different neural organoids and spheroids have been developed ranging from brain organoids containing multiple different brain regions, termed ‘cerebral organoids’, to brain region-specific organoids, including forebrain, midbrain, cerebellar and hippocampal and hypothalamic organoids, through the use of patterning factors [
120,
121,
122,
123,
124,
125,
126,
127,
128]. Protocols to generate these brain spheroids/organoids differ in several aspects, such as the use of ECM, patterning factors or the initial cells used, which are either PSCs or neural stem/progenitor cells derived from PSCs. These differences can have implications on the complexity of the model, making them more or less suitable for certain specific applications. The use of these organoids has proven extremely useful for the study of neurodevelopment and associated pathologies, such as microcephaly, ZIKA virus infection and autism spectrum disorders [
122,
127,
129,
130,
131]. Other applications include neurodegenerative disease modelling and neurotoxicity testing [
124,
132,
133,
134,
135]. Though current brain spheroids and organoids are already useful tools gaining popularity in different biomedical fields, they are subject to continuous research aimed at improving their resemblance to the human brain. One of the major limitations of current organoid and spheroid models is the lack of vascularisation, causing the development of a hypoxic, necrotic core and further hampering the growth and maturation of neural organoids and spheroids [
115,
118,
119,
136,
137]. Researchers are therefore trying to develop vascularised brain organoids [
138,
139,
140] or implement microfluidic technologies (further described in section ‘microfluidics technologies’). Besides vasculature, organoids generally lack microglia [
115,
118,
119,
136,
137], which have important roles in immune defence and maintenance of CNS homeostasis [
141]. Recently developed differentiation protocols of iPSC-derived microglia [
69,
70,
71,
72,
73] are paving the way to develop state-of-the-art immune-competent brain organoids and spheroids [
142,
143,
144], more closely mimicking the human brain. Ischaemic stroke research would also greatly benefit from the generation of brain organoids containing vasculature (preferably with specialised BBB properties), and microglia, since it is a cerebrovascular disease with neuroinflammation being an important aspect of secondary injury after stroke. Finally, the heterogeneity of organoids, especially the cerebral organoids, in terms of size, shape and composition pose another major limitation [
137,
145,
146]. Lower heterogeneity and enhanced reproducibility are crucial for controlled experiments and future potential screening approaches [
145]. Several ways to reduce variability have been proposed, such as the use of bioreactors, avoidance of natural hydrogels (e.g., Matrigel) containing undefined factors, the use of patterning factors and starting from iPSC-derived neural stem/progenitor cells instead of iPSC to exclusively obtain cells of neuroectodermal lineage [
115,
127,
145].
Only a few articles have been published so far, in which brain organoids or spheroids were subjected to hypoxic stimuli. To date, most studies that exposed neural organoids to low oxygen tension envisaged to study the effect of hypoxia on neurodevelopment and corticogenesis. For instance, Pasça et al. subjected brain region-specific organoids called human cortical spheroids (hCS) to hypoxia to determine the effect of oxygen deprivation on corticogenesis, to model injury in the developing brain. They found that intermediate progenitors, a specific population of cortical progenitors that are thought to contribute to the expansion of the primate cerebral cortex, were reduced following hypoxia and subsequent reoxygenation. Moreover, Kim et al. studied the effect of hypoxia on neurodevelopment [
147]. They used human neural organoids, derived from neural stem cells (NSCs), and found that after hypoxia, reoxygenation was able to restore neuronal proliferation but no neuronal maturation, as shown by the retained decrease in TBR1+ cells. Similarly, Daviaud et al. subjected human cerebral organoids with dorsal forebrain specification to transient hypoxia, as a model for prenatal hypoxic injury, and demonstrated the distinct vulnerability and resilience of different neuroprogenitor subtypes [
148]. They demonstrate that outer radial glia (FMA107+) and differentiating neuroblasts/immature neurons (TBR2+ and DCX+) are highly vulnerable to hypoxic injury, whereas NSCs displayed relative resilience to hypoxic injury and even provide a mechanism to replenish the stem cell pool, by shifting the cleavage plane angle favouring symmetric division. The results of the last-mentioned study were also replicated by our own studies. With the aim of developing a human neurospheroid model for ischaemic stroke, we equipped iPSC-derived neurospheroids with intrinsic bioluminescence to enable the real-time monitoring of the viability of neurospheroids subjected to OGD and were able to model OGD-mediated neurotoxicity [
149]. By comparing 1-week-old with 4-week-old neurospheroids, containing a high proportion of undifferentiated NSCs and intermediate progenitors/immature neurons, respectively, it was demonstrated that 1-week-old neurospheroids were able to completely and spontaneously recover from the initial OGD-induced damage over the course of one week, unlike 4-week-old neurospheroids. These dynamics of OGD-mediated neurotoxicity of different ages of neurospheroids underscore the need for older, more mature neurospheroids for in vitro stroke research.
Furthermore, cerebral organoids have also been employed to further unravel the mechanisms underlying ischaemic injury. Iwasa et al. subjected cerebral organoids to OGD and reoxygenation and identified peroxisome proliferator-activated receptor (PPAR) signalling and pyruvate kinase isoform M2 (PKM2) as key markers of neuronal cells in response to OGD and reoxygenation [
150]. In addition, Ko et al. described 3D cortical spheroids derived from primary rat cortical cells treated with OGD and reoxygenation as a model for cerebral ischaemia [
151]. They demonstrated that their model successfully mimicked the ischaemic response as evidenced by the upregulated mRNA expressions of the key markers for stroke, S100B, IL-1β and MBP and additionally substantiate the role of transient cell-substrate interactions herein. Lastly, spheroid models have also been exposed to hypoxia to study the integrity of the BBB under pathological conditions. Nzou et al. made cortical spheroids with a functional BBB by mixing human primary brain endothelial cells, pericytes, astrocytes, and human iPSC-derived microglia, oligodendrocytes and neurons at a certain ratio in a hanging drop culture environment. They challenged the spheroids with a hypoxic stimulus and demonstrated that hypoxia resulted in BBB disruption, as evidenced by the altered localisation of tight and adherens junctions [
106]. This further indicates the usefulness of the organoid/spheroid model in studying ischaemia in a physiologically relevant environment.
Alternative to neurospheroids and organoids, recently, scaffold-based 3D systems have also been proposed as a potential in vitro model for CNS injury, including stroke. Here, cells are embedded in a polymer-based scaffold that mimics the ECM of the brain. Lin et al. seeded SH-SY5Y cells onto a patterned gelatin scaffold and investigated the neuroprotective effects of resveratrol, an AMP-activated protein kinase (AMPK) activator, when subjected to OGD [152]. Vagaska et al. describe a model consisting of primary human NSCs dispersed in a hydrogel (i.e., Collagen-I/Matrigel) subjected to OGD or to thapsigargin, an inducer of intracellular calcium release [31]. In the same study, the difference in human NSC phenotype and damage response between 2D and 3D cultures of NSCs was assessed, suggesting that 3D models may be better predictors of the in vivo response to damage and compound cytotoxicity.
Finally, these brain spheroids/organoids and scaffold-based 3D cultures of CNS cells can take advantage of from microfluidic systems, to generate so called brain-on-a-chip models, forming the final category of existing 3D cell cultures of the brain. Brain-on-a-chip models and microfluidics technology are further discussed in the next section of ‘microfluidics technology’.
2.4. Implementation of Microfluidics Technology in In Vitro Models of Ischaemic Stroke
Besides the factors described above, new technologies may also help to increase the complexity and predictive power of in vitro ischaemic stroke models (
Figure 1). The newly developed ‘brain-on-a-chip’ models employ microfluidics technology to create a more physiologically relevant microenvironment for the culture of CNS cells. Through the spatial control over fluids in micro-meter sized channels, microfluidics enable (i) the co-culture of cells in a spatially controlled manner, (ii) generation of and control over (signalling) gradients and (iii) perfusion flow, contributing to an increase in physiological relevance of in vitro models [
154]. These applications will be further discussed hereafter.
First, microfluidics facilitate physical separation of cellular populations and/or components on a microscale as a basis for mechanistic studies [
83]. For instance, using microfluidic devices, the interaction between neuronal populations derived from different brain regions can be studied. This way, cortico-thalamic, cortico-hippocampal interactions and even interactions between three different brain regions (cortex, hippocampus and amygdala) have been established to model the brain’s complex neuronal architecture and functionality [
83]. The studies using microfluidic systems to investigate brain region interactions are nicely described in the review by Nikolakopoulou et al. [
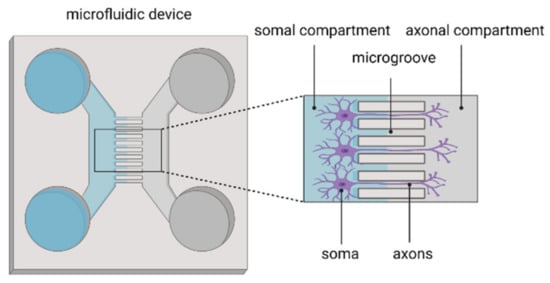
83]. Besides the physical isolation of different cell populations, microfluidics are also used to separately study axons and cell bodies of neurons (
Figure 2). Axons are directed to grow in microgrooves, thereby isolating axons from the cell soma. This platform allows the study of axonal biology, injury, regeneration and myelination but also synapse formation and modulation as well as viral spreading after axonal infection [
155,
156,
157,
158,
159]. Specifically in the context of the stroke, a similar microfluidic set-up has been used to study the spreading neurotoxicity into undamaged brain areas [
160]. Hereto, hippocampal neurons were cultured in each chamber and synaptically connected via axons traversing the microchannels. An isolated excitotoxic insult (i.e., glutamate) was delivered to neurons in one chamber, and the spreading toxicity of other synaptically connected neuronal populations could be monitored [
160]. This system thus allows one to recapitulate focal ischaemia, which has been considered difficult to mimic in in vitro models.
Figure 2. Microfluidic device for isolating axons from the neuronal soma.
Second, since microfluidics enable spatial control over fluids, gradients can be generated and precisely controlled [
154]. This has proven particularly useful for studying angiogenesis, invasion and migration, as all are associated with molecular gradients in vivo [
154]. Biochemical gradients of growth factors and cytokines also dictate differentiation patterning in vivo, making microfluidic devices suitable tools for studying early neurodevelopment [
83,
161,
162,
163]. Likewise, different microfluidic devices have been developed to establish oxygen gradients in cell and tissue cultures [
164,
165,
166,
167,
168,
169,
170,
171]. By flowing gas mixtures with desired oxygen concentrations through gas-permeable polydimethylsiloxane (PDMS) gas channels, cellular platforms, including adherent cells, brain slices and even 3D scaffold-based or spheroid models, can be rapidly and efficiently exposed to a range of oxygen concentrations as low as 0.1% O
2 [
164,
165,
166,
167,
168,
169,
170,
171], which are of relevance for future ischaemic stroke research. Compared to a hypoxic chamber, where all cultures are exposed to the same oxygen tension, this microfluidic based system allows one to apply multiple oxygen concentrations or gradients to cultures, representing another possible approach to induce focal ischaemia by means of microfluidics technology [
37,
171].
Last but not least, the compartmentalisation of microfluidic devices allows the perfusion of media adjacent or through (3D) cell cultures on microfluidic chips. This perfusion ensures stable nutrient and oxygen supply and removal of waste metabolites and mimics physiological flows, such as interstitial or blood flow. Moreover, accompanying the fluid flow, physiological shear stresses are introduced, which have been demonstrated to be essential for cellular morphology and the gene expression of endothelial cells, when modelling vascularity [
154]. The perfusion feature of microfluidics has also been exploited to specifically support the perfusion of brain spheroids and organoids generated on a microfluidic chip [
172,
173,
174,
175,
176]. Evidently, this microfluidic platform is also ideal to recapitulate the BBB, and even the complete NVU, which is of particular interest for stroke research. The different BBB/NVU models will be described in section ‘BBB/NVU models’.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23137140