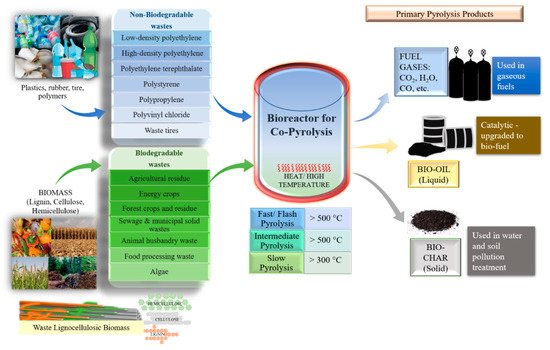
1. Introduction
To convert biomass into biofuels, various thermochemical and biological processes are used. Among these, pyrolysis is one of the most convenient methods because it has several advantages, such as easy optimization, variety in product formation, complete utilization of feedstocks, and diversification in feedstocks (both biodegradable and non-biodegradable) that can undergo pyrolysis (
Figure 1) [
20]. There are three categories of pyrolysis products formed: bio-oil (liquid), biochar (solid), and fuel gas [
21]. The yield of pyrolytic products is generally governed by the composition of biomass and the operating parameters [
22]. There are three forms of pyrolysis: slow, fast, and flash. Flash pyrolysis operates with a higher heating rate and shorter reaction time than fast pyrolysis, and the main product formed is bio-oil [
23,
24], whereas slow pyrolysis is done at low temperature, a low heating rate, and longer vapor residence time. The main product formed from slow pyrolysis is biochar [
24]. Fast pyrolysis is commonly used and operates at controlled temperatures (~500 °C) for a short residence period (<2 s) and high heating speed (>200 °C s
−1). Its main product is bio-oil [
23].
Figure 1. Co-pyrolysis of biodegradable and non-biodegradable feedstocks and its products.
Fuel gas is an undesirable product, but its production is unavoidable during pyrolysis; hence, it can be used to preheat the biomass, but its combustion byproducts are environmentally undesirable and require neutralization before being released. Pretreatment technologies (such as NOX scrubbers, electrostatic precipitators, adsorption systems for volatile organic compounds using activated carbon, SOX fuel gas desulfurization systems, flares, and biofilters) are used before the release of gasses, and/or optimization to lower its production during pyrolysis is implemented. On the other hand, biochar has been used to heat biomass and generate power for the pyrolysis plant, but recently it was observed that it can also be used as a soil enhancer, compost bulking agent, activated carbon, bioremediater of water and soil, bio-catalyst, energy source, and for carbon sequestration because of its composition [
25,
26,
27,
28,
29]. Bio-oil produced from pyrolysis exists as a dark brown, highly viscous liquid comprised of anhydro sugars, acids, alcohols, aldehydes, phenols, and oligomers. However, since bio-oils produced from biodegradable wastes are chemically unstable owing to high oxygen and water contents, culminating in reduced calorific value, high viscosity, and problems with corrosion and stability, its upgradation or enhancement is essential for its use as an alternative to crude oil [
30]. Due to these unfavorable properties, it is incapable of being employed as transportation fuel directly [
15]. In addition, pyrolysis of biomass results in minor aromatic yield and major coke production as a result of a decreased hydrogen/carbon effective ratio (H/C
eff) in most biodegradable biomasses [
31]. There are several routes for bio-oil enhancement depending on product requirements, such as emulsification, catalytic deoxygenation/hydrogenation, co-pyrolysis, thermal cracking, physical extraction, esterification, or gasification [
24]. Among all, co-pyrolysis has clearly shown potential for long-term business implementation due to improved results and cost-effectiveness.
Co-pyrolysis constitutes the pyrolysis of more than one type of feedstock, resulting in a positive synergistic effect. For instance, co-pyrolysis of biomass along with non-biodegradable polymer waste (such as plastic waste, e-waste, waste tyres, etc.) has often increased the amount of hydrogen produced and also reduced CO content [
32]. In addition, without much alteration to the system, co-pyrolysis results in favorable performance and cost-savings in comparison to other upgradation methods, such as hydrodeoxygenation (HDO) and catalytic cracking [
19]. It also results in improved quality and quantity of oil with a high calorific value [
33]. This positive synergistic interaction due to the mixing of feedstocks during pyrolysis has led to efficient oil content in combination with a secure and alternate way to handle non-biodegradable waste, such as plastic, tires, and lubricant oil [
34]. Thus, co-pyrolysis is an easy, efficient, and feasible strategy for high-quality fuel extraction with the potential to enhance the world’s energy protection, facilitate faster waste management, and decrease dependence on fossil fuels while preserving a healthy climate and ecosystem. The reason that co-pyrolysis has achieved a lot of recognition and success in recent years is that the pyrolysis of biomass and polymer/plastic waste individually and then mixing of their respective bio-oil is uneconomical, as more energy would be required to stabilize the mixture, and there is the possibility of separation after a short period due to the polar characteristic of the biomass pyrolysis oil. However, co-pyrolysis is more consistent in generating homogeneous and stable bio-oil compared to directly blending oil, as the interplay of the radicals leads to the formation of a stable bio-oil and avoids separation. In addition, co-pyrolysis offers a platform to treat a large volume of waste concurrently, which if treated separately would increase the overall cost. Therefore, it not only decreases waste treatment cost, but also solves various environmental issues accompanying traditional treatment methods, such as landfilling and incineration [
33].
In the past few years, much research related to co-pyrolysis has been documented in various peer-reviewed journals. For instance, Salvilla et al. [
35] studied the co-pyrolysis of polyolefin plastics synergistically with wood and agricultural wastes and concluded that activation energy of plastic decomposition in co-pyrolysis was reduced, and the results could be used in polyolefin plastic and lignocellulosic waste co-pyrolysis for biofuels production. In another study, co-pyrolysis of woody biomass with plastic waste at an analytical and pilot scale by Johansson et al. [
36] showed that the addition of plastics considerably impacts the composition and characteristics of the bio-oil. On the other hand, Wang et al. [
37] considered sewage sludge co-pyrolysis with rice husk and concluded that pyrolysis behavior was improved synergisticically. Several other latest findings on the co-pyrolysis of different biomasses are listed in
Table 1.
2. Mechanism of Co-Pyrolysis
Pyrolysis and co-pyrolysis are basically the same. However, in pyrolysis, only one feedstock is used, while in co-pyrolysis two or more feedstocks are used. Co-pyrolysis is carried out inside a closed reactor in an anoxygenic environment having low operating temperatures (350–600 °C) [
38]. Co-pyrolysis essentially requires four basic steps or processes for bio-oil production: selection of feedstocks, sample preparation/pretreatment, co-pyrolysis, and condensation [
33]. This section deteails the selection of feedstock and pretreatment strategies for co-pyrolysis, while the main co-pyrolysis step is discussed in the subsequent section.
2.1. Selection of Feedstocks for Co-Pyrolysis
Employing biodegradable and non-biodegradable wastes as raw materials in co-pyrolysis varies extensively among investigations. A list of different biodegradable and non-biodegradable wastes is given in
Table 2. Co-pyrolysis has not only emerged as an alternative to manage these biodegradable and non-biodegradable wastes, but has also provided an alternative way to produce energy. This will not only reduce our dependence on fossil fuels, but can also help resolve global warming, eutrophication, acidification, and eco-toxicity problems that the whole world is facing right now. In addition, independent pyrolysis of biomass and non-biodegradable polymers such as plastics (or waste tires) needs more energy, which in turn leads to significantly increased production costs. Therefore, the coincidence of plastic or tire waste during the pyrolysis of various biomass types potentiates a positive influence to the heating rate through synergy [
19,
39]. In this section, the choice and accessibility of feedstocks that can be employed in co-pyrolysis are discussed.
Table 1. Composition of different biomasses used for pyrolysis.
2.1.1. Biodegradable Wastes
Biomass is a biodegradable and renewable organic waste that comes from plants and animals. It is already known to be the world’s only truly viable source of organic carbon and the ideal petroleum equivalent for the commercial production of fuels with net carbon zero emissions [
68]. It accounts for about 14% of the globe’s energy supply and can even be assumed to be the predominant form of renewable energy as it may be transformed into a variety of fuels (liquid, gaseous, and solid) and useful chemicals [
69]. It includes agricultural residues, forest residues, biogenic parts of industrial and municipal solid wastes (MSW), algal biomass, and dedicated energy crops [
70]. Biodegradable waste can be categorized as following:
Lignocellulose Wastes
It is clear from
Table 1 that almost all the biomasses (such as agriculture residue, forest residue, industrial wastes, municipal solid wastes, energy crops, etc.) are rich in lignocellulose. Therefore, the most-investigated and successful feedstocks for producing renewable biofuels have been lignocellulosic biomasses. Cellulose, hemicellulose (polysaccharides), and lignin, together with some resins and minerals, are the major components of lignocellulosic biomass [
71]. The linear polysaccharide cellulose, a glucose polymer having strong β-1,4-glycosidic linkages, functions as the framework of lignocellulosic cell walls [
72,
73]. In cellulose chains, an assortment of hydroxyl groups is present, leading to the establishment of hydrogen bonds within the same chains or in neighboring chains [
74]. Hemicellulose is a short, highly branched, amorphous heteropolymer formed from monomeric sugars and sugar acids [
75]. The role of hemicellulose is to link lignin and cellulose. There are numerous hexoses (galactose, glucose, rhamnose, mannose, and fucose), pentoses (xylose and arabinose), and uronic acids (methyl glucuronic acid, glucuronic acid, and galacturonic acid) in the structure of hemicelluloses [
76]. The amorphous, heterogeneous, cross-linked aromatic polymer lignin has a three-dimensional structure composed of trans-sinapyl, trans-coniferyl, and trans-p-coumaryl alcohols [
77]. Cellulose is also crosslinked by lignin along with hemicellulose to generate three dimensional cell wall structures. Further, because of its large carbon content, lignin stores around 40 % of the energy in lignocellulosic biomass [
78].
Lignocellulose waste pyrolysis normally occurs in three major phases: (i) Temperature <200 °C (required for evaporation of moisture); (ii) Primary decomposition at 475 °C to 655 °C; (iii) Reduction in decomposition reaction at temperature >600 °C. Cellulose and hemicellulose decomposition refers to the second stage, and lignin decomposition happens at temperatures between 455 °C and 1175 °C [
79]. Unlike cellulose, hemicellulose decomposition at low temperature ranges (i.e., 200–260 °C) generates increased volatile components and decreased tar and char. Hemicellulose decomposition consists of two phases: dehydration and cracking of the side unit in the first phase, which occurs below 100 °C. Decomposition of the main chain is during the second phase, arising within a temperature range of 240–290 °C [
80]. Hydrocellulose and levoglucosan are the primary products of the conversion of cellulose between 320 °C and 380 °C. Lignin degradation at a higher temperature range (i.e., 200–500 °C) generates adequate oxygenates, such as phenols, derived from benzene rings [
69]. Especially in comparison to hemicellulose (32 wt.%) and cellulose (5 wt.%), lignin decomposition generates the maximum solid residue (42 wt.%) due to the condensation of lignin fragments through free radicals [
80].
Table 2 lists the pyrolysis of a few selected lignocellulosic feedstocks.
Table 2. Typical properties of bio-oil obtained from pyrolysis of different biodegradable (lignocellulosic) and non-biodegradable feedstock.
| Raw Material |
Properties of Bio-Oil Produced by Pyrolysis |
Reference |
| Acacia nilotica (Babool) Seeds |
Rich in hydrocarbons, alcohols, phenols but further upgradation to remove oxygen is required |
[81] |
| Cedrus deodara |
Rich in acids, alcohols, aromatic ethers,
carbonyl compounds, hydrocarbons, phenols, but further refining is required |
[82] |
| Corn Cob |
Acids, furans, lignin-derived phenols, nonaromatic aldehydes, non-aromatic ketones,
sugars, but further upgradation for removing moisture is required |
[40] |
| Cotton Residue |
Bio-oil contains phenolic compounds but is highly oxygenated |
[83] |
| HDPE |
Rich in aliphatic hydrocarbon (C8 to C12); lower proportion of aromatic hydrocarbons |
[84] |
| LDPE |
Rich in aliphatic and simple aromatic hydrocarbons |
[85] |
| Mixed Plastic |
Heating Value 44.40 MJ/kg |
[39] |
| Oil Palm Empty Fruit Bunches |
Rich in phenol, furan, ketone, alcohol, acids, pyrans |
[40] |
| Palm Fronds |
Rich in acids, phenols, ketones, aldehydes, alcohols, but this conversion is still not optimal |
[86] |
| PE |
High aromatic content having other hydrocarbon compounds and some aliphatic content; higher heating values than conventional diesel |
[87] |
| PET |
Rich in aromatic hydroxyl groups; lack oxygen, carboxyl, and aliphatic hydroxyl groups |
[88] |
| Pinewood |
Rich in acids, phenols; however, further optimization and upgradation is required. |
[89] |
| Poplar |
Bio-oil collected under two fractions: |
[90] |
- (a)
-
lighter (85 wt.%)—high water content made chiefly of acids and ketones, and
- (b)
-
heavier (15 wt.%)—lower water content and rich in phenols
|
| PP |
Rich in the naphtha range hydrocarbons. |
[91] |
| PS |
Heating value 43.0 MJ/kg; flash point: 26.1 °C |
[39] |
| PVC |
Heating value 43.22 MJ/kg; flash point: 40 °C |
[39] |
| Rice Husk |
Rich in acids, aromatic, heterocyclic compounds, but to use as vehicle fuel it requires some refining |
[40] |
| Spruce |
Rich in non-aromatic aldehydes, sugars, non-aromatic ketones, guaiacols, acids, furans, pyrans |
[40] |
| Sugarcane Bagasse |
Bio-oil has significantly less oxygen and higher calorific value due to pressure applied during pyrolysis |
[90] |
| Sweet Sorghum Bagasse |
Properties of bio-oil were found to vary across the fractional condensers. |
[90] |
| Waste Tires |
Contains aromatic, aliphatic, polar and hetero-atom fractions. |
[92] |
The low hydrogen/carbon effective ratio (H/C
eff) of biomass results in decreased aromatic yield, more coke production, and increased water, oxygen content, viscosity, and corrosiveness of the bio-oil produced; hence, it cannot be employed commercially for transportation. Additionally, biomass exhibits a low hydrogen/carbon effective ratio (H/C
eff), which results in low aromatic yield and high coke production [
15]. All these factors reduce the conversion efficiency of the obtained bio-oil into advanced biofuel. Therefore, effective approaches are desired to enhance the quality of bio-oil and make it identical to those hydrocarbon fuels. A few of the procedures for upgrading bio-oil are catalytic cracking, which involves the introduction of a solid acid catalyst, and hydrodeoxygenation, which involves metal catalysts with a pressurized hydrogen atmosphere. Nevertheless, both processes seem to be unpromising due to great operating costs associated with the use of noble catalysts and substantial catalyst deactivation. Zhang et al. [
93] have evaluated catalytic pyrolysis involving the addition of catalysts before quenching volatiles. Catalytic pyrolysis results in highly stable bio-oil, although, even in the existence of extremely efficient catalysts, catalytic pyrolysis of lignocellulosic biomass still results in low carbon yield and great quantities of solid residues, together with biochar and coke. Such a large content of coke can result in deactivation of the catalyst and thus make the whole process impractical to commercialize. Thus, in order to increase carbon yield and curtail coke generation, researchers recently evaluated the amalgamation of high H/C
eff ratio co-reactants (such as non-biodegradable wastes such as plastics) with lignocellulosic biomass to mitigate the issues that arise due to the upgradation of bio-oil produced solely from lignocellulose wastes [
94].
Non-Lignocellulosic Wastes
Apart from lignocellulosic biomass, there are some non-lignocellulosic sources, such as algal biomass, which have shown potential for biofuel production through pyrolysis [
95]. Algal biomass is comprised mainly of three components: lipids, proteins, and polysaccharides [
96]. There are several advantages of using algal biomass as a substrate for co-pyrolysis/pyrolysis such as ease of cultivation and, compared to land plants, relatively lower inputs. Moreover, algae can be grown in fresh, brackish, and marine waters as well as wastewater [
97,
98]. For instance, pyrolysis of microalgae
Scenedesmus dimorphus was found to produce bio-oil rich in aromatics, phenols, heterocyclic hydrocarbons, amides, and indole with the potential to be converted into bioenergy and biomaterial after a few upgradations [
99]. In another study, blue–green algae blooms underwent pyrolysis in a fixed-bed reactor and produced bio-oil with great heating value and controlled eutrophication [
90]. Other traditional non-lignocellulosic biomasses are sewage sludge and manure, which are usually managed by landfilling, burning, agricultural use, and so on. Therefore, converting sewage sludge and manure to bio-oil through pyrolysis with biochar as a value-added byproduct is an excellent way to manage these non-lignocellulosic wastes [
63]. For instance, Ma et al. [
61] investigated sewage sludge supercritical water pyrolysis and established that bio-oil formed can be utilized as fuel after refining. However, the conversion of non-lignocellulose waste through pyrolysis still requires further investigation, and not much research is available as of now.
2.1.2. Non-Biodegradable Wastes
The consumption of plastics and related products has increased enormously over the years due to their various applications. However, non-biodegradable wastes are more voluminous than biodegradable wastes, and most parts of non-biodegradable wastes take millions of years to degrade. Therefore, the massive production and consumption of non-biodegradable materials has created an environmental emergency. Simultaneously, the existing high energy demand in our society is also a matter of great concern. Non-biodegradable wastes usually include materials such as high-density polyethylene (HDPE), polyethylene terephthalate (PET), low-density polyethylene (LDPE), polystyrene (PS), polypropylene (PP), polyamide (PA), polyacrylate (PAC), polyvinyl–chloride (PVC), waste tires, e-waste, etc. Among all, plastic polymers and tire waste are generally available in abundance. To dispose of these wastes, incineration and landfilling are the traditional methods adopted by many countries if recycling is not possible. However, landfills consume massive space and contaminate soil and water of nearby areas, and incineration leads to unacceptable emission of harmful compounds [
39,
100]. Therefore, pyrolysis of non-biodegradable waste is one of the alternative methods to manage these wastes as well as to recover energy from them. Since plastics and tires are prepared from petroleum, they have the same physical characteristics as fuel. Therefore, their wastes need additional consideration in terms of management for their potential application in the production of second generation biofuels through pyrolysis.
Plastics are non-renewable synthetic materials originating from crude oil. They only contain carbon and hydrogen and lack elemental oxygen. In the pyrolysis of plastics, their polymeric macromolecular structures are cracked down into minor molecules or monomers, and further degradation depends upon the operating conditions and presence of catalysts. The oil formed from pyrolytic degradation of plastics displays a high calorific value similar to conventional fuel. It also has lower oxygen and higher hydrogen content than oil from biomass [
19]. Therefore, the presence of plastics during pyrolysis of various biomasses, such as lignocellulosic wastes, can have a desirable synergistic influence on the heating value, and sole pyrolysis of plastic waste can also serve as an alternative management technique and resource/energy recovery option.
Tire waste has also been considered as another excellent candidate for pyrolysis. It contains rubber (60–65 wt.%) and carbon black (25–35 wt.%). Styrene–butadiene rubber, nitrile rubber, natural rubber (polyisoprene), polybutadiene rubber, and chloroprene rubber are some of the natural and synthetic rubbers. The pyrolytic oil generated from the pyrolysis of tire waste has energy up to 44 MJ/kg and shows decreased oxygen levels, an increased H/C atomic ratio (~1.5), and contains both aromatic and aliphatic compounds [
15,
33].
As already discussed, researchers are finding cost-effective ways to improve the carbon yield in bio-oil formed from biodegradable biomasses. However, the blending of pyrolytic oil from non-biodegradable wastes with bio-oil generated from biomass is uneconomical and next to impossible. Further, the distinct pyrolysis of biomass and non-biodegradable wastes also increases the overall cost. Thus, co-pyrolysis has come out as a more reliable option [
19]. In co-pyrolysis, there is an interaction of radicals that can encourage the creation of consistent pyrolysis oil that evades phase separation [
32]. Most non-biodegradable wastes can be characterized as potential co-feeds in co-pyrolysis because of their abundant availability. Higher energy content and the creation of high-quality bio-oils occur in co-pyrolysis due to synergism between the diverse materials, especially during co-pyrolysis of biodegradable waste with non-biodegradable wastes. Additionally, the key advantage of co-pyrolysis is that an increased amount of waste is consumed as raw material, reducing the need for landfills and separate waste treatment techniques.
Table 2 has already shown the co-pyrolysis of a few raw materials and the properties of the oil produced.
2.2. Sample Preparation/Pretreatment
Prior to co-pyrolysis, feedstocks must be prepared and pre-treated. Pretreatment is another approach to improving the synergistic interactions between biodegradable wastes and non-biodegradable wastes. Pretreatment is an essential step for the generation of bio-oil with higher quality and quantity from co-pyrolysis. Pretreatment of biomass is necessary due to the coincidence of alkali and alkaline earth metals that negatively influence the performance of co-pyrolysis [
101]. Numerous pretreatment steps and their consequences on productivity and quality are discussed below.
2.2.1. Physical/Mechanical Pretreatment
Drying and grinding are some common physical pretreatment methods that have been found to make the transformation process easier and more effective [
19]. Grinding samples to 2–3 mm in size along with drying is essential to reach high biomass heating levels [
33]. Drying is done by oven heating for 24 h at 105 °C. The dried feed material should have a moisture level less than or equal to 10% because higher moisture content generates inferior quality oil with high water content [
102]. At an industrial level, char and fuel gasses are employed as sources of energy for drying and grinding [
19,
33]. Apart from drying and grinding, sorting and dewatering are used for pretreatment and sample preparation [
33]. Most recently, microwave irradiation has been used for pretreatment. Zhang et al. [
103] explored co-pyrolysis of microwave pre-treated chili straw and PP over HZSM-5 catalyst to yield hydrocarbon-rich bio-oil, and aromatic content increased from 4.46 to 17.34%. Further, thermal biomass pretreatment by dry torrefaction, in which temperatures ranging from 200 °C–290 °C were applied, improved biomass structure and consequently produced improved bio-oil [
90]. Torrefaction of biomass into small oxygenates and augmentation of the ring opening resulted in the generation of aliphatic intermediates [
15].
2.2.2. Chemical Pretreatment
Chemical pretreatment has been shown to alter product yield and cause structural changes in the raw materials [
15]. Acid pretreatment of biomass is one of the common techniques to reduce the harmful effects of alkaline earth metals. Several acid pretreatment studies of co-pyrolysis have been discussed in various articles. Xue and Bai [
104] investigated the co-pyrolysis of corn stover treated with sulphuric acid (98%) and PE in a tandem micro-pyrolyzer at 600 °C, and a considerable improvement in the levels of levoglucosan and phenolic monomers were observed. The investigators discussed the synergistic effects of robust Diel–Alder reactions between biomass-derived furans and plastic-derived olefins [
104]. Another study investigated acid-washed (aqueous nitric acid (3 wt.%) corn stover–HDPE co-pyrolysis performed in a drop-tube reactor with N
2 as a carrier gas at 550 °C, and a high liquid yield of 51% was reported, with enhancement in the bio-oil eminence [
105]. Another group of researchers studied co-pyrolysis in a fixed-bed reactor with HCl-treated cotton stalk biomass and waste tires at 550 °C and observed a reduction in light oxygenates. Furthermore, liquid yields were increased from 45 wt% [
106]. Alkali pretreatment has also been used in some studies and has improved bio-oil yield by untethering ester and glycosidic bonds within lignin [
90]. Krutof and Hawboldt [
107] reviewed co-pyrolysis of different feedstocks and stated alkali washing also leads to demineralization.
2.2.3. Physio–Chemical Pretreatment
Wet torrefaction (hydrothermal), ammonia fiber expansion, steam explosion, and CO
2 explosion are some of the common physio–chemical pretreatment methods. Wet torrefaction, also identified as hydrothermal pretreatment, is implemented using hot (150–260 °C), compressed water [
15]. The group of researchers performed co-pyrolysis of hydrothermally treated (175 °C) high-protein
Chlorella sp. (microalgae) and PP (550 °C), and an excellent upsurge in the generation of cyclohexane derivatives and alkenes was observed due to synergistic interactions amongst both feedstocks; further, a reduction in heterocyclic N-containing compounds was also observed [
108]. In a separate study, microwave assisted co-pyrolysis of torrefied lignin and LDPE at 550 °C was performed, and a drastic increase in aromatic content was observed in the bio-oil [
109]. Dai et al. [
110] concluded that hydrothermal pretreatment has great potential to improve aromatic production during biomass–polyethylene co-pyrolysis. Further, ammonia fiber expansion (AFEX) is known to be an effective pretreatment technique to improve the biomass structure [
90]. On the other hand, steam explosion has been developed for wood pretreatment, in which wood chips in a vessel are subjected to 285 °C and 35 bar for 2 min, then the pressure increases to 70 bar within 5 s. This process provides more-accessible feedstock for secondary conversion [
90].
2.2.4. Biological Pretreatment
In comparison to most of the above-mentioned pretreatments, biological pretreatment is found to be more environment friendly for several reasons, such as nonrequirement of additional chemicals, lesser energy requirements, and very low or negligible production of inhibitory compounds. Moreover, there are no corrosion-related problems, and noninvolvement of harsh chemicals also reduces the burden of harmful waste [
111]. This can be done with help of microorganisms or by directly applying the enzyme cocktails. The main constraint of using biological pretreatment is that the rate of degradation is very slow, so researchers need to optimize the strain in such a way that the maximum yield can be obtained in a limited amount of time [
90]. In addition, not many studies are present in the literature where biological pretreatment is performed before co-pyrolysis.
This entry is adapted from the peer-reviewed paper 10.3390/en15114168