Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Primary ureteral neuroendocrine neoplasms (NENs) are rare. Small-cell neuroendocrine cancer (NEC) of the ureter is usually observed in elderly patients, and around 15 cases have been observed in females so far.
- neuroendocrine tumors
- female neuroendocrine neoplasms
- urethral neoplasm
1. Epidemiology, Presentation, and Pathogenesis
Primary ureteral neuroendocrine neoplasms (NENs) are rare, and no more than 50 cases of small-cell ureteral neuroendocrine cancer (NEC) have been reported in the literature [1][2]. Small-cell NEC of the ureter is usually observed in elderly patients, and around 15 cases have been observed in females so far [1][2][3]. Less than 10 cases of large-cell NEC of the ureter [4][5] have been reported in the literature. Neuroendocrine cells are not usually found in the urinary tract. The pathogenesis of ureteral NENs is still disputed and hypothesized to be similar to that of bladder NENs [6][7]. Most of the NENs arise from the lower ureteral segment. Collision tumors are rare, in which tumors of two or more types coexist in the same organ and do not intermix. Approximately 50% of ureteral small-cell NECs account for collision tumors [8][9]. Around 62% of patients demonstrate small-cell NEC associated with urothelial carcinoma [10].
Clinical presentation is varied, ranging from asymptomatic to complete ureteral obstruction resulting in hydronephrosis [11]. In contrast to bladder masses obstructing bilateral ureters, ureteral NENs is unilateral and does not usually cause oligo-anuria in bi-nephric individuals. Gross hematuria and flank pain are the most common symptoms of small-cell ureteral NEC. Endoscopy provides the biopsy specimen, which aids in diagnosing the mass by revealing malignant neuroendocrine cells [5][12]. A few may also present with paraneoplastic syndromes, which indicate advanced or extensive disease [2]. Ouzzane et al. reported that 72% of patients presented with pT3 or pT4 stages, and 54% developed metastases within 13 months [10].
2. Imaging
Screening of ureteral NENs is challenging due to non-specific symptoms, such as ureteral obstruction or hematuria. As the most common presentation includes hematuria and hydronephrosis, ultrasonography (USG) is the initial investigation of choice to exclude urinary tract stones. USG, although it detects ureteral distention, is insensitive to ureteral tumors. Retrograde urography can detect the luminal mass indirectly by showing a filling defect in the ureteral lumen [11]. Finally, grossly, ureteral NENs are solid and sessile; they have gray to white cut surfaces, firm consistency, and peritumoral mural thickening (Figure 1) [5][12]. Microscopically, the tumor has a desmoplastic stromal reaction, a high ki-67 index (>50–60%), necrosis, adjacent structural invasion, and lymphovascular invasion, in addition to specific cellular characteristics described in the other sections (Figure 1) [5][12][13][14].
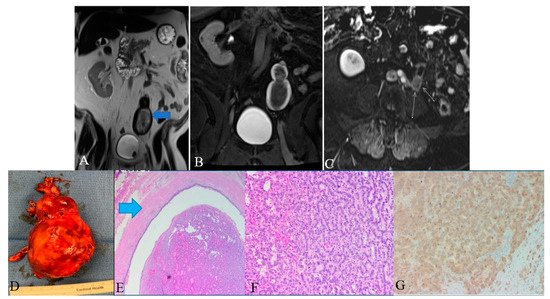
Figure 1. A 48-year-old female with ureteral NENs. (A) Coronal T2 nonfat sat image shows a large expansible hematoma in the ureteric stump (arrows). (B) Coronal T1 postcontrast delayed fat sat image: Shows a large expansible hematoma with intrinsic high T1 signal. (C) Axial T1 postcontrast delayed fat sat subtraction image: a small enhancing nodule (arrows) along the superior aspect of the stump. (D) Left lower ureter mass consists of a segment of the ureter with an attached firm, tan-brown, irregular, mass, measuring 11.5 × 7.0 × 6.0 cm. (E) E&H staining: 20× shows polypoid tumor bulging into the ureter lumen. Normal urothelial mucosa and muscle layer can be seen on the top half (arrow). (F) H&E straining: 200× high-power view shows tumor cells arranged in a trabecular and tubular pattern. (G) Synaptophysin staining: 200× shows tumor cells expressing synaptophysin (brown staining), which is a marker of neuroendocrine differentiation.
Histologic examination of small-cell NEC of the ureter may reveal small palisading cells with scant to moderate cytoplasm, high nuclear-cytoplasmic ratios, finely speckled chromatin, and high mitotic counts (Table 1). The specimen stains positive for CAM5.2, chromogranin A, synaptophysin, neuron-specific enolase, pan-cytokeratin (AE1/AE3), and CD56 (Figure 1). Around 44% of extrapulmonary small-cell cancers stain positive for thyroid-transcription factor-1 [15][16]. Although ureteral metastases are rare, it is prudent to exclude metastatic small-cell NEC to the upper urinary tract during the diagnosis [3][17][18].
Table 1. Morphological features of small-cell ureteral NEC.
| Features | Small-Cell NEC |
|---|---|
| Gross | Well-defined firm-greyish mass protruding into the ureteral lumen; hemorrhagic areas |
| Histology | |
| Cellular arrangement | Solid sheets; rosette; nests |
| Cellular characteristics | Small to medium sized cells with scant cytoplasm and granular chromatin |
| Additional features | Frequent necrotic areas, mitosis and vascular invasion |
| Immunohistochemistry | |
| Neuroendocrine stains | Chromogranin A, synaptophysin, CD56, neuron-specific enolase |
| Epithelial stains | Cytokeratin-7, epithelial membrane antigen, and pan-cytokeratin |
| Differential stains | Uroplakin III-negative (positive in umbrella cells of urothelium and transitional cell carcinoma) |
The diagnostic criteria for large-cell NEC of the ureter are based on the pulmonary counterpart: (i) Morphological characteristics of a neuroendocrine tumor (palisading, organoid nesting, trabeculae, or rosettes). (ii) High mitotic activity (>11/HPF with a median of 70/HPF). (iii) Large necrotic zone. (iv) Microscopic cellular characteristics of a non-small-cell carcinoma (large cells with low nuclear-cytoplasmic ratio, vesicular/fine/coarse chromatin, and frequent nucleoli). (v) Positive immunohistochemistry for ≥1 neuroendocrine marker (except neuron-specific enolase) and neuroendocrine granules (100–200 nm membrane-bound cytoplasmic granules) [5]. The differential diagnosis of large-cell ureteral NEC and its distinguishing features are described in Table 2.
| Differential Diagnosis | Distinguishing Feature |
|---|---|
| Carcinoid tumors | Small cells with low-grade nuclear atypia; low mitotic activity (usually <2/HPF); low Ki-67 index |
| Small-cell carcinoma | Small cells (usually less than the diameter of the three small lymphocytes) with scant cytoplasm; fine granular chromatin; absent or inconspicuous nuclei; high miotic activity (≥11/HPF with a median of 80/HPF); frequent large areas of necrosis |
| High-grade urothelial carcinoma | Poorly differentiated cells with centrally located nuclei and thick, rough nuclear membranes; Identifiable nucleoli; Irregular chromatin; Positive immunohistochemistry for uroplakin (57–81% of cases) and negative or neuroendocrine markers; |
| Primary or metastatic adenocarcinoma | Diffuse glandular morphology; negative neuroendocrine markers on immunohistochemical analyses |
3. Prognosis and Management
Ureteral NENs are aggressive, among which small-cell NEC has a rapid progression and dismal prognosis with a median overall survival of 17 months [15][19]. Most of the patients present with locally advanced disease, and 20% have had lymph node involvement at diagnosis [11]. The small-cell ureteral NEC’s 1- and 3-year overall survival rates are 52% and 30%, respectively [8]. The pathologic stage is the most important prognostic factor. The median survival times of patients with stages pT1–pT2 and pT3–pT4 are around 31 and 8 months, respectively [15]. Zhong et al. reported regional recurrences and distant metastasis in 9.4% and 25% of patients with early-stage disease, respectively, during a short follow-up [8]. Vimentin positive small-cell ureteral NEC had a poor prognosis in a study by Chuang et al.; however, further studies are required to determine the efficacy of vimentin in predicting the prognosis [20].
There are no well-established strategies for treating small-cell ureteral NEC due to limited data, and hence, decision-making depends on small-cell lung carcinoma treatment strategies. Oncologists consider a multimodality approach with surgery, adjuvant chemotherapy, and radiation (Figure 2) [21]. The staging of ureteral cancers is described in Table 2 and Figure 3. Radical resection is preferred in early-stage, small-cell ureteral NEC (Figure 2). However, if the patient has upper urinary tract urothelial carcinoma, nephroureterectomy with bladder cuff excision is recommended. The median survival ranges from 8.2 months with surgery alone to 24 months with adjuvant platinum-based chemotherapy [13][15][16]. Platinum-based chemotherapy with EP (etoposide and cisplatin) or CE (carboplatin and etoposide) has shown a response rate of 69% [22]. The irinotecan and cisplatin have also been shown to achieve the tumor reduction [13][23]. Studies show that adjuvant chemotherapy has more favorable outcomes compared to surgery alone. The median overall survival times for patients who received adjuvant chemotherapy and surgery alone were 24 and 12 months, respectively (p = 0.56) [10][15]. The efficacy of immunotherapy has not been studied in the case of small-cell ureteral NEC. Immunotherapies targeting EGFR, BCL-2, C-kit, CD56, and PDGFR-α might be promising approaches [3][24][25].
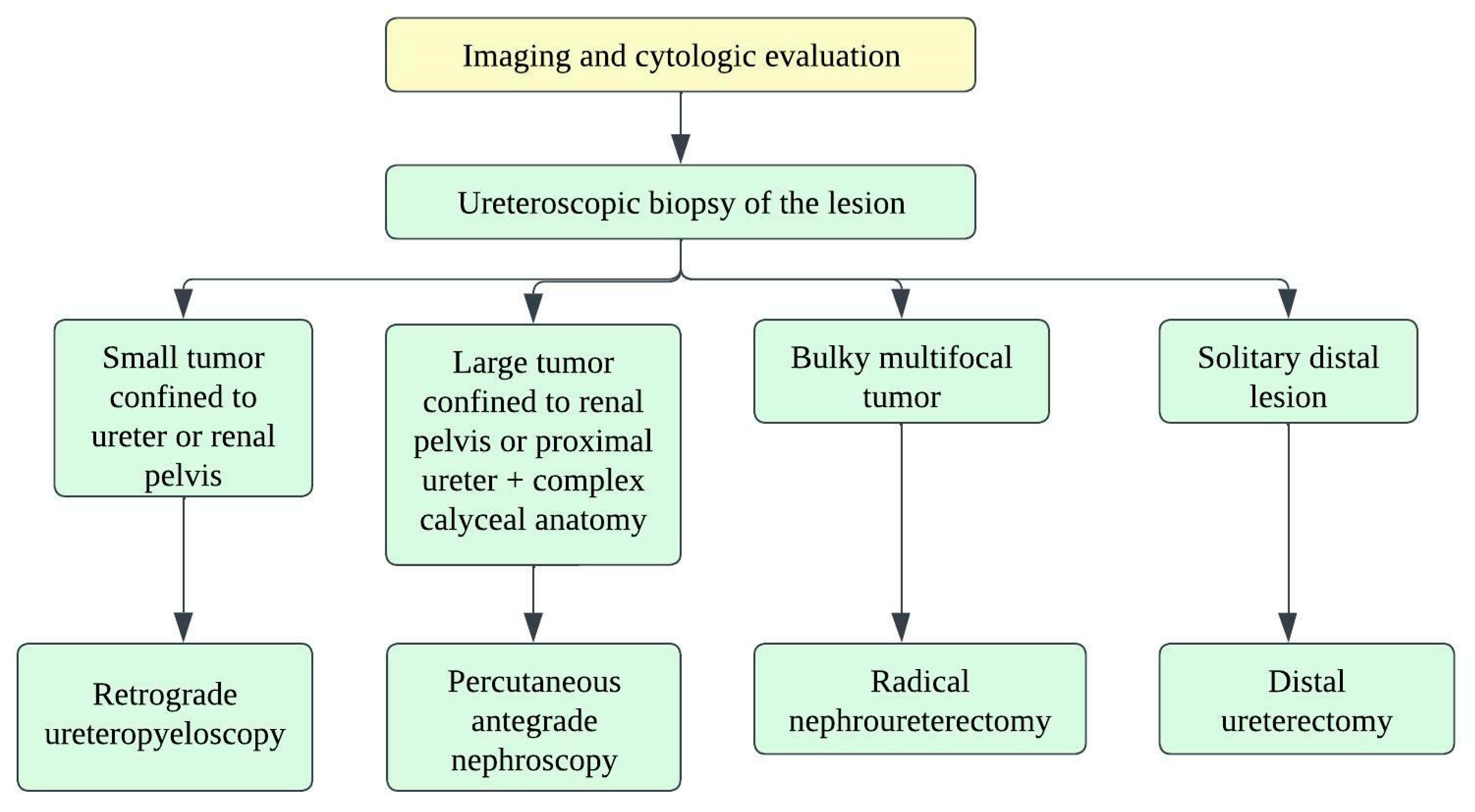
Figure 2. Treatment algorithm for upper urinary tract carcinoma.
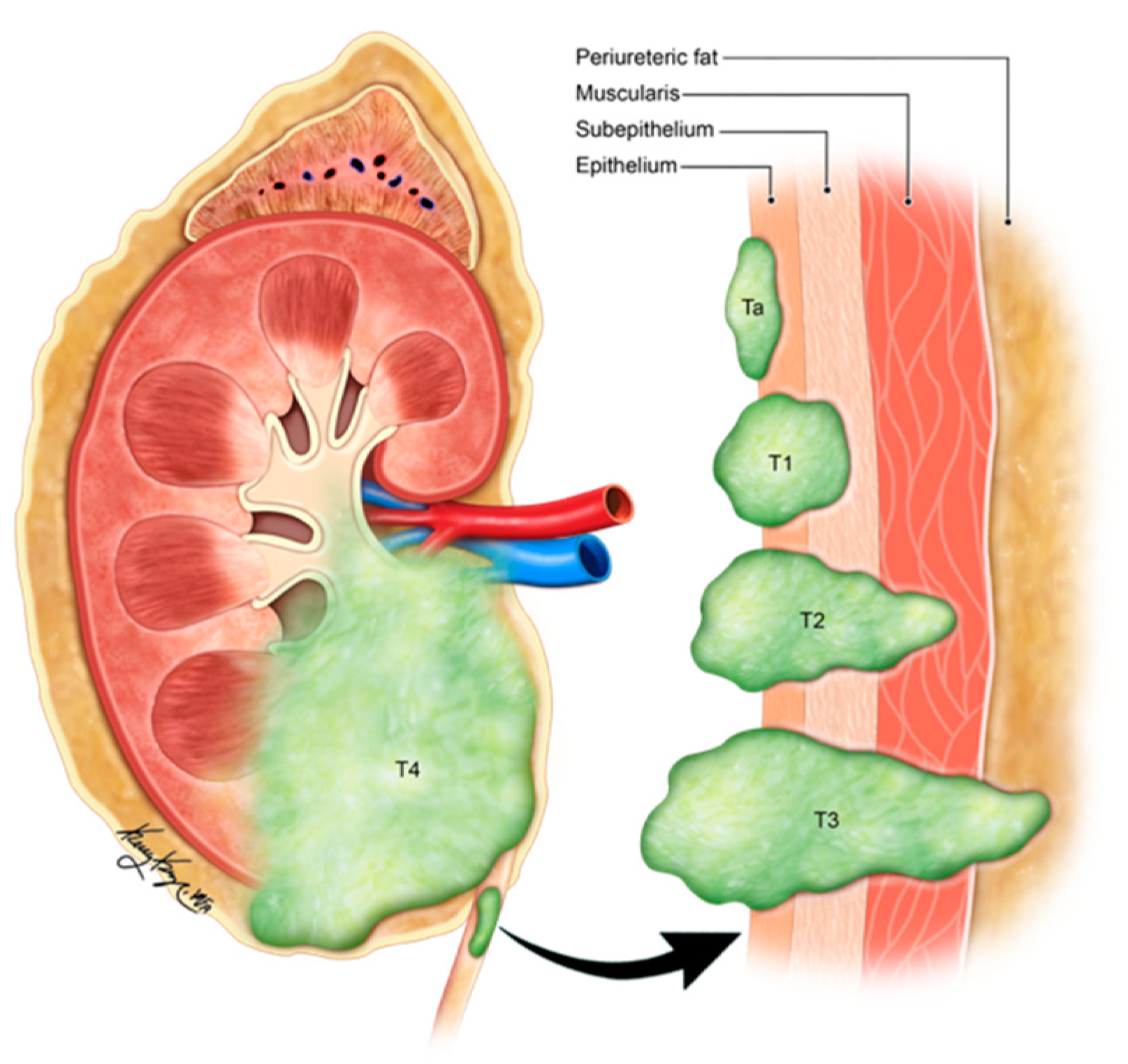
Figure 3. Illustration demonstrating the staging of ureteral carcinoma. Ta: non-invasive papillary tumor; T1: tumor invasion into sub-epithelial connective tissue through lamina propria; T2: tumor invasion into muscularis propria; T3: tumor invasion into periureteric fat beyond muscularis propria; T4: tumor invades adjacent organs or through the kidney into the perinephric fat.
Table 2. TNM staging of ureteral malignancies [26].
| Stage | TNM Staging | Description |
|---|---|---|
| 0 | Ta N0 M0 | Non-invasive papillary tumor |
| Tis N0 M0 | Carcinoma in-situ | |
| I | T1 N0 M0 | Tumor invasion into sb-epithelial connective tissue through lamina propria |
| II | T2 N0 M0 | Tumor invasion into muscularis propria |
| III | T3 N0 M0 | Tumor invasion into periureteric fat beyond muscularis propria |
| T4 N0 M0 | Tumor invades adjacent organs or through the kidney into the perinephric fat | |
| IV | T4 Any N Any M | N1-metastasis in a single lymph node ≤ 2 cm in greatest dimension N2-Metastasis in a single lymph node > 2 cm; or multiple lymph node involvement N3-Metastasis in a lymph node, more than 5 cm in greatest dimension M1-Distant metastasis |
| Any T N1-3 Any M |
This entry is adapted from the peer-reviewed paper 10.3390/cancers14133218
References
- Farci, F.; Manassero, F.; Baldesi, R.; Bartolucci, A.; Boldrini, L.; Selli, C.; Faviana, P. Primary small cell carcinoma of the ureter: Case report and review of the literature. Medicine 2018, 97, e11113.
- Qing, D.; Peng, L.; Cen, F.; Huang, X.; Wei, Q.; Lu, H. Hyperprogression After Immunotherapy for Primary Small Cell Neuroendocrine Carcinoma of the Ureter: A Case Report. Front. Oncol. 2021, 11, 696422.
- Rupert, V.; Clifton, M.M.; Fulmer, B.R.; Mori, R.L.; Williams, H.; Park, A. Primary small cell carcinoma of the upper urinary tract: A case report. Urol. Case Rep. 2019, 27, 100995.
- Choi, J.H.; Sol, Y.; Lee, S.W.; Jeh, S.U.; Hwa, J.S.; Hyun, J.S.; Chung, K.H.; Seo, D.H.; Yang, J.W.; Song, D.H.; et al. Primary large-cell neuroendocrine carcinoma of the upper ureter: A case report. Medicine 2019, 98, e15613.
- Oshiro, H.; Odagaki, Y.; Iobe, H.; Ozu, C.; Takizawa, I.; Nagai, T.; Matsubayashi, J.; Inagaki, A.; Miyake, S.; Nagao, T. Primary large cell neuroendocrine carcinoma of the ureter. Int. J. Clin. Exp. Pathol. 2013, 6, 729.
- Fetissof, F.; Dubois, M.; Lanson, Y.; Jobard, P. Endocrine cells in renal pelvis and ureter, an immunohistochemical analysis. J. Urol. 1986, 135, 420–421.
- Harada, K.; Sato, Y.; Ikeda, H.; Hsu, M.; Igarashi, S.; Nakanuma, Y. Notch1-Hes1 signalling axis in the tumourigenesis of biliary neuroendocrine tumours. J. Clin. Pathol. 2013, 66, 386–391.
- Zhong, W.; Lin, R.; Zhang, L.; Jin, C.; Li, X.; He, Q.; Gong, K.; He, Z.; Zhou, L. Clinicopathologic characteristics, therapy and outcomes of patients with primary ureteral small cell carcinoma: A case series and systematic review of the literature. OncoTargets Ther. 2017, 10, 4105.
- Xu, S.; Xu, L.; Cao, P.; Yao, S.; Wu, T.; Hu, X.; Chen, H.; Gu, J.; Che, X. Collision Carcinoma Involving Small Cell Neuroendocrine Carcinoma and Squamous Cell Carcinoma of the Ureter: A Case Report and Review of the Literature. Front. Oncol. 2021, 11, 663119.
- Ouzzane, A.; Ghoneim, T.P.; Udo, K.; Verhasselt-Crinquette, M.; Puech, P.; Betrouni, N.; Rouprêt, M.; Villers, A.; Leroy, X.; Colin, P. Small cell carcinoma of the upper urinary tract (UUT-SCC): Report of a rare entity and systematic review of the literature. Cancer Treat. Rev. 2011, 37, 366–372.
- Acosta, A.M.; Kajdacsy-Balla, A. Primary Neuroendocrine Tumors of the Ureter: A Short Review. Arch. Pathol. Lab. Med. 2016, 140, 714–717.
- Banerji, J.S.; Korula, A.; Panicker, J.B. Multicentric small cell neuroendocrine neoplasm of the renal pelvis and ureter with concomitant focal high-grade urothelial carcinoma of the ureter: A case report. Indian J. Urol. IJU J. Urol. Soc. India 2008, 24, 571.
- Ping, J.H.; Chen, Z.X.; Jiong, Q.; Han, Y.Q.; Nong, X. Small cell neuroendocrine carcinoma of the ureter: A case report and literature review. Oncol. Lett. 2014, 7, 728–730.
- Yang, J.; Zhao, Z.; Ni, J.; Dong, W.; Wang, N.; Wang, B. Urography and CT features of primary small cell carcinoma of the ureter: A case report. Iran. J. Radiol. 2013, 10, 160.
- Hensley, P.J.; Bhalodi, A.A.; Gupta, S. Primary Upper Urinary Tract Small Cell Carcinoma: A Case Series and Literature Review. J. Endourol. Case Rep. 2017, 3, 165–168.
- Acosta, A.M.; Hamedani, F.S.; Meeks, J.J.; Wu, S. Primary ureteral thyroid transcription factor 1–positive small cell neuroendocrine carcinoma: Case report and review of the literature. Int. J. Surg. Pathol. 2015, 23, 472–477.
- Miller, R.J.; Holmäng, S.; Johansson, S.L.; Lele, S.M. Small cell carcinoma of the renal pelvis and ureter: Clinicopathologic and immunohistochemical features. Arch. Pathol. Lab. Med. 2011, 135, 1565–1569.
- DiPietro, M.; Zeman, R.K.; Keohane, M.; Rosenfield, A.T. Oat cell carcinoma metastatic to ureter. Urology 1983, 22, 419–420.
- Zhu, J.; Feng, Y.; Fan, M.; He, X.; Wang, L. Primary small cell neuroendocrine carcinoma combined with squamous carcinoma of the ureter after renal transplantation: A rare case report. Int. J. Clin. Exp. Pathol. 2019, 12, 1357.
- Wang, H.; Ma, C.; Wu, J.; Zhao, F.; Zou, Y.; Zhang, W.; Jiang, Y. Clinicopathologic features of the ureteral neuroendocrine tumors. Pathol. Res. Pract. 2020, 216, 152788.
- Essenfeld, H.; Manivel, J.C.; Benedetto, P.; Albores-Saavedra, J. Small cell carcinoma of the renal pelvis: A clinicopathological, morphological and immunohistochemical study of 2 cases. J. Urol. 1990, 144, 344–347.
- Sato, R.; Ishikawa, T.; Imagawa, M.; Yonemasu, H.; Narimatsu, T.; Murakami, K. Primary small cell carcinoma of the ureter with hydronephrosis: A case report. Urol. Case Rep. 2020, 29, 101099.
- Sood, A.; Williamson, S.R.; Leavitt, D.A. Neuroendocrine Tumor of the Ureter: A Zebra among Horses. J. Endourol. Case Rep. 2016, 2, 204–208.
- Yao, J.L.; Madeb, R.; Bourne, P.; Lei, J.; Yang, X.; Tickoo, S.; Liu, Z.; Tan, D.; Cheng, L.; Hatem, F. Small cell carcinoma of the prostate: An immunohistochemical study. Am. J. Surg. Pathol. 2006, 30, 705–712.
- Terada, T. Primary small cell carcinoma of the ureter: A case report involving immunohistochemical and molecular genetic analyses of KIT and PDGFRA genes. Pathology 2010, 42, 101–102.
- Arora, H.C.; Fascelli, M.; Zhang, J.H.; Isharwal, S.; Campbell, S.C. Kidney, Ureteral, and Bladder Cancer: A Primer for the Internist. Med. Clin. N. Am. 2018, 102, 231–249.
This entry is offline, you can click here to edit this entry!
