Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Immune checkpoint inhibitors (ICIs), antibodies that target the checkpoints in immune cells, work to activate inhibited T-cells and other cells of the innate and adaptive arms, resulting in the robust activation of the immune system and productive antitumor immune responses. However, ICIs-related cardiotoxicity has been recognized as a rare but fatal consequence. Although there has been extensive research based on different types of ICIs, these studies have not indicated whether cardiotoxicity is specific to a type of cancer.
- immune checkpoint inhibitors
- cardiotoxicity
- cardio-oncology
- cancer-type-specific
1. Introduction
Cardiovascular disease (CVD) and cancer are global health issues with high morbidity and mortality [1], and numerous studies suggest that there is an overlap in epidemiology, risk factors, and pathophysiologic processes (Figure 1) .
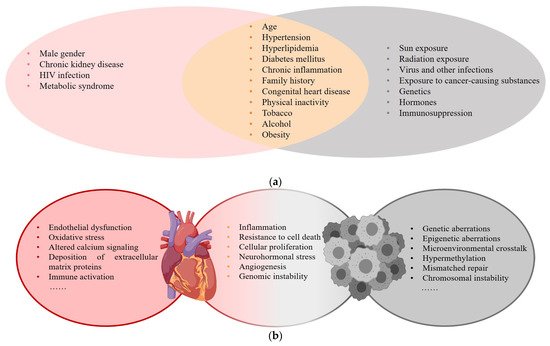
Figure 1. (a) Risk factors for CVD and cancer; (b) Common pathophysiologic processes of CVD and cancer.
With the widespread application of anticancer drugs, the survival of patients has significantly improved, but the related cardiotoxicity affects long-term therapeutic outcomes, and this has attracted considerable attention. Immune checkpoint inhibitors (ICIs), antibodies that target the checkpoints in immune cells, work to activate inhibited T-cells and other cells of the innate and adaptive arms, resulting in the robust activation of the immune system and productive antitumor immune responses. This new type of immunotherapy drug has significantly improved the survival of cancer patients [2][3][4]. However, their use is associated with adverse side effects involving different organs [5][6]. ICIs-related cardiotoxicity, which may develop even without a history of significant cardiac risk factors, includes myocarditis, pericarditis, heart failure, arrhythmias, and vasculitis [7]. In reported cases of adverse ICIs-related events, 6.2% were cardiac adverse events (CAEs), which can be the main determinants of quality of life and increased mortality [8][9][10].
2. Cardiotoxicity in Melanoma
In 16 studies, 24 of 6710 patients on ICIs [11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26] developed CAEs. This corresponded with an incidence of 0.20–4.93% in which grade 3–5 CAEs accounted for 41.7%. Commonly encountered cardiotoxicities included hypertension (50%), hypotension (16.7%), and myocarditis (8.3%). Treatment-related hypertension was linked to the application of lambrolizumab (58.3%) (PD-1). Nivolumab may have had a correlation with ICIs-related hypotension. Patients treated with a higher dose of ipilimumab, particularly 10 mg/kg × 4 doses/3 weeks, were more prone to fatal adverse events such as cardiac arrest (Table 1).
Table 1. Cardiotoxicity in melanoma.
| Author, Year | Study Type | Phase | Sample Size | Drug | Dose and Frequency | Non-CAE | CAE | Manifestation | 3–5 Grade CAE |
|---|---|---|---|---|---|---|---|---|---|
| Omid Hamid et al., 2017 [11] | Prospective study | II | 528 (178 vs. 179 vs. 171) | Pembrolizumab vs. Pembrolizumab vs. chemotherapy | 2 mg/kg/3 weeks vs. 10 mg/kg/3 weeks vs. standard dose | 528 | 0 | 0 | 0 |
| Caroline Robert et al., 2014 [12] | Prospective study | III | 418 (210 vs. 208) | Nivolumab vs. Dacarbazine |
3 mg/kg/2 weeks vs. standard dose | 308 (153 vs. 155) | 5 | Hypotension 1 vs. 4 | 0 |
| Jeffrey S Weber et al., 2015 [13] | Prospective study | III | 370 (268 vs. 102) | Nivolumab vs. ICC (Dacarbazine al) | 3 mg/kg/2 weeks vs. standard dose | 362 (181 vs. 81) | 0 | 0 | 0 |
| Paolo A Ascierto et al., 2017 [14] | Prospective study | III | 726 (364 vs. 362) | Ipilimumab | 10 mg/kg/4 doses/3 weeks vs. 3 mg/kg/4 doses/3 weeks | 514 (286 vs. 228) | 3 | Hypertension 1 vs. 0; Heart arrest 1 vs. 0; Pericarditis 1 vs. 0 | 3 |
| F Stephen Hodi et al., 2016 [15] | Prospective study | II | 142 (95 vs. 47) | Nivolumab + Ipilimumab vs. Ipilimumab + placebo | 1 mg/kg + 3 mg/kg/4 doses/3 weeks vs. 3 mg/kg + placebo/4 doses/3 weeks | 140 (94 vs. 46) | 7 | Hypotension 3 vs. 0; Ventricular arrhythmia 1 vs. 0; Ventricular tachycardia 1 vs. 0; Atrial fibrillation 1 vs. 0; Myocardial infarction 1 vs. 0 | 5 |
| Caroline Robert et al., 2015 [16] | Prospective study | III | 834 (278 vs. 277 vs. 256) | Pembrolizumab vs. Pembrolizumab vs. Ipilimumab | 10 mg/kg/2 weeks/doses vs. 10 mg/kg/3 weeks/ doses vs. 3 mg/kg/3 weeks/4 doses | 610 (221 vs. 202 vs. 187) | 4 | Hypertension 3 vs. 1 vs. 0 |
2 |
| J. Weber, M. et al., 2017 [17] | Prospective study | III | 906 (453 vs. 453) | Nivolumab vs. Ipilimumab | 3 mg/kg/4 doses/2 weeks vs. 10 mg/kg/4 doses/3 weeks | 884 (438 vs. 446) | 0 | 0 | 0 |
| J.D. Wolchok et al., 2017 [18] | Prospective study | III | 937 (313 vs. 313 vs. 311) | Nivolumab + Ipilimumab vs. Nivolumab + p vs. Ipilimumab + p p(placebo) |
1 mg/kg+3 mg/kg /3 weeks/4 doses vs. 3 mg/kg/2 weeks + placebo vs. 3 mg/kg/3 weeks/4 doses + placebo |
847 (300 vs. 279 vs. 268) | 0 | 0 | 0 |
| Jedd D Wolchok et al., 2010 [19] | Prospective study | II | 217 (73 vs. 72 vs. 72) | Ipilimumab | 10 mg/kg vs. 3 mg/kg vs. 0.3 mg/kg/3 weeks/4 doses | 115 (50 vs. 46 vs. 19) | 0 | 0 | 0 |
| Ines Pires da Silva et al., 2021 [20] | Retrospective study | NR (Not Reported) | 355 (193 vs. 162) | Ipilimumab + Nivolumab/Pembrolizumab/Atezolizumab vs. Ipilimumab | 3 mg/kg/3 weeks/4 doses + standard dose vs. 3 mg/kg/3 weeks/4 doses | 287 (163 vs. 124) | 1 (0 vs. 1) | Myocarditis 0 vs. 1 | 1 |
| Patrick Schöffski et al., 2022 [21] | Retrospective study | I/II | 255 (134 vs. 121) | LAG-3 inhibitor Ieramilimab vs. Ieramilimab + Spartalizumab |
Ieramilimab (escalating 1–15 mg/kg)/2 weeks or once/4 weeks vs. Ieramilimab + Spartalizumab q2w or q3w or q4w or Ieramilimab q2w + Spartalizumab q4w | 159 (75 vs. 84) | 0 | 0 | 0 |
| Alexander M.M. et al., 2020 [22] | Prospective study | III | 1011 (509 vs. 502) | Pembrolizumab vs. placebo | 200 mg/3 weeks for 18 doses | 235 (190 vs. 45) | 1 (1 vs. 0) | Myocarditis 1 vs. 0 | NR |
| Omid Hamid et al., 2013 [23] | Prospective study | I | 135 (57 vs. 56 vs. 22) | Lambrolizumab | 10 mg/kg/2 weeks vs. 10 mg/kg/3 weeks vs. 2 mg/kg/3 weeks | 132 (55 vs. 55 vs. 22) | 7 (2 vs. 4 vs. 1) | Hypertension (2 vs. 4 vs. 1) | NR |
| Margaret K. et al., 2018 [24] | Retrospective study | I | 94 (53 vs. 41) | Ipilimumab + Nivolumab Nivolumab (Niv) Ipilimumab (Ipi) |
Niv+Ipi(escalating doses)/3 weeks for four doses, followed by Niv 3 weeks for four doses, then Niv + Ipi/12 weeks for eight doses vs. Niv 1 mg/kg + Ipi 3 mg/kg/3 weeks for 4 doses, followed by Niv 3 mg/kg/2 weeks |
87 | 0 | 0 | 0 |
| Ulrich Keilholz et al., 2019 [25] | Prospective study | I | 51 | Avelumab | 10 mg/kg for one-hour intravenous infusion/2 weeks | 39 | 0 | 0 | 0 |
| Hussein A et al., 2022 [26] | Retrospective study | II-III | 714 (355 vs. 359) | Relatlimab + Nivolumab vs. Nivolumab | Relatlimab 160 mg + Nivolumab 480 mg vs. Nivolumab 480 mg | 504 (288 vs. 216) | 0 | 0 | 0 |
3. Cardiotoxicity in Lung Cancer
A total of 11 studies [27][28][29][30][31][32][33][34][35][36][37] included 5404 patients on ICIs, and 101 developed CAEs for an incidence of 0.15–37.78% in which grade 3–5 CAEs accounted for 55.4%. Commonly encountered cardiotoxicities included arrhythmia (32.7%), cardiac-related chest pain (24.8%), elevated cTnI or myocarditis (23.8%), cardiomyopathy (20.8%), pericardial disease (11.9%), and acute coronary syndrome (10.9%). One study indicated that major adverse cardiovascular events (MACEs) were dose-independent of nivolumab and pembrolizumab in lung cancer patients [27]. Those treated with a higher dose of durvalumab, particularly 10 mg/kg × 4 doses/2 weeks, were more prone to fatal adverse events such as a cardiac arrest and cardiogenic shock [31]. One patient treated with pembrolizumab at 10 mg/kg for 3 weeks underwent a myocardial infarction, which led to death (Table 2) [33].
| Author, Year | Study Type | Phase | Sample Size | Drug | Dose and Frequency | Non-CAE | CAE | Manifestation | 3–5 Grade CAE |
|---|---|---|---|---|---|---|---|---|---|
| Kalyan R et al., 2019 [37] | Retrospective study | NR | 252 (117 vs. 135) | Non-ICI vs. ICI (Nivolumab/Pembrolizumab) Nivolumab (Niv) Pembrolizumab (Pem) |
Standard dose vs. increasing dose (Niv < 540 mg; 540~1440 mg; > 1440 mg Pem < 600 mg; 600~1707 mg; >1707 mg) | NR | 93 (42 vs. 51) | Arrhythmia 31 vs. 25; Cardiac-related chest pain 12 vs. 25; Valvular heart disease 4 vs. 2; Cardiomyopathy 13 vs. 20; Myopericardial disease 11; Pericardial disease 8; Myocarditis 1; Valvular-disease 2; Venous arterial thromboembolic events 8 | 40 (major CAE) |
| Scott N et al., 2015 [38] | Prospective study (NSCLC) | I | 129 (33 vs. 37 vs. 59) | Nivolumab | 1 mg/kg vs. 3 mg/kg vs. 10 mg/kg intravenously/2 weeks in 8-week cycles for up to 96 weeks. | 91 (21 vs. 25 vs. 45) | 0 | 0 | 0 |
| Tony S K Mok et al., 2019 [39] | Prospective study (NSCLC) | III | 1251 (636 vs. 615) | Pembrolizumab vs. platinum-based chemotherapy | 200 mg/3 weeks for up to 35 cycles vs. platinum-based chemotherapy for four to six cycles. | 1112 (515 vs. 597) | 1 (1 vs. 0) | Myocarditis 1 vs. 0 | 1 |
| Achim Rittmeyer et al., 2017 [40] | Prospective study (NSCLC) | III | 1187 (609 vs. 578) | Atezolizumab vs. Docetaxel | 1200 mg/3 weeks vs. 75 mg/m2/3 weeks | 886 (390 vs. 496) | 0 | 0 | 0 |
| S.J. Antonia et al., 2017 [41] | Prospective study (NSCLC) | III | 718 (475 vs. 234) | Durvalumab vs. Placebo |
10 mg/kg/2 weeks for up to 12 months vs. placebo |
421 (301 vs. 120) | 26 (21 vs. 5) | ACS 9 vs. 2; Arrhythmia 7 vs. 1; Heart failure 7 vs. 0; Cardiac arrest 2 vs. 1; Cardiogenic shock 1 vs. 0; Cardiomyopathy 1 vs. 0; Myocarditis 0 vs. 1; Pericardial effusion 2 vs. 0 | NR |
| Yuequan Shi et al., 2021 [42] | Observational study (NSCLC/SCLC) | NR | 1905 (1162 vs. 743) (598 vs. 455 vs. 273 vs. 176 vs. 125 vs. 81 vs. 62 vs. 34 vs. 23) |
ICI (Pembrolizumab/Nivolumab/Camrelizumab/Treprizumab/Tisilizumab/Atezolizumab/Durvalumab/Ipilimumab) only vs. combination therapy | at least one dose | 647 | 22 (22 vs. 0) | Elevated cTnI or myocarditis 22 | 9 |
| Roy S Herbst et al., 2016 [43] | Prospective study (NSCLC) | II/III | 991 (339 vs. 343 vs. 309) | Pembrolizumab vs. Docetaxel | Pem 2 mg/kg, Pem 10 mg/kg vs. Docetaxel 75 mg/m2/3 weeks | 690 (215 vs. 225 vs. 250) | 1 (0 vs. 1 vs. 1) | Myocardial infarction 0 vs. 1 vs. 0; Acute cardiac failure 0 vs. 0 vs. 1 | 1 |
| Martin Reck et al., 2016 [44] | Prospective study (NSCLC) | III | 304 (154 vs. 150) | Pembrolizumab vs. platinum-based chemotherapy |
200 mg/3 weeks vs. standard dose | 52 (45 vs. 7) | 0 | 0 | 0 |
| H. Borghaei et al., 2015 [45] | Prospective study (NSCLC) | III | 555 (278 vs. 268) | Nivolumab vs. Docetaxel | 3 mg/kg/2 weeks vs. 75 mg/m2/3 weeks | 432 (196 vs. 236) | 3 (3 vs. 0) | Cardiac tamponade 1 vs. 0; Pericardial effusion 1 vs. 0 Tachycardia 1 vs. 0 |
3 |
| Julie Brahmer et al., 2015 [46] | Prospective study (NSCLC) | III | 272 (135:137) | Nivolumab vs. Docetaxel | 3 mg/kg/2 weeks vs. 75 mg/m2/3 weeks. | 187 (76 vs. 111) | 0 | 0 | 0 |
| D.P. Carbone et al., 2017 [47] | Prospective study (NSCLC) | III | 530 (267 vs. 263) | Nivolumab vs. Chemotherapy(platinum-based) | 3 mg/kg/2 weeks vs. standard dose for six cycles. | 431 (188 vs. 243) | 2 (2 vs. 0) | Myocardial infarction 1 vs. 0; Pericardial effusion malignant 1 vs. 0 | 2 |
4. Renal Cell Carcinoma
In seven studies [48][49][50][51][52][53][54] comprising 1971 patients with renal cell carcinomas on ICIs, 14 developed CAEs with an incidence of 0.20–2.19% in which grade 3–5 CAEs accounted for 35.7%. Commonly encountered cardiotoxicities included hypertension (85.7%) and myocarditis (7.1%). Treatment-related hypertension was linked to a nivolumab plus ipilimumab therapy (100%). Compared with melanomas and lung cancer, the ICI therapy caused mild cardiotoxicity in renal cell carcinomas. Fatal CAEs were not found.
5. Urothelial Carcinoma
In Seven studies [55][56][57][58][59][60][61] 111 of 2550 patients with urothelial carcinomas on ICIs developed CAEs with an incidence of 0.22–10.60% in which grade 3–5 CAEs accounted for 52.3%. Commonly encountered cardiotoxicities included hypertension (28.8%), arrhythmia (14.4%) and hypotension (6.3%). The fluctuation of blood pressure was linked to treatment with atezolizumab. Hypertension was observed in 21 patients and hypotension was observed in 7 after application of atezolizumab. Patients treated with 200 mg pembrolizumab for 3 weeks (maximum 35 cycles) or at 1200 mg every three weeks were more prone to fatal adverse events such as a cardiac arrest.
6. Other Types of Cancer
The most commonly encountered ICIs-related type of cardiotoxicity in hematological malignancies was hypertension [62][63][64][65]. In other cancers, such as hepatocellular carcinomas and malignant pleural mesotheliomas, the relevant research did not present many cases [66][67][68][69][70][71]; these were almost all case reports of myocarditis [72][73][74].
This entry is adapted from the peer-reviewed paper 10.3390/jcdd9070203
This entry is offline, you can click here to edit this entry!
