Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Urinary bladder neuroendocrine neoplasms (NENs) are classified into well-differentiated NENs, small-cell NENs, large-cell NENs, and paragangliomas.
- neuroendocrine tumors
- female neuroendocrine neoplasms
1. Epidemiology, Presentation, and Pathogenesis
Urinary bladder neuroendocrine neoplasms (NENs) are classified into well-differentiated NENs, small-cell NENs, large-cell NENs, and paragangliomas (Table 1) [1]. They comprise <1% of the urinary bladder tumors and are more common in males with a male-to-female ratio of 3.3:1.0 [2][3]. A small-cell variant is the common NENs of the urinary bladder, with an estimated 500 cases per year [2]. It constitutes 0.3–0.7% of primary bladder malignancies [4]. Small-cell neuroendocrine cancer (NEC) of the urinary bladder was first described by Cramer et al. in 1981 [4]. Note that 88% of small-cell NEC patients presented with hematuria in a study by Cheng et al. [3]. Around 60% of patients are diagnosed with metastatic disease at diagnosis [5]. Cigarette smoking is a risk factor in 50–70% of small-cell bladder NECs [6]. Additionally, small-cell NEC is predominant in the sixth to seventh decades of life. In contrast, well-differentiated NENs of the urinary bladder are rare and are usually seen in middle-aged individuals; there is a slight predominance in males [7][8]. Large-cell NEC constitutes <0.5% of all the bladder urothelial carcinomas and is usually reported in male (77.1% vs. 23%), elderly patients (>60 years) [9][10][11]. In the urinary tract, the bladder is the most common site for the origin of large-cell NEC [12][13]. The urinary bladder (79.2%), followed by urethra (13%), pelvis (5%), and ureter (3%) are the most common sites of paraganglioma in the genitourinary tract [14][15]. The first case of urinary bladder paraganglioma was reported in 1953 by Zimmerman et al. [16]. Bladder paragangliomas account for 0.05–0.1% of all bladder tumors and 6% of paragangliomas [17][18]. Females are three times more commonly affected than males [19]. Functional paragangliomas secrete catecholamines resulting in hypertension, diaphoresis, headache, palpitations, and post-micturition syncope [20]. The characteristic triad of paroxysmal or sustained hypertension, gross hematuria, and micturition syncope can be observed in >75% of patients [21][22].
Table 1. Histological characteristics of urinary bladder NENs [2].
| Histological Features | Well-Differentiated NET | Small-Cell NEC | Large-Cell NEC | Paraganglioma |
|---|---|---|---|---|
| Cellular arrangements | Anastomosing cords; Glandular; Cribriform structures | Diffuse sheets; Nests | Sheets; Solid nests; Trabeculae; Rosettes | Nests; Diffuse growth; Pseudo rosettes |
| Cellular characteristics | Intermediate cuboidal/columnar monomorphic cells with moderate to abundant cytoplasm and eosinophilic granules | Small/intermediate fusiform cells with scant cytoplasm | Large polygonal cells with abundant cytoplasm | Large polygonal cells with moderate cytoplasm |
| Nuclei | Small round to oval nuclei with finely stippled chromatin and inconspicuous nucleolus | Small round to oval nuclei with finely granular chromatin, molding and crush artifact; Inconspicuous salt and pepper nucleolus | Large oval nuclei with coarse, granular, vesicular chromatin and prominent nucleolus | Medium round to oval nuclei with smudged, hyperchromatic chromatin and prominent nucleolus |
| Additional findings | Infrequent mitotic activity and absent necrosis | High mitotic activity and foci of necrosis; Lymphovascular invasion | Very high mitotic activity and large areas of necrosis | Rare mitotic activity and necrosis |
Well-differentiated NENs are derived from the neuroendocrine cells in the normal urothelial and reactive urothelial basement membrane. There are three proposed theories to explain the cells of origin of small-cell urinary bladder NENs: (i) From undifferentiated and multipotent cells or stem cells in the urothelium. This theory supports the frequent co-existence of small-cell NEC and urothelial carcinoma. (ii) From neuroendocrine cells of the regular or metastatic urothelium and (iii) from undefined submucosal neuroendocrine cells [23]. While 10–30% of patients present with a pure form of small-cell NEC, around 70–90% of patients, small-cell carcinoma coexists with adenocarcinoma, squamous cell carcinoma, or urothelial carcinoma [3][24]. Large-cell NEC of the urinary bladder can occur as a pure variant or associated with squamous cell carcinoma, carcinosarcoma, primary adenocarcinoma of the bladder, or urothelial carcinoma [25]. Pheochromocytomas at the extra-adrenal sites are termed paragangliomas. They arise from the sympathetic chain in the detrusor muscle and may occur anywhere in the urinary bladder [21][22]. Although most of the cases are sporadic, a few cases of bladder paraganglioma are associated with hereditary syndromes, such as neurofibromatosis, Sturge–Weber syndrome, von Hippel–Lindau syndrome, tuberous sclerosis, and the Carney triad (pulmonary chondromas, paragangliomas, and gastrointestinal stromal tumors) [26].
2. Imaging
Well-differentiated NENs of the urinary bladder appear as polyps or submucosal nodules with occasional inflammatory changes in cystoscopy (Figure 1) [7]. Most of the tumors are small, ranging from 2 to 10 mm, and involve neck and trigone regions of the bladder [7]. The CT and MR imaging of a small-cell NEC of the urinary bladder shows a broad-based polypoid mass with or without necrotic and cystic areas (Figure 2 and Figure 3). The mass displays patchy enhancement with contrast administration and exhibits invasion of the entire thickness of the bladder wall at the time of diagnosis. Bladder wall invasion is typical in tumors ranging from 3 to 8 cm in size. Kim et al. reported that the small-cell NEC of the urinary bladder is hypointense on both T1 and T2-weighted MR imaging due to the high tumor cellularity [27]. The aggressiveness of small-cell NEC of the urinary bladder is reflected in its adjacent structural invasion of the vagina, uterus, ureters, peritoneum, and abdominal muscles. Lymph node involvement can be noticed in 66% of the patients with distant metastases in the lung, liver, and bone [26][27].
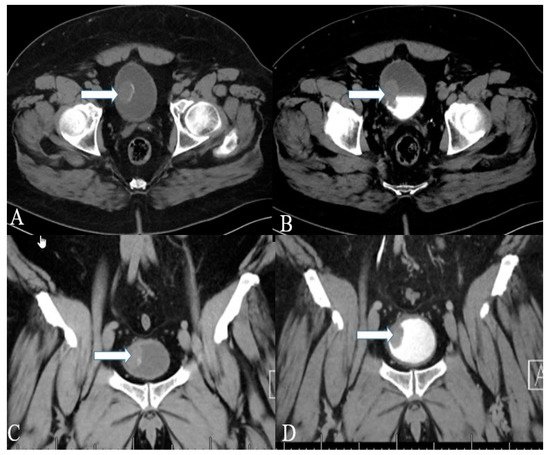
Figure 1. A 40-year-old female with urinary bladder NENs. Axial CT (A) pre-contrast and (B) delayed post-contrast images with coronal reconstruction (C) pre-contrast and (D) post-contrast reveal a 4.8 × 2.5 cm mass (arrows) at the proper aspect of the urinary bladder, showing faint peripheral calcification. Pathology revealed a neuroendocrine tumor of the urinary bladder.
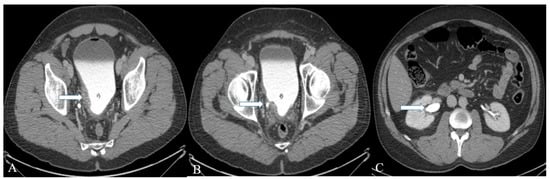
Figure 2. A 32-year-old female with urinary bladder NENs. Axial CT post-contrast (A) shows soft tissue thickening (arrow) mounting to mass formation involving the right lateral and posterior urinary bladder wall. (B,C) It is seen narrowing (arrow in (B)) the right ureterovesical junction with secondary mild right hydroureteronephrosis (arrow in (C)). This mass seems to infiltrate the perivesical fat. A catheter balloon is seen within the bladder lumen. Pathology of the group revealed urothelial carcinoma with neuroendocrine features and focal glandular differentiation, high grade.
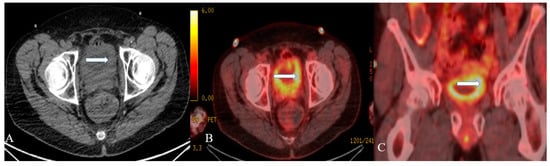
Figure 3. A 48-year-old female with urinary bladder NENs. Axial CT without contrast (A), axial (B), and coronal (C) reconstruction fused PET/CT images show thickened urinary bladder wall (arrows), which is most evident on the left side. The maximum thickness measures 2.4 cm and has an SUV of 8.2. Pathology revealed small-cell neuroendocrine carcinoma of the urinary bladder.
Paraganglioma appears as a sub-mucosal homogeneous or heterogeneous hyper-enhancing lobulated mass on contrast-enhanced CT. A ring-like peripheral calcification is highly suggestive of a paraganglioma [28]. MR imaging is superior to CT and demonstrates low-signal intensity on T1 and moderately high signal intensity on T2-weighted imaging sequences [26]. Multiple signal voids with hyperintense foci can be seen on MR imaging, resulting in a “salt-and-pepper appearance” [17]. The enlarged feeding arteries and intense tumor blush are observed on angiography. Iodine-131 metaiodobenzylguanidine (MIBG) scan has higher specificity (96%) than CT or MRI to detect paraganglioma and aids in evaluating metastatic disease [26]. At the same time, MR imaging (88%) is more sensitive than CT (64%) or iodine-131 MIBG (64%) when identifying the extra-adrenal paragangliomas [29]. Metastatic lymph nodes are visualized by PET scan with 6-[18F] fluorodopamine scan, which is superior to iodine-131 MIBG [30]. Most of the tumors are benign, whereas 10–15% of tumors exhibit malignant behavior [31].
Immunohistochemistry reveals positivity for synaptophysin, chromogranin A, and cytokeratin AE1–AE3 [32]. Diagnosis of small-cell NEC of the urinary bladder is based on WHO diagnostic criteria for small-cell lung carcinoma, which include morphological characteristics (Table 1) [33]. Small-cell NEC of the urinary bladder stain positive for chromogranin, CD57 CD56, synaptophysin, TTF-1, neuron-specific enolase, CAM5.2, keratin7, and the epithelial membrane antigen GATA3 in immunohistochemical analysis. A dot-like reactivity to CAM5.2 can also be noticed. Synaptophysin (64.3%) and CD56 (71%) have higher sensitivity than chromogranin A (29%) in the tumor differentiation [34]. Up to 33–39% of small-cell NECs of urinary bladder demonstrate positive staining for TTF1 [35][36]. The paraganglioma appears as a well-circumscribed nodule on gross examination and shows cells arranged in the Zellballen pattern on microscopic examination (Table 1) [37]. Immunohistochemistry of the tumor reveals positive synaptophysin and chromogranin staining in chief cells, and positive S-100 and negative cytokeratin staining in sustentacular cells. Negative cytokeratin differentiates paragangliomas from urothelial and carcinoid tumors, which usually stain positive for cytokeratin [17]. Paragangliomas with succinate dehydrogenase B mutations are likely to exhibit malignant behavior [1].
3. Prognosis and Management
The pure form of well-differentiated NENs of the urinary bladder is associated with good outcomes. Most of the tumors are so small that they can be wholly resected during a biopsy. Nonetheless, 25% of patients exhibit distant or regional lymph nodal metastases [22]. The stage is the most important prognostic factor in small-cell NEC. Five-year survival rates range from 8 to 16% in low–high stage disease (Figure 4) [3][32][38]. Patients with large-cell NEC present at late stages and have a median survival of 20–23 months [6]. The presence of features such as hypertension micturition attacks, presentation at a younger age, and invasion through the bladder wall, indicates a high potential for malignant progression [22]. The differentiation solely based on gross or histological characteristics is challenging; however, the deep tissue invasion, lymph node involvement, and distant metastases indicate the malignant activity of the tumor [37]. The staging of the urinary bladder malignancies is described in Table 2 and Figure 4. Chemotherapy with/without radiation is the preferred treatment, alongside surgery, in small-cell NEC of the urinary bladder. The standard treatment of bladder paragangliomas is surgical resection with appropriate pre-and post-operative adrenergic blockade to prevent a hypertensive crisis (Figure 5). The tumor can be contracted through transurethral or open/laparoscopic radical or partial cystectomy in conjunction with lymph node dissection [17][39]. Adjuvant radiation therapy can provide better survival for individuals enduring malignant tumors [18].
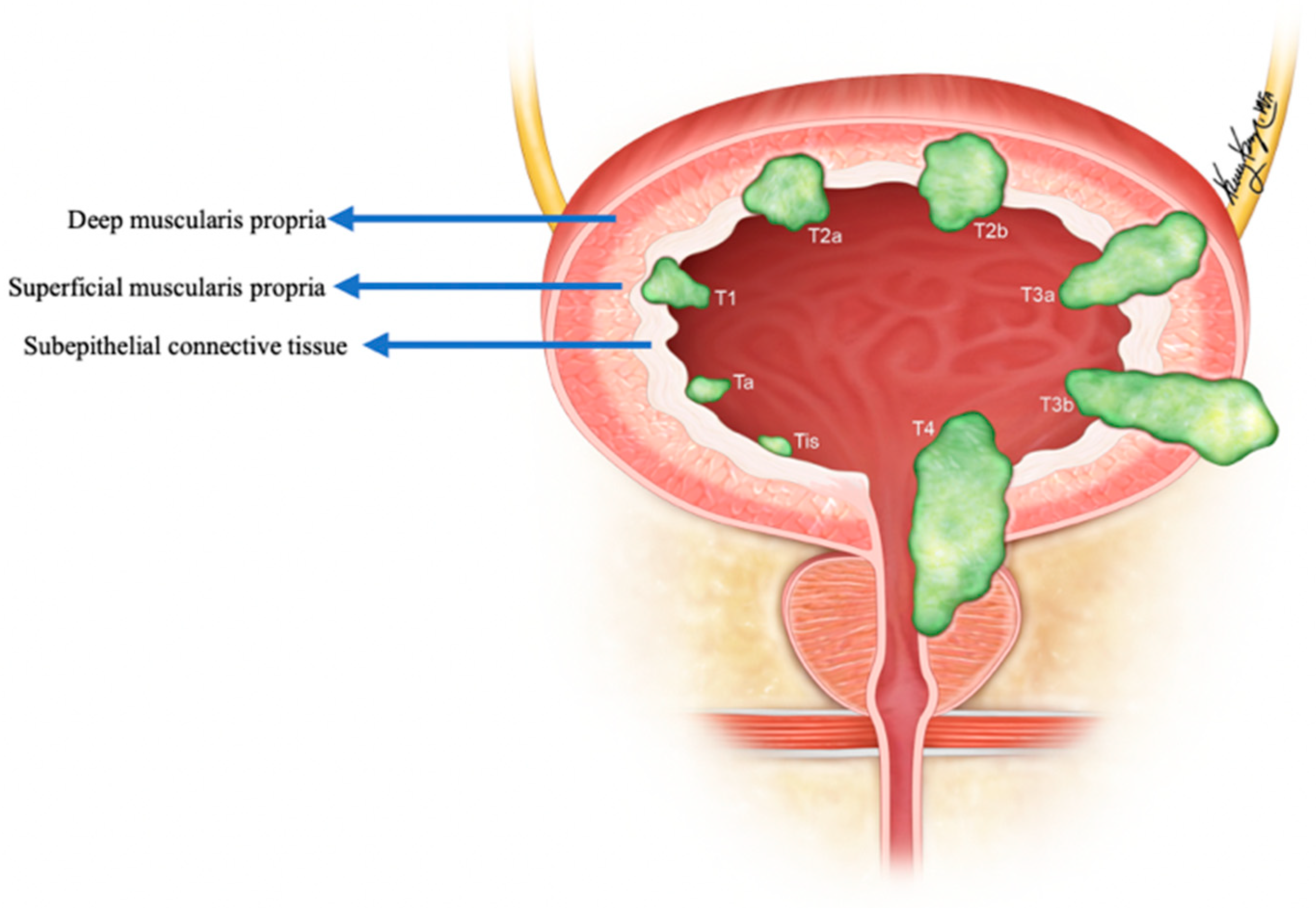
Figure 4. Staging of urinary bladder neuroendocrine carcinoma. Ta: non-invasive carcinoma; Tis: carcinoma in situ (Flat-tumor); T1: tumor invasion into subepithelial connective tissue; T2a: tumor invasion into superficial muscularis propria; T2b: umor invasion into deep muscularis propria; T3a: microscopic invasion of peri-vesical tissue; T3b: macroscopic invasion of peri-vesical tissue (extravesical mass); T4: tumor invasion of adjacent structures, pelvic or abdominal wall.
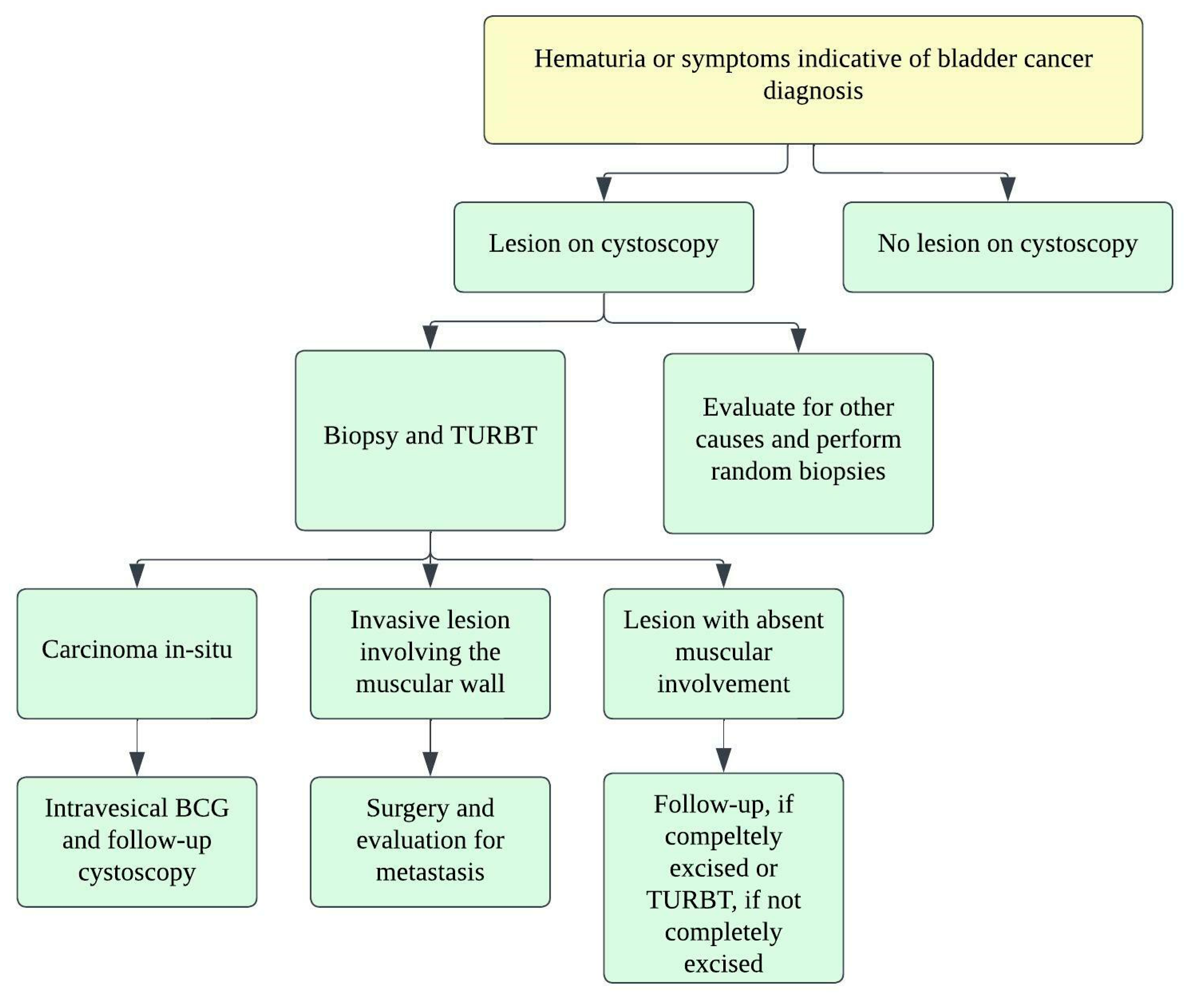
Figure 5. Treatment algorithm for urinary bladder carcinoma.
| Stage | TNM Category | Description |
|---|---|---|
| 0a | Ta N0 M0 | Non-invasive papillary carcinoma; No lymph node involvement; No distant metastasis |
| 0is | Tis N0 M0 | Carcinoma in-situ (“Flat-tumor”) |
| I | T1 N0 M0 | Tumor invasion into subepithelial connective tissue (lamina propria) |
| II | T2a N0 M0 | Tumor invasion into superficial muscularis propria (inner half of detrusor muscle) |
| T2b N0 M0 | Tumor invasion into deep muscularis propria (outer half of detrusor muscle) | |
| IIIA | T3a N0 M0 | Microscopic invasion of peri-vesical tissue |
| T3b N0 M0 | Macroscopic invasion of peri-vesical tissue (extravesical mass) | |
| T4a N0 M0 | Tumor invades any of: prostatic stroma, seminal vesicles, uterus, vagina | |
| T1-4a N1 M0 | Single regional lymph node involvement: peri-vesical, obturator, internal and external iliac, or sacral lymph nodes | |
| IIIB | T1-4a N2 or N3 M0 | N2–Multiple regional lymph node involvement; N3–Common iliac lymph node involvement |
| IVA | T4b Any N M0 | T4b–Tumor invasion into pelvic or abdominal wall |
| Any T Any N M1a | M1a–Distant metastases to lymph nodes beyond common iliac arteries | |
| IVB | Any T Any N M1b | M1b–Distant metastases to sites such as bones, liver or lungs |
This entry is adapted from the peer-reviewed paper 10.3390/cancers14133218
References
- Shehabeldin, A.N.; Ro, J.Y. Neuroendocrine tumors of genitourinary tract: Recent advances. Ann. Diagn. Pathol. 2019, 42, 48–58.
- Kouba, E.; Cheng, L. Neuroendocrine Tumors of the Urinary Bladder According to the 2016 World Health Organization Classification: Molecular and Clinical Characteristics. Endocr. Pathol. 2016, 27, 188–199.
- Cheng, L.; Pan, C.X.; Yang, X.J.; Lopez-Beltran, A.; MacLennan, G.T.; Lin, H.; Kuzel, T.M.; Papavero, V.; Tretiakova, M.; Nigro, K.; et al. Small cell carcinoma of the urinary bladder: A clinicopathologic analysis of 64 patients. Cancer 2004, 101, 957–962.
- Qayoom, S.; Chakrabarti, D.; Khan, F.; Goel, M.M. Primary small cell carcinoma of the urinary bladder. BMJ Case Rep. 2019, 12.
- Reyes, C.V.; Soneru, I. Small cell carcinoma of the urinary bladder with hypercalcemia. Cancer 1985, 56, 2530–2533.
- Sehgal, S.S.; Wein, A.J.; Bing, Z.; Malkowicz, S.B.; Guzzo, T.J. Neuroendocrine tumor of the bladder. Rev. Urol. 2010, 12, e197.
- Chen, Y.-b.; Epstein, J.I. Primary carcinoid tumors of the urinary bladder and prostatic urethra: A clinicopathologic study of 6 cases. Am. J. Surg. Pathol. 2011, 35, 442–446.
- Holmang, S.; Borghede, G.; Johansson, S.L. Primary small cell carcinoma of the bladder: A report of 25 cases. J. Urol. 1995, 153, 1820–1822.
- Sanguedolce, F.; Calo, B.; Chirico, M.; Tortorella, S.; Carrieri, G.; Cormio, L. Urinary Tract Large Cell Neuroendocrine Carcinoma: Diagnostic, Prognostic and Therapeutic Issues. Anticancer Res. 2020, 40, 2439–2447.
- Amin, M.B. Histological variants of urothelial carcinoma: Diagnostic, therapeutic and prognostic implications. Mod. Pathol. 2009, 22, S96–S118.
- Watson, G.A.; Ahmed, Y.; Picardo, S.; Chew, S.; Cobbe, S.; Mahony, C.; Crotty, J.; Wallis, F.; Shelly, M.J.; Kiely, P. Unusual sites of high-grade neuroendocrine carcinomas: A case series and review of the literature. Am. J. Case Rep. 2018, 19, 710.
- Choi, J.H.; Sol, Y.; Lee, S.W.; Jeh, S.U.; Hwa, J.S.; Hyun, J.S.; Chung, K.H.; Seo, D.H.; Yang, J.W.; Song, D.H.; et al. Primary large-cell neuroendocrine carcinoma of the upper ureter: A case report. Medicine 2019, 98, e15613.
- Oshiro, H.; Odagaki, Y.; Iobe, H.; Ozu, C.; Takizawa, I.; Nagai, T.; Matsubayashi, J.; Inagaki, A.; Miyake, S.; Nagao, T. Primary large cell neuroendocrine carcinoma of the ureter. Int. J. Clin. Exp. Pathol. 2013, 6, 729.
- Hanji, A.M.; Rohan, V.S.; Patel, J.J.; Tankshali, R.A. Pheochromocytoma of the urinary bladder: A rare cause of severe hypertension. Saudi J. Kidney Dis. Transplant. 2012, 23, 813.
- Dahm, P.; Gschwend, J.E. Malignant non-urothelial neoplasms of the urinary bladder: A review. Eur. Urol. 2003, 44, 672–681.
- Zimmerman, I.J.; Biron, R.E.; MacMahon, H.E. Pheochromocytoma of the urinary bladder. N. Engl. J. Med. 1953, 249, 25–26.
- Dwethi, G.; Vikas, C.; Ramesh, T. Urinary bladder paraganglioma. Appl. Radiol. 2019, 48, 48A–48C.
- Alberti, C. Urology pertinent neuroendocrine tumors: Focusing on renal pelvis, bladder, prostate located sympathetic functional paragangliomas. G. Chir. 2016, 37, 55.
- Yadav, R.; Das, A.K.; Kumar, R. Malignant non-functional paraganglioma of the bladder presenting with azotemia. Int. Urol. Nephrol. 2007, 39, 449–451.
- Sheps, S.G.; Jiang, N.S.; Klee, G.G.; van Heerden, J.A. Recent developments in the diagnosis and treatment of pheochromocytoma. Mayo Clin Proc 1990, 65, 88–95.
- Fine, S.W. Neuroendocrine lesions of the genitourinary tract. Adv. Anat. Pathol. 2007, 14, 286–296.
- Eble, J.N. Classification of tumours: Pathology and genetics of tumours of the urinary system and male genital organs. World Health Organ. Classif. Tumours 2004, 255–257.
- Cheng, L.; Jones, T.D.; McCarthy, R.P.; Eble, J.N.; Wang, M.; MacLennan, G.T.; Lopez-Beltran, A.; Yang, X.J.; Koch, M.O.; Zhang, S.; et al. Molecular genetic evidence for a common clonal origin of urinary bladder small cell carcinoma and coexisting urothelial carcinoma. Am. J. Pathol. 2005, 166, 1533–1539.
- Abrahams, N.; Moran, C.; Reyes, A.; Siefker-Radtke, A.; Ayala, A. Small cell carcinoma of the bladder: A contemporary clinicopathological study of 51 cases. Histopathology 2005, 46, 57–63.
- Akamatsu, S.; Kanamaru, S.; Ishihara, M.; Sano, T.; Soeda, A.; Hashimoto, K. Primary large cell neuroendocrine carcinoma of the urinary bladder. Int. J. Urol. 2008, 15, 1080–1083.
- Wong-You–Cheong, J.J.; Woodward, P.J.; Manning, M.A.; Sesterhenn, I.A. Neoplasms of the urinary bladder: Radiologic-pathologic correlation. Radiographics 2006, 26, 553–580.
- Kim, J.C.; Kim, K.H.; Jung, S. Small cell carcinoma of the urinary bladder: CT and MR imaging findings. Korean J. Radiol. 2003, 4, 130–135.
- Asbury, W., Jr.; Hatcher, P.; Gould, H.; Reeves, W.; Wilson, D. Bladder pheochromocytoma with ring calcification. Abdom. Imaging 1996, 21, 275–277.
- Jalil, N.D.; Pattou, F.N.; Combemale, F.; Chapuis, Y.; Henry, J.F.; Peix, J.L.; Proye, C.A. Effectiveness and limits of preoperative imaging studies for the localisation of pheochromocytomas and paragangliomas: A review of 282 cases. French Association of Surgery (AFC), and The French Association of Endocrine Surgeons (AFCE). Eur. J. Surg. 1998, 164, 23–28.
- Hwang, J.J.; Uchio, E.M.; Patel, S.V.; Linehan, W.M.; Walther, M.M.; Pacak, K. Diagnostic localization of malignant bladder pheochromocytoma using 6-18F fluorodopamine positron emission tomography. J. Urol. 2003, 169, 274–275.
- Halefoglu, A.M.; Miroglu, C.; Uysal, V.; Mahmutoglu, A. Malignant paraganglioma of the urinary bladder. Eur. J. Radiol. Extra 2006, 58, 53–58.
- Dhillon, J. Neuroendocrine Tumors of the Urinary Bladder and Molecular Features. In Neuroendocrine Tumors: Review of Pathology, Molecular and Therapeutic Advances; Nasir, A., Coppola, D., Eds.; Springer: New York, NY, USA, 2016; pp. 359–367.
- Zhao, X.; Flynn, E.A. Small cell carcinoma of the urinary bladder: A rare, aggressive neuroendocrine malignancy. Arch. Pathol. Lab. Med. 2012, 136, 1451–1459.
- Erdem, G.U.; Özdemir, N.Y.; Demirci, N.S.; Şahin, S.; Bozkaya, Y.; Zengin, N. Small cell carcinoma of the urinary bladder: Changing trends in the current literature. Curr. Med. Res. Opin. 2016, 32, 1013–1021.
- Jones, T.D.; Kernek, K.M.; Yang, X.J.; Lopez-Beltran, A.; MacLennan, G.T.; Eble, J.N.; Lin, H.; Pan, C.-X.; Tretiakova, M.; Baldridge, L.A. Thyroid transcription factor 1 expression in small cell carcinoma of the urinary bladder: An immunohistochemical profile of 44 cases. Hum. Pathol. 2005, 36, 718–723.
- Cheuk, W.; Kwan, M.Y.; Suster, S.; Chan, J.K. Immunostaining for thyroid transcription factor 1 and cytokeratin 20 aids the distinction of small cell carcinoma from Merkel cell carcinoma, but not pulmonary from extrapulmonary small cell carcinomas. Arch. Pathol. Lab. Med. 2001, 125, 228–231.
- Kairi-Vassilatou, E.; Argeitis, J.; Nika, H.; Grapsa, D.; Smyrniotis, V.; Kondi-Pafiti, A. Malignant paraganglioma of the urinary bladder in a 44-year-old female: Clinicopathological and immunohistochemical study of a rare entity and literature review. Eur. J. Gynaecol. Oncol. 2007, 28, 149–151.
- Choong, N.W.; Quevedo, J.F.; Kaur, J.S. Small cell carcinoma of the urinary bladder: The Mayo Clinic experience. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2005, 103, 1172–1178.
- Kempasiddaiah, G.M.; Thimmaiah, R.; Gurappa, M. A rare case of early pregnancy with paraganglioma of urinary bladder. Int. J. Curr. Res. Rev. 2014, 6, 15–20.
- Magers, M.J.; Lopez-Beltran, A.; Montironi, R.; Williamson, S.R.; Kaimakliotis, H.Z.; Cheng, L. Staging of bladder cancer. Histopathology 2019, 74, 112–134.
- Mirmomen, S.M.; Shinagare, A.B.; Williams, K.E.; Silverman, S.G.; Malayeri, A.A. Preoperative imaging for locoregional staging of bladder cancer. Abdom. Radiol. 2019, 44, 3843–3857.
This entry is offline, you can click here to edit this entry!
