2. COVID-19 Specific Dermatological Manifestations
2.1. Exanthematous (Morbilliform) Rash
Exanthematous rashes exist as a broad category, but are classically described as viral exanthems or drug-induced type IV hypersensitivity reactions [
46]. Morbilliform rash is the most common cutaneous reaction associated with COVID-19 infection [
23,
47,
48]. It accounts for 11% to 47% of all dermatological cases related to COVID-19 [
23,
48]. Additionally, retrospective studies and case series reported a higher rate of hospitalization (45% to 80%) in COVID-19 patients with morbilliform eruptions compared to COVID-19 patients without the rash [
23,
48,
49,
50,
51].
The temporal association between the onset of morbilliform rash and other COVID-19-related symptoms varies among patients. Morbilliform rashes tend to occur concurrently or after the emergence of other COVID-19-related symptoms [
23]. Morbilliform eruptions are associated with moderate to severe COVID-19 symptoms [
23,
49,
52,
53]. Among COVID-19 patients, the most common anatomical locations for morbilliform eruptions include the chest, abdomen, back, arms, and legs (
Figure 1A–C) [
23,
50,
52,
54].
Histological examinations of skin biopsies from patients with morbilliform rash and concomitant SARS-CoV-2 infection reveal spongiosis and perivascular inflammatory infiltrates. Such lesions may present with edematous thrombosed vessels surrounded by neutrophils and eosinophils [
13]. However, the SARS-CoV-2 spike protein has not been detected in biopsy samples [
13,
55,
56]. These findings suggest morbilliform eruptions are not directly caused by the virus but rather may be secondary to the inflammatory response of the infection [
55].
COVID-19-induced morbilliform exanthem treatment protocols are limited to those described in case reports. Most cases of morbilliform eruptions are self-resolving and do not require treatment. However, in severe symptomatic cases, treatment may be necessary for symptomatic relief (i.e., pruritus). For example, one case study reported a 58-year-old male who experienced complete resolution upon treatment with topical triamcinolone 0.1% cream [
57]. Another case reported positive treatment outcomes with the administration of topical corticosteroids (type unspecified) and oral antihistamines [
54].
2.2. Pernio (Chilblain)-like Acral Lesions
Chilblains-like lesions typically present as violaceous, erythematous to purpuric plaques with or without edema. While chilblains lesions can present in multiple body regions, these lesions are typically seen on the fingers or toes (
Figure 1D,E) [
58,
59]. Chilblains lesions were relatively rare before the COVID-19 pandemic, with approximately nine to ten cases reported annually between 2000 and 2011 [
60]. However, since the onset of the COVID-19 pandemic, cases of chilblains-like rashes have been on the rise. Multiple studies reported that chilblains-like eruptions comprised approximately 19% to 38% of all dermatological manifestations related to COVID-19 infections [
23,
48,
61].
The term “chilblains-like” lesion was coined to delineate skin lesions that mimicked primary chilblains in patients with COVID-19 infection. Primary chilblains develop after exposure to low temperatures. In contrast, many COVID-19 patients with “chilblains-like” lesions had no cold exposure or history of similar rash [
62]. Several mechanisms have been proposed to describe the pathogenesis of chilblains-like rashes. One prominent hypothesis suggests that during the active infection phase, Type 1 interferon promotes the production of cryofibrinogen. Cryofibrinogen is an acute phase reactant that can induce perniosis at acral sites [
60].
Histopathological examinations of COVID-19 chilblains-like lesions reveal the presence of superficial and deep lymphocytic inflammatory infiltrates in a lichenoid, perivascular, and perieccrine pattern [
13,
60,
63,
64,
65]. Additionally, SARS-CoV-2 spike proteins were detected in the endothelium of blood vessels in some samples but absent in others [
66,
67,
68]. This discrepancy likely demonstrates complex relationships with the SARS-CoV-2 spike protein and the resulting inflammatory cascade. These findings indicate a direct causal relationship between SARS-CoV-2 and chilblains-like eruptions [
69,
70].
Among COVID-19 patients, chilblains-like rashes typically spontaneously resolve within two to eight weeks [
23,
24]. However, underlying persistent inflammation may have contributed to prolonged cases lasting over six months [
71]. Patients should avoid cold exposure to prevent flare-ups. In refractory cases, corticosteroids, or calcium channel blockers (e.g., nifedipine) can provide therapeutic relief [
72,
73].
2.3. Urticaria
Urticaria is characterized by well-circumscribed, edematous, raised, pruritic, and erythematous plaques (
Figure 1F) [
74]. Causes of urticaria include but are not limited to allergens, insects, medications, and infections [
75]. In addition, viruses such as rhinovirus, rotavirus, hepatitis A, hepatitis B, and Epstein-Barr virus (EBV) are known to trigger urticarial eruptions [
75]. The pathogenesis of urticaria involves the degranulation of mast cells or basophils and the release of histamine in the upper dermis. Histamine released in the deeper dermis increases vasculature permeability, leading to angioedema [
76].
SARS-CoV-2 infection appears to induce urticaria [
48,
77,
78,
79]. Several international studies report urticaria among 8–19% of skin lesions related to COVID-19 [
23,
48,
80]. Several studies proposed associations between the severity of urticarial eruptions and COVID-19 infections, but the data are mixed. Casas et al. reported high morbidity and mortality in a subset of their cohort (2%) with urticaria. However, Dastoli et al. demonstrated that COVID-19 patients with urticaria have a better prognosis, potentially due to eosinophilia [
81,
82]. Currently, the protective mechanism of eosinophilia against COVID-19 is unknown [
83,
84].
Histological findings of urticarial eruptions related to COVID-19 are nonspecific but consistent with viral urticarial exanthems. Such findings consist of papillary dermal edema and mild perivascular lymphocytic infiltrate with some eosinophils, though neutrophilia predominate in early urticaria [
13]. One case that appeared clinically consistent with urticaria had histopathologic findings comparable to erythema multiforme, with slight vacuolar interface dermatitis and occasional necrotic basal keratinocytes [
13]. Given the uncertain clinicopathological mechanism, biopsies of future urticarial eruptions in the setting of COVID-19 should be considered for further evaluation [
85].
Urticarial eruptions associated with SARS-CoV-2 infection have variable presentations. One case reported a 27-year-old woman who developed angioedema and urticarial rash one week after confirmed diagnosis of SARS-CoV-2 infection [
86]. The urticaria persisted for 12 weeks despite treatment with cetirizine 20 mg twice daily [
86]. Other studies have reported urticaria as the initial or only clinical sign in several patients with COVID-19 infection [
48,
87,
88,
89]. As such, physicians should recognize symptoms of urticaria and fever in patients to limit the spread of COVID-19 [
90].
The primary treatment of urticaria in patients with COVID-19 includes second-generation oral antihistamines. One case report depicts the resolution of a unilateral upper-extremity urticarial rash within 24 h after treatment with oral antihistamines and topical corticosteroids [
91]. Other investigations have proposed low-dose systemic corticosteroids as a viable treatment option, though more clinical data are needed [
92].
2.4. Livedo Reticularis
Livedo reticularis (LR) is distinguished as a transient or persistent vascular, violaceous, net-like skin discoloration (
Figure 1G). LR occurs secondary to physiological or pathological reduction in blood flow to the skin [
93]. According to the AAD’s Dermatology Registry, livedo reticularis comprised 5.3% of confirmed COVID-19 cases with cutaneous manifestations [
23]. Histologic findings of livedo reticularis in a patient with confirmed COVID-19 revealed pauci-inflammatory thrombotic vasculopathy [
23]. However, most reported cases of livedo reticularis in COVID-19 patients were mild, transient, and without thromboembolic complications [
23].
Limited reports depict the treatment of LR in patients with COVID-19. One case study described a patient who developed LR on the trunk and bilateral proximal upper extremities following COVID-19 infection. Therapy with acetaminophen, heparin, hydroxychloroquine, and oxygen resolved LR in the patient [
94]. In contrast, other cases of COVID-19-related LR have resolved spontaneously [
95]. Additional research is needed to elucidate the best management strategies for these patients.
2.5. Livedo Racemosa/Retiform Purpura
Livedo racemosa and retiform purpura are vaso-occlusive lesions of the superficial microvasculature. These lesions are associated with elevations in d-dimer and disseminated intravascular coagulopathy (DIC), as seen in those with severe COVID-19 infections [
96,
97]. Rather than the fine net-like pattern of livedo reticularis [
98]. In contrast to the transient nature of livedo reticularis, livedo racemosa presents as a violaceous mottling of the skin in a disorganized pattern or broken circular segments. Livedo racemosa is pathologic, persistent, and associated with increased severity of ischemia. Differentiation between the two lesions is often distinguished based on pattern, histological examination, and location on the body. In terms of anatomical location, livedo racemosa is more commonly found on the trunk, limbs, and buttocks [
93]. Upon biopsy with immunochemical staining, these vaso-occlusive lesions demonstrate evidence of complement activation with immunoglobulins and microthrombi within the vasculature of the superficial and mid-dermis [
96].
According to the AAD Data Registry, retiform purpura and livedo racemosa presented in 6.4% and 2.3% of COVID-19 patients with dermatological conditions, respectively [
23]. These lesions correlate with increased severity of COVID-19 infection and mortality. One hundred percent of patients with documented retiform purpura were hospitalized, and 82% developed acute respiratory distress syndrome (ARDS) [
23,
96]. These vaso-occlusive conditions are consistent with the highest mortality rate of all cutaneous manifestations at 18.2%, with urticaria-like lesions ranking the lowest at 2.2% [
99]. Management incorporates treatment to address underlying pathology, anticoagulation, a biopsy of skin lesions, and wound care [
100].
Purpuric pressure ulcers have been observed during the COVID-19 pandemic on the back, buttocks, and other pressure-dependent locations [
97]. In a case series by Chand et al., a biopsy of lesions presented with no evidence of thrombotic vasculopathy. The mechanism of necrosis may have been due to pressure occlusion rather than inflammation or thrombosis. Additionally, those with purpuric pressure ulcers appeared with no laboratory evidence of DIC [
96,
97].
2.6. Vesicular (Varicella-like) Eruptions
Vesicular eruptions are among the most common COVID-19-associated dermatological manifestations. International case series and retrospective cohort studies identified varicella-like rashes in 9% to 13% of COVID-19 patients with cutaneous eruptions [
23,
48,
101,
102]. In relation to other specific COVID-19 symptoms, the onset of varicella-like rashes varies among patients. The majority of lesions appear three days after systemic symptoms such as fever, cough, and fatigue [
48,
102]. On average, resolution occurs by day eight without residual scarring [
23,
48,
102]. However, a minority of patients developed vesicular lesions prior to the onset of COVID-19 symptoms [
23,
48].
Varicella-like eruptions are classified into two morphological patterns: (1) diffuse (more common) and (2) localized [
103]. The diffuse pattern consists of small papules, vesicles, and pustules of varying sizes. These lesions appear at different stages simultaneously, starting on the trunk (most commonly) with further spreading to palms, soles, and other corporal areas [
103]. The localized monomorphic pattern typically involves the trunk and back (
Figure 1H) [
103]. In contrast to the diffuse pattern, varicella-like lesions associated with the localized pattern are monomorphic and appear at the same stage of evolution. These lesions affect one central area involving the chest, upper abdomen, or back [
103].
Similar to varicella-exanthems, varicella-like eruptions associated with COVID-19 can be diffuse or localized and also predominantly involve the trunk [
96,
102]. Unlike true varicella exanthems, most cases of varicella-like eruptions related to COVID-19 have been documented as non- or mildly pruritic [
96,
102].
Histological findings of varicella-like lesions vary between patients. Most studies report histological patterns consistent with viral exanthems, such as vacuolar degeneration of the basal layer with multinucleate, hyperchromatic keratinocytes, and dyskeratotic cells [
102,
104]. Contrastingly, other studies have documented the presence of non-ballooning acantholysis with eosinophilic dyskeratosis [
105]. Despite the histological variations, the consistent clinical morphology and presentation of these lesions assists in identifying patients with COVID-19 infection [
103].
The data surrounding the treatment of varicella-like eruptions in the literature is limited. A case series (
n = 22) presented by Marzano et al. demonstrated lesions spontaneously resolved within eight days of systemic symptoms [
103]. At this time, watchful waiting is recommended as an effective treatment.
2.7. Papulosquamous Rashes and Pityriasis Rosea
Pityriasis rosea (PR) manifests as typical papulosquamous eruptions characterized by ovoid and raised scaly patches on the body [
96]. Classically, pityriasis rosea presents as a solitary lesion (herald patch) and progresses along the Langer lines as a generalized rash over the trunk and limbs [
106]. Papulosquamous eruptions comprised 9.9% of cutaneous manifestations related to COVID-19 in the AAD Dermatology Registry [
23]. Additional studies have documented an increased incidence of PR-like lesions in association with SARS-CoV-2 infection [
107,
108,
109,
110,
111].
However, cases of PR in patients with COVID-19 infection may not present with the classic herald patch. In one case report, a 49-year-old female patient with COVID-19 pneumonia developed a papulosquamous rash resembling pityriasis rosea three days prior to the development of COVID-19-specific symptoms. Supportive treatment improved her respiratory and dermatological symptoms on day 5 of hospitalization [
112]. In another case report, Sanchez et al. documented an atypical digitate papulosquamous variant in an elderly patient (age not specified) with a confirmed diagnosis of COVID-19. The lesions were clinically similar to pityriasis rosea, however, without the presence of a herald patch [
113]. The rash resolved within one week. However, the patient expired due to an infection-related sequelae.
The specific etiology of papulosquamous eruptions in patients with COVID-19 remains unknown. It is postulated that increased inflammatory cytokines may contribute to rash development [
113]. Other potential causes include viral reactivation of Human Herpes Virus-6 and 7 (HHV-6 and 7) [
114], direct SARS-CoV-2 spike protein infection of the endothelium of dermal blood vessels [
115], or viral EBV reactivation [
113,
114].
Histological findings of papulosquamous eruptions are characterized by spongiosis with focal parakeratosis in the epidermis with aggregates of lymphocytes and Langerhans cells [
23,
113,
116]. Treatment for papulosquamous eruptions is unnecessary and warrants watchful waiting. For patients who present with papulosquamous eruptions, it is recommended to test for COVID-19, EBV, and HHV-6/7 [
96]. It remains uncertain whether a direct association exists between COVID-19 infection and the development of papulosquamous eruptions. Further research is needed to extrapolate a potential pathomechanism between SARS-CoV-2 infection and such skin lesions.
2.8. Multisystem Inflammatory Syndrome in Children
Children infected with SARS-CoV-2 are often asymptomatic or exhibit mild symptoms [
117,
118]. Although quite rare, a small percentage of MIS-C cases in children with COVID-19 required hospitalization, secondary to shock and multiorgan system failure. These children had symptoms comparable to toxic shock syndrome (TSS) and Kawasaki disease (KD) [
119,
120,
121,
122,
123]. Consequently, the CDC released an official report and used the term Multisystem Inflammatory Syndrome in Children (MIS-C) to define this novel condition. In the United States, the CDC and WHO diagnostic guidelines for this condition require the presence of all of the following:
Most children who met the criteria for MIS-C presented with a multitude of mucocutaneous findings [
126]. Thus, it is vital for dermatologists to be aware of this condition and its dermatological manifestations and potential sequelae. The mucocutaneous findings of MIS-C include conjunctival injection, palmoplantar erythema, lip hyperemia, periorbital erythema and edema, strawberry tongue, and malar erythema [
127,
128].
Patients diagnosed with MIS-C require immunomodulatory treatment and multidisciplinary care. In the United States, the CDC published management guidelines that specify IVIG and glucocorticoids as first-line treatments. The interleukin-1 (IL-1) receptor antagonist anakinra is recommended for refractory cases of MIS-C. Low-dose aspirin, enoxaparin, and tocilizumab can also be prescribed in certain cases [
129].
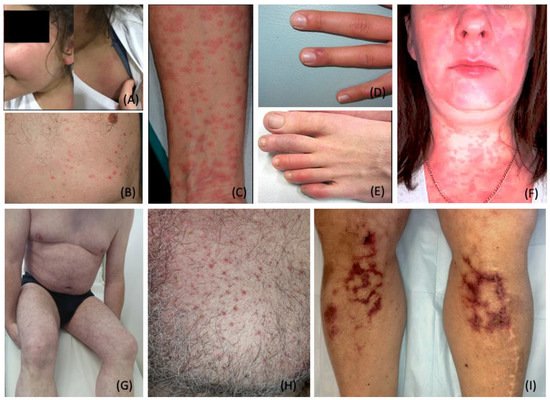
Figure 1. Cutaneous Manifestations Related to COVID-19 Infection. (
A). Maculopapular rash observed on the face and shoulder of an 11-year-old child; (
B). Varicella-like crusted papular lesions of the trunk; (
C). Maculopapular lesions coalesced into small plaques on the anterior forearm; (
D,
E). Chilblains-like erythema of the fingers (
D) and toes (
E); (
F). Urticarial erythematous eruption of the face, neck, and upper chest; note: no mild angioedema on the lower lip, due to excess interstitial fluid in the dermis and subcutaneous tissue; (
G). Livedo reticularis: a symmetric regular lace-like network of the trunk and extremities (
H). Monomorphic vesicles and pustules of the chest; (
I). Retiform purpura of the lower extremities. Photo credit is acknowledged to the following original reports: Burcu Bursal Duramaz [
140], Raffaele Gianotti [
141], Michel Verheyden [
94], Khalid Hassan [
87], Xavier Bosch-Amate [
135].
3. Dermatological Conditions Associated with Personal Protective Equipment and Hygiene Products
The COVID-19 pandemic has led to increased personal protective equipment (PPE) use among the general population and healthcare workers. Personal protective equipment protects the wearer from the transfer of disease through air, fluids, or direct contact. Pandemic healthcare workers have developed dermatological conditions due to prolonged use of personal protective equipment (PPE). Increased use of personal protective equipment (e.g., goggles, gloves, N95 face masks, and alcohol-based hand rubs) has led to a multitude of dermatological conditions: acne, periorificial dermatitis, papulopustular rosacea, pressure injury, irritant contact dermatitis, allergic contact dermatitis, hand eczema, and seborrheic dermatitis [
28,
29,
30,
31,
32]. (
Figure 2A–E) Long-term usage of protective clothing and contact disinfectants may disrupt the skin barrier and increase the risk of infection and autoimmune conditions [
33]. Dermatoses related to the prolonged wearing of PPE can be managed with prophylactic hygiene measures, conservative treatment, and pharmacological therapies.
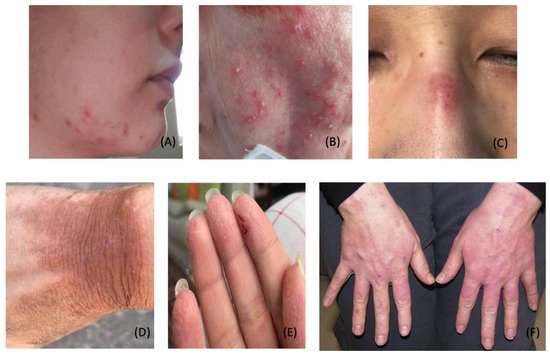
Figure 2. Dermatological Conditions Related to PPE and Hygiene Products. (
A). Acne vulgaris; (
B). Rosacea; (
C). Pressure injury; (
D,
E). Irritant contact dermatitis; (
F). Hand eczema; Photo credit is acknowledged to the following original reports: Mohammed Shanshal [
154], Anca E. Chiriac [
155], Z Q Yin [
156], Mohammed Shanshal [
154], Chandler W. Rundle [
157].
4. COVID-19 Vaccine-Induced Dermatological Manifestations
COVID-19 vaccinations were developed to reduce the spread and severity of SARS-CoV-2 infection. At this time, mRNA and DNA-based COVID-19 vaccinations are available worldwide. In the United States, COVID-19 mRNA vaccinations have been widely available to the public through FDA approval, conditional marketing approval, and emergency use authorization pathways [
158,
159,
160].
In the United States, adverse vaccine reactions are documented in a federal vaccine surveillance registry known as Vaccine Adverse Events Reporting System (VAERS). As a passive reporting system, VAERS data has been estimated to be largely underreported with incomplete data [
161]. The emergence of mass worldwide SARS-CoV-2 vaccination has resulted in an increased number of diverse adverse vaccine reactions. The greatest absolute risks related to COVID-19 vaccinations include, but are not limited to, allergic, constitutional, cardiovascular, dermatological, gastrointestinal, neurological, and localized pain.
A research study compared the documented adverse events of the COVID-19 vaccines administered between 2020 and 2021 to those of the influenza vaccines administered within the same year in the United States and Europe. All individuals who received either the COVID-19 or influenza vaccines were 18 years and older. A similar number of people received either COVID-19 vaccines (
n = 451 million) or the influenza vaccines (
n = 437 million). Results of the study revealed that more serious adverse events were associated with the COVID-19 vaccines in comparison to the influenza vaccines. Greater adverse events were quantified as allergic reactions, arrhythmias, general cardiovascular events, coagulation, hemorrhage, constitutional, ocular, sexual organ, and gastrointestinal reactions, with a greater relative risk for thromboembolic events [
161].
Anaphylactic urticaria and other dermatological manifestations currently comprise a smaller percentage of COVID-19 vaccination adverse events [
161,
162]. Anaphylaxis is a serious and life-threatening complication associated with COVID-19 vaccines. Anaphylaxis presents as an acute, life-threatening hypersensitivity reaction that affects multiple organ systems. Symptoms range from mild wheals, pruritus, and urticaria to severe respiratory symptoms [
163]. An increasing number of cases of anaphylaxis continues to be reported worldwide, and should be adequately addressed by regulatory authorities and pharmaceutical manufacturers [
164,
165].
The Pfizer and Moderna mRNA vaccines are packaged in lipid nanoparticles (LPNs) that contain polyethylene glycol (PEG), proteins, and other lipids [
166,
167]. PEG has been suggested as a potential allergen in vaccines [
166,
167]. Multiple cases of severe allergies to PEG have been reported [
168,
169,
170,
171]. Due to low awareness of PEG allergy, many cases have been misdiagnosed as idiopathic anaphylactic reactions [
170,
171,
172].
The proposed mechanisms of anaphylaxis to PEG are defined as IgE-mediated or complement-mediated [
169,
173,
174]. Currently, no approved allergy testing exists for PEG. Although skin testing has been used to investigate reactions to PEG, severe allergic reactions have been reported after the intradermal injection of this agent [
171]. The underlying immunological mechanism of allergic reactions to the COVID-19 vaccines remains poorly understood. More research is needed to devise appropriate, cost-effective pre-screening methods for vulnerable populations.
Another emerging complication of COVID-19 vaccines includes COVID arm. (
Figure 3A) “COVID arm” is a term coined to describe a post-vaccination reaction characterized by an erythematous rash surrounding the injection site [
175]. Multiple case series revealed the majority of COVID arm cases develop after the first dose of the Moderna vaccine with a median onset of eight days post-vaccination [
38,
175,
176,
177]. Histological findings of these skin lesions revealed a predominance of CD4+ helper T cells with variable eosinophils, consistent with delayed-type hypersensitivity reactions (DTHR) [
38,
175,
176].
As discussed earlier, Moderna and Pfizer vaccines contain excipients such as polyethylene glycol, which is proposed to elicit DTHR [
178]. In the United States, Pfizer vaccines have been administered in similar amounts to the Moderna vaccine [
179]. However, the incidence of COVID arm after the administration of the Pfizer vaccine is significantly lower compared to the Moderna vaccine [
175,
176,
177,
180]. Underreporting of vaccine reactions in the United States VAERS may account for the difference in numbers [
161]. COVID arm symptoms resolve spontaneously after three to eight days [
177]. Treatment with topical steroids, ice, or oral antihistamines can be implemented for expedited recovery and symptom relief [
177,
180].
Chilblains-like rash (“COVID toes”) is another cutaneous reaction that has been associated with the Pfizer and Moderna vaccines. (
Figure 3B) COVID toes typically appear four to seven days post-vaccination [
38,
181]. This localized reaction is hypothesized to manifest as an immune response to the vaccination. On histological examination, interstitial lymphocytic inflammatory infiltrates have been observed on punch biopsy [
181,
182]. COVID toes have been documented to occur after the first or second dose of the vaccine [
181,
182].
Other cutaneous manifestations associated with Moderna, Pfizer, and AstraZeneca vaccines include urticaria, morbilliform rash, papulovesicular rash, pityriasis rosea-like rash, and purpuric reactions [
37]. (
Figure 3C) Furthermore, eruptions of rosacea have been documented after receiving COVID-19 vaccinations.
Figure 3D, E illustrates a documented case of rosacea in a 45-year-old woman after receiving the Sinovac-CoronaVac COVID-19 DNA vaccine. Other reported cases of morbilliform rash progressed to severe dermatological reactions, including erythroderma, bullous pemphigoid, acute generalized exanthematous pustulosis, vasculitis, and urticaria [
37]. The patterns of vaccine-induced rashes are similar to rashes described in association with SARS-CoV-2 infection [
38,
48,
183]. It has been proposed that the host immune response, rather than direct SARS-CoV-2 damage, can cause these skin lesions [
37,
161]. Additionally, DTHR to vaccine excipients has been shown to play a role in the pathogenesis of these skin lesions [
37].
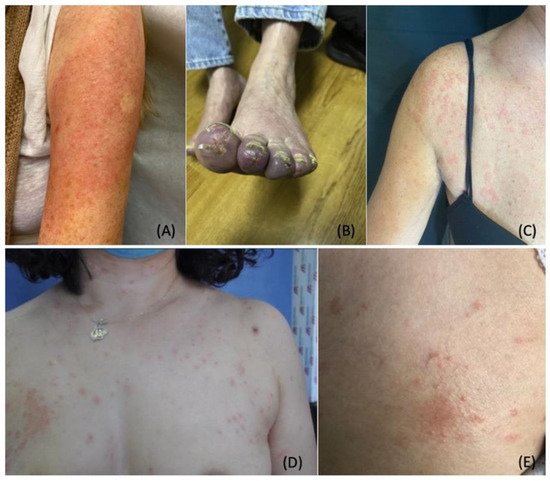
Figure 3. Cutaneous Manifestations Associated with COVID-19 Vaccination. (
A). Pruritic and erythematous rash was observed on the left arm of a 74-year-old woman eight days following the inoculation of the Moderna vaccine. (
B). Pernio/chilblains-like lesion of the toes observed in a 76-year-old man one week after receiving the second dose of the Moderna vaccine. (
C). Urticarial wheals on the trunk of a 48-year-old woman three hours after receiving the second dose of the Oxford-AstraZeneca vaccine. (
D,
E). Oval salmon-colored plaques on the trunk and herald patch on the right breast of a 45-year-old woman four days after receiving the first dose of the CoronaVac vaccine. Photo credit is acknowledged to the following original reports: Nancy Wei [
180], John M. Kelso [
181], Enes Yağız Akdaş [
184], Martina Burlando [
185].
5. Stress-Induced Dermatological Conditions
The COVID-19 pandemic has been a major disturbance to people’s lives globally. With lockdowns and restricted quarantine measures, people worldwide are concerned about their safety, job security, lack of access to treatment, commodities, and adverse socioeconomic consequences [
186]. An increased prevalence of psychiatric illnesses during the COVID-19 pandemic has been reported and follows a similar pattern observed in the populations following natural disasters [
186]. Pandemic-associated psychiatric conditions include anxiety disorders, depression, post-traumatic stress disorder (PTSD) symptoms, sleep disorders, somatic symptoms, and suicidal behavior [
186,
187,
188,
189]. Furthermore, epidemiological data conducted during previous pandemics (e.g., severe acute respiratory syndrome (SARS), Ebola, H1N1 influenza, Middle East Respiratory Syndrome (MERS), equine influenza) suggest an increased incidence of psychopathological disorders. Psychological stress, anxiety, and depression have been associated with an increased exacerbation of stress-responsive dermatological conditions [
190]. As such, it can be logically presumed that a higher incidence of comorbid psychopathology and psychodermatological disorders will be observed during the COVID-19 pandemic.
Likewise, it is prudent for dermatologists to thoroughly evaluate patients with a history of psychological illnesses or psychiatric diagnoses. When identified, these patients should be referred for appropriate psychosocial support to improve psychosocial outcomes and reduce potential dermatological exacerbations. The consistent epidemiological trend of psychopathological cutaneous disorders justifies a more specialized standard of care for at-risk individuals. Further research is recommended to help identify potential strategies to improve the identification and management of at-risk individuals.
5.1. Telogen Effluvium
Telogen effluvium (TE) is a common, self-limiting hair loss condition usually seen in women with a history of a recent stressor. Stressors associated with TE include systemic disease, infections, stressful events, drugs, nutritional deficiencies, postpartum hormonal changes, and major surgeries [
44]. TE usually presents three months following the onset of the stressor. It is classified as acute (hair loss lasting up to six months) or chronic (hair loss exceeding six months) [
193]. Coined as “COVID scalp,” increased cases of TE have been associated with the COVID-19 pandemic [
194]. The cause of TE during the COVID-19 pandemic is multifactorial, and it is thought to be stress-induced or a direct manifestation of the inflammatory process during the infection phase.
6. Pathomechanisms: Cutaneous Manifestations in SARS-CoV-2
International reports have identified a range of potential cutaneous manifestations related to COVID-19. The frequency and timing of these lesions to COVID-19 infection are difficult to ascertain. Multiple established associations in the scientific literature offer valuable insights into plausible mechanisms behind cutaneous manifestations of SARS-CoV-2. One proposed pathomechanism explains the presence of ACE2 in keratinocytes [19]. Another promising association describes the significance of androgen levels in the increased expression of the TMPRSS2 gene [13,14].
A strong association between increased androgens and increased TMPRSS2 gene expression has been identified in the literature [13,14]. ACE2 receptors and TMPRSS2 are expressed in multiple tissues throughout the body including the skin. In conjunction, the presence of ACE2 receptors in keratinocytes may play a significant role in the manifestation of dermatological conditions related to COVID-19. Furthermore, the association between androgen levels and TMPRSS2 can further contribute to the development of skin lesions [13,14,19]. As such, high levels of androgens combined with ACE2 distribution in the skin can provide invaluable clinical insight into the pathomechanism of skin manifestations.
Androgens have been shown to uniquely activate the TMPRSS2 gene. TMPRSS2 plays an essential role in the activation of spike protein and facilitates viral entry through ACE2 receptors. This correlation helps explain the greater susceptibility to COVID-19 in males, as males typically maintain higher levels of androgens. Moreover, it has been shown that males with prostate cancer receiving androgen deprivation therapy (ADT) have a lower risk of COVID-19 compared to prostate cancer patients who did not receive ADT [
14]. As such, ADT may play a role in reducing the severity of symptoms in patients with COVID-19 [
13,
14]. However, more research is needed to confirm these findings. Additionally, it is well established that children have a lower rate of COVID-19 infection compared to adult males and females. The less symptomatic disease in children may be explained by overall low expressions of androgen receptors and androgen levels [
7,
13,
14,
15,
16,
17].
7. The Role of Vitamin D in SARS-CoV-2
Additional susceptibility to COVID-19 has been strongly correlated with low vitamin D levels. Studies continue to identify and analyze the increased severity of COVID-19 infection with concomitant low vitamin D levels [
224,
225,
226]. Vitamin D can be administered in various forms including (but not limited to) cholecalciferol, calcifediol, and calcitriol. Vitamin D supplementation has been proposed to reduce the risk of COVID-19 infection through multiple protective mechanisms. Such mechanisms include modulation of the host immune system, upregulation of ACE2 concentration, vitamin D receptor activation [
226], reduction in endothelial damage, and reduction in proinflammatory cytokines [
224,
225,
226].
Research studies have demonstrated that vitamin D receptors (VDRs) are expressed in high concentration levels in cuboidal alveolar type II cells (ACII) within the pulmonary system. Calcitriol, also known as 1,25 dihydroxyvitamin D (1,25(OH)
2 D
3), binds to VDRs in ACII cells. Calcitriol binding activates multiple intracellular signals which inhibit inflammatory cytokines and chemokines involved in Acute Respiratory Distress Syndrome (ARDS) [
226].
Additional investigations have revealed that vitamin D signaling pathways prevent pulmonary vessel constriction, a manifestation associated with increased COVID-19 mortality [
224,
225,
226]. VDR activation promotes vasodilatory effects through two mechanisms: inhibitions of angiotensin II (a potent vasoconstrictor) and upregulation of ACE2. Decreased expression of angiotensin II promotes pulmonary vasodilation [
224,
225,
226]. Additionally, the induction of ACE2 expression in pulmonary tissues further dampens the effect of angiotensin II, thereby reducing respiratory distress symptoms. Prevention of pulmonary vasoconstriction greatly improves respiratory symptoms associated with SAR-CoV-2 infection. Therefore, ACE2 acts as an anti-inflammatory factor in the etiology of ARDS [
224,
225,
226]. VDR activation has also been shown to inhibit Skp2 protein, which is utilized by SARS-CoV-2 to replicate inside cells. Thus, the binding of calcitriol and VDR activation reduce viral replication in pulmonary tissues and reduce the disease severity of COVID-19 [
226].
Calcifediol, a vitamin D3 analog, rapidly increases serum levels of vitamin D 25-hydroxyvitamin D (25-OH-D), thereby promoting the protective properties associated with vitamin D [
225]. A parallel randomized open label, double-masked clinical trial evaluated the effect of calcifediol on the severity of COVID-19 disease. The randomized clinical trial was conducted on 76 consecutive patients hospitalized with COVID-19 infection. All 76 patients clinically presented with acute respiratory infections, confirmed by radiographic patterns of viral pneumonia. Likewise, all 76 patients tested positive for SARS-CoV-2 through PCR tests. Lastly, all 76 patients were confirmed for appropriate hospital admission via the CURB65 Severity Scale [
225]. All hospitalized patients received the best available therapy at the same standard of care. Of the 50 patients treated with calcifediol, one patient required intensive care unit (ICU) admission, and of the 26 untreated patients, 13 patients required ICU admission, with two deaths in the ICU. The remaining 11 untreated patients who did not receive calcifediol were discharged. Of all 50 patients treated with calcifediol, none died, and all patients were discharged with no complications [
225]. A larger-scale observational cohort study with 930 patients also revealed significantly reduced ICU admissions and mortality rates associated with early vitamin D administration [
227]. These clinical trials underscore the clinical value of vitamin D in a significant reduction in disease severity and disease mortality [
225,
227].
8. The Role of Vitamin D in Cutaneous Manifestations Associated with COVID-19
With regards to infection-related cutaneous manifestations, most conditions have been attributed to the host’s inflammatory response to SARS-CoV-2. Likewise, the immune-boosting, anti-inflammatory properties of vitamin D alleviate and reduce the spread of cutaneous lesions [
224,
225,
226,
227]. In addition, vitamin D bioavailability and efficacy have been shown to increase with magnesium supplementation. Magnesium has been shown to facilitate vitamin D-related processes by activating vitamin D processing enzymes [
228]. Vitamin D and magnesium supplementation are cost-effective measures that help prevent infection, reduce disease severity, and improve prognosis [
228]. Likewise, patient education and adherence are essential factors for favorable clinical outcomes. Therefore, further investigations are warranted to identify the ideal maintenance dosage of vitamin D and magnesium to prevent infection. Additional research is recommended to elucidate an effective dosing regimen to reduce infection-related cutaneous lesions.
Throughout the pandemic, clinical management for most COVID-19-associated and non-COVID-19 cutaneous manifestations have been similar in nature. For example, chilblain-like lesions in COVID-19 patients do not require treatment, but topical corticosteroids can relieve discomfort [
62,
64,
229]. Other rashes, such as varicelliform-like/vesicular lesions, are self-limiting and therefore do not require treatment [
48,
102]. In contrast, maculopapular and urticarial eruptions can occur concomitantly or separately in moderate to severe cases of COVID-19. These lesions present with pruritus and pain which necessitates prompt treatment with therapeutic agents such as topical corticosteroids, oral antihistamines, oral corticosteroids, and vitamin C [
48,
230,
231,
232,
233]. In addition to rash therapies, early treatment interventions have been shown to improve the overall prognosis for infected patients and reduce patient mortality [
234,
235].
9. Global Implications of the COVID-19 Pandemic
The COVID-19 pandemic has caused an insurmountable disturbance on a global scale. The strict quarantine measures have caused significant psychosocial distress. Resultantly, many individuals have developed new or worsening pre-existing dermatological conditions, such as telogen effluvium, psoriasis, eczema, urticaria, and atopic dermatitis. Exacerbated lesions require supportive management, targeted treatment for underlying issues, and relevant psychosocial support. Additionally, interdisciplinary treatment involving mental health professionals should be implemented to help relieve stress, anxiety, and depression.
As the pandemic continues, strict measures and mandates have been implemented to promote mass vaccination. With increased immunizations, additional cases of anaphylaxis and other allergic reactions such as COVID toes, COVID arm, and urticaria have been documented in response to immunizations [37,38,175,176,177,180]. It has been proposed that lipid nanoparticles (LNPs) containing messenger RNA vaccines trigger allergic and anaphylactic reactions [240]. LNPs are composed of positively charged lipids at low pH to stabilize the messenger RNA [241]. Likewise, LNPs contain high amounts of polyethylene glycol (PEG), a highly hydrophilic molecule [239,242]. PEG helps to increase the hydrophilicity of LNPs and stabilize the mRNA. However, PEG within LNPs has been shown to trigger inflammatory responses through complement-mediated and direct mast cell activation [240]. Furthermore, when inoculated into the bloodstream, LNPs trigger nonclassical allergic reactions in certain patients. Such reactions involve preformed antibodies to PEG and other components of LNPs [240]. Moreover, LNPs destabilize during the freeze and thaw cycle of immunization preparation [240]. When injected, destabilized LNPs release the naked mRNA into the bloodstream. Naked mRNA is proinflammatory and has been shown to induce allergic and anaphylactic reactions [239,242]. Additionally, classical allergic reactions to PEG may be IgE-mediated [240]. As such, skin prick testing with PEG should be performed before receiving the vaccine to avoid anaphylactic reactions [168]. At this time, allergy testing has not been implemented to screen susceptible individuals and should be considered to maximize safety and minimize adverse events. Moreover, novel measures on vaccine risk reduction can improve outcomes and maximize safety.
10. Conclusions
The COVID-19 pandemic has posed considerable challenges across the entirety of medicine. As evidenced, the COVID-19 pandemic has triggered a significant range of dermatologic sequela. Various factors surrounding the pandemic have resulted in a multitude of other dermatological manifestations. Dermatologists play an integral role in the proper diagnosis and treatment of COVID-related lesions. Several years into the pandemic, there is still much to learn and understand. As more information is collected and assessed, our comprehension of the pathogenesis and treatment of dermatologic manifestations will continue to evolve and guide the dermatological standard of care.
Early treatment regimens and timely prophylaxis have been shown to improve prognosis and reduce further infection-related sequelae. Moreover, novel measures on vaccine risk reduction can improve outcomes and maximize safety. Given the evolutionary nature of the pandemic, intentional observation and research are prudent in the diagnosis, management, and treatment of these cutaneous manifestations. Robust investigations are necessary to identify underlying dermatological pathomechanisms and improve lesion diagnosis. Data collection will help reveal pertinent risk factors and boost public health outcomes. Such studies will reduce disease burden and optimize quality of life as society continues to adapt and adjust to life after SARS-CoV-2.