Several eye-catching techniques have been developed to implement high-quality optical thin films for light-guiding applications. Thin films are the foundation for innovative technologies in various areas, including optical devices, environmental applications, telecommunications devices, and energy storage devices. The morphology and reliability of thin films are critical issues in all applications. Deposition techniques have a major influence on thin-film morphology. Physical and chemical deposition methods can be used to deposit high-quality thin films. A thin film is a thin layer of material with a thickness ranging from a few nm to a few μm. Thin films, like all materials, are classified as amorphous or polycrystalline based on the preparation conditions and the quality of the target material. Glass WGs display highly attractive properties due to the straightforward technology, the low propagation losses, and the flexible index matching to glass fibers. It is highly desirable to have low-loss glasses, reliable and enabling low-cost WG fabrication procedures. An overall requirement is that manufacturing technologies are proficient in high yield, and have guaranteed duplicability within the quantified tolerances, and fundamentally low operating costs.
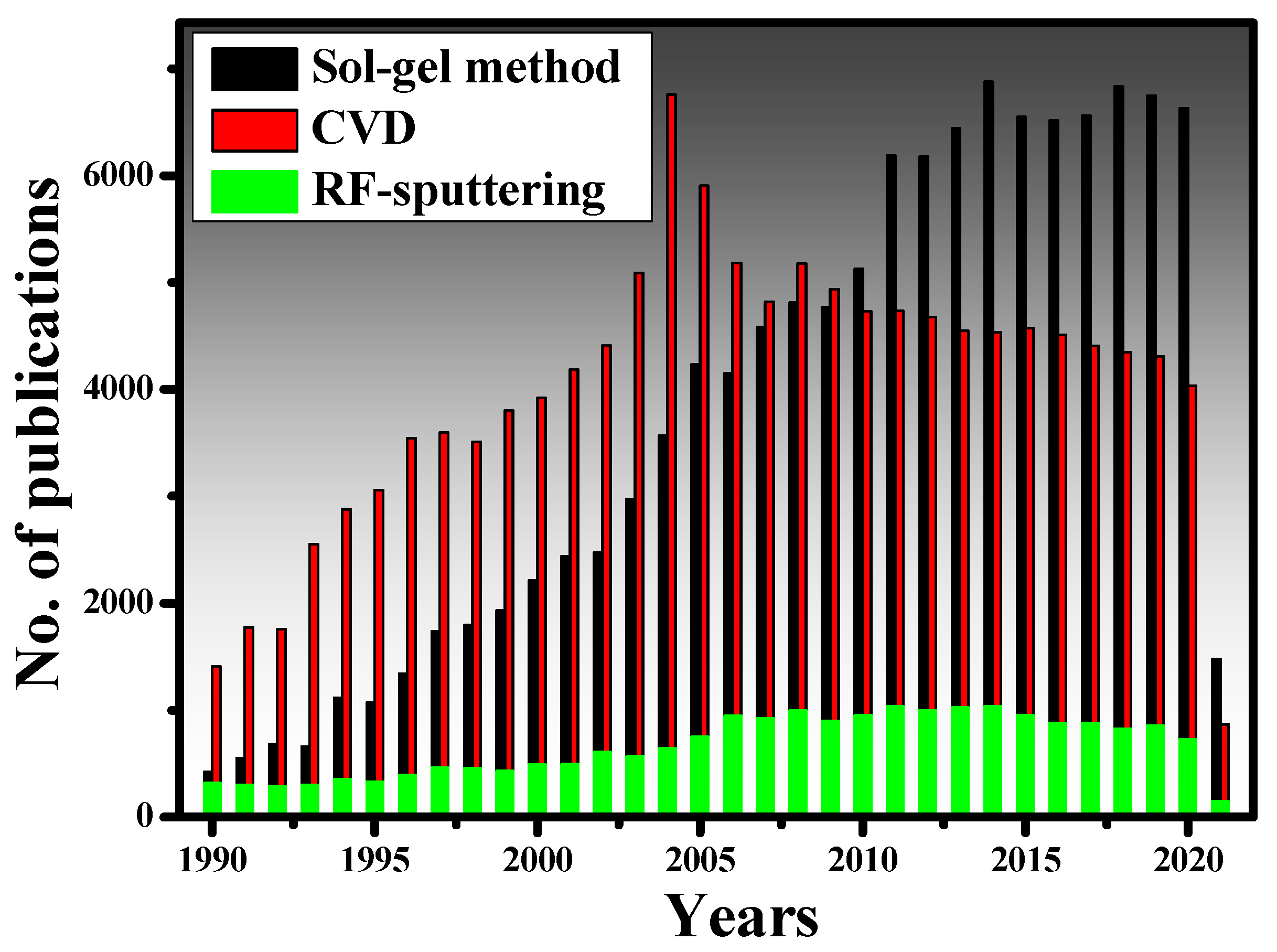
- sol-gel dip-coating method
- chemical vapor deposition
- silica-titania waveguide platform
- ion exchange
1. Thin-Film Deposition Techniques
1.1. Physical Vapor Deposition Techniques (PVD)
- A.
-
Vacuum
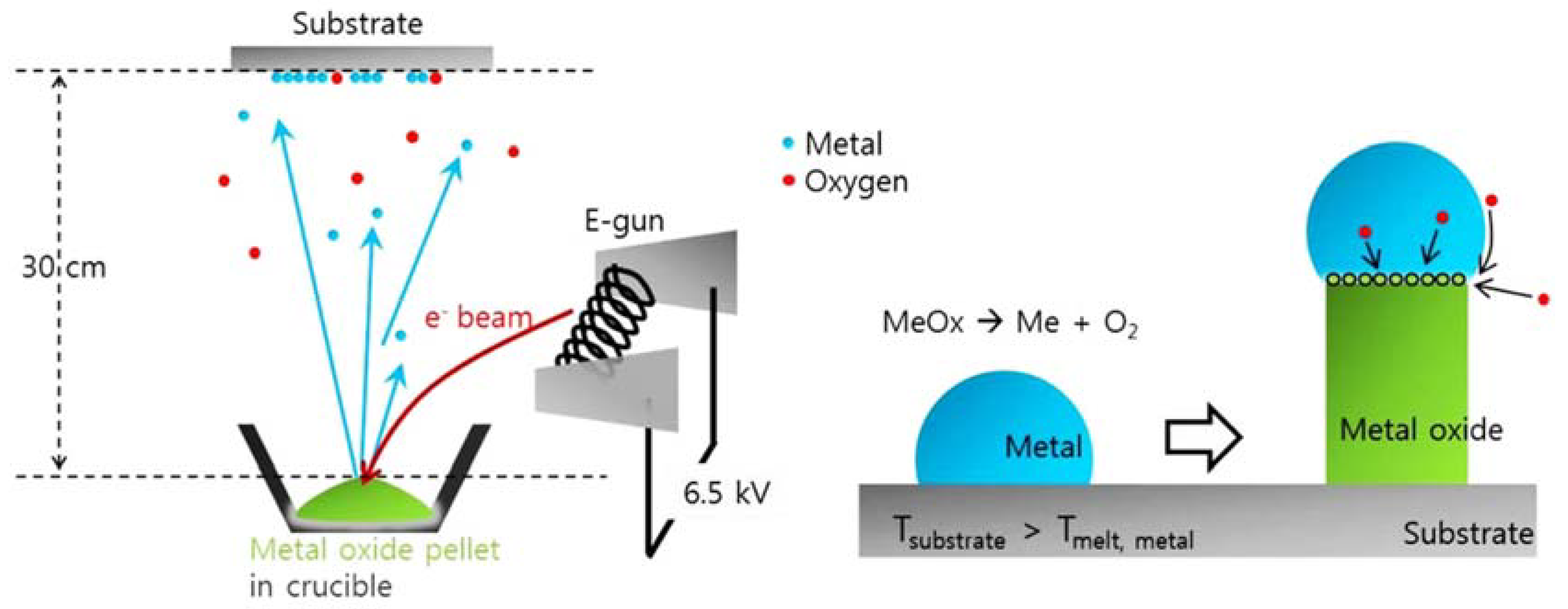
- B.
-
Sputtering techniques
1.2. Chemical Deposition Techniques
- A.
-
Gas-phase
- B.
-
Liquid-phase deposition
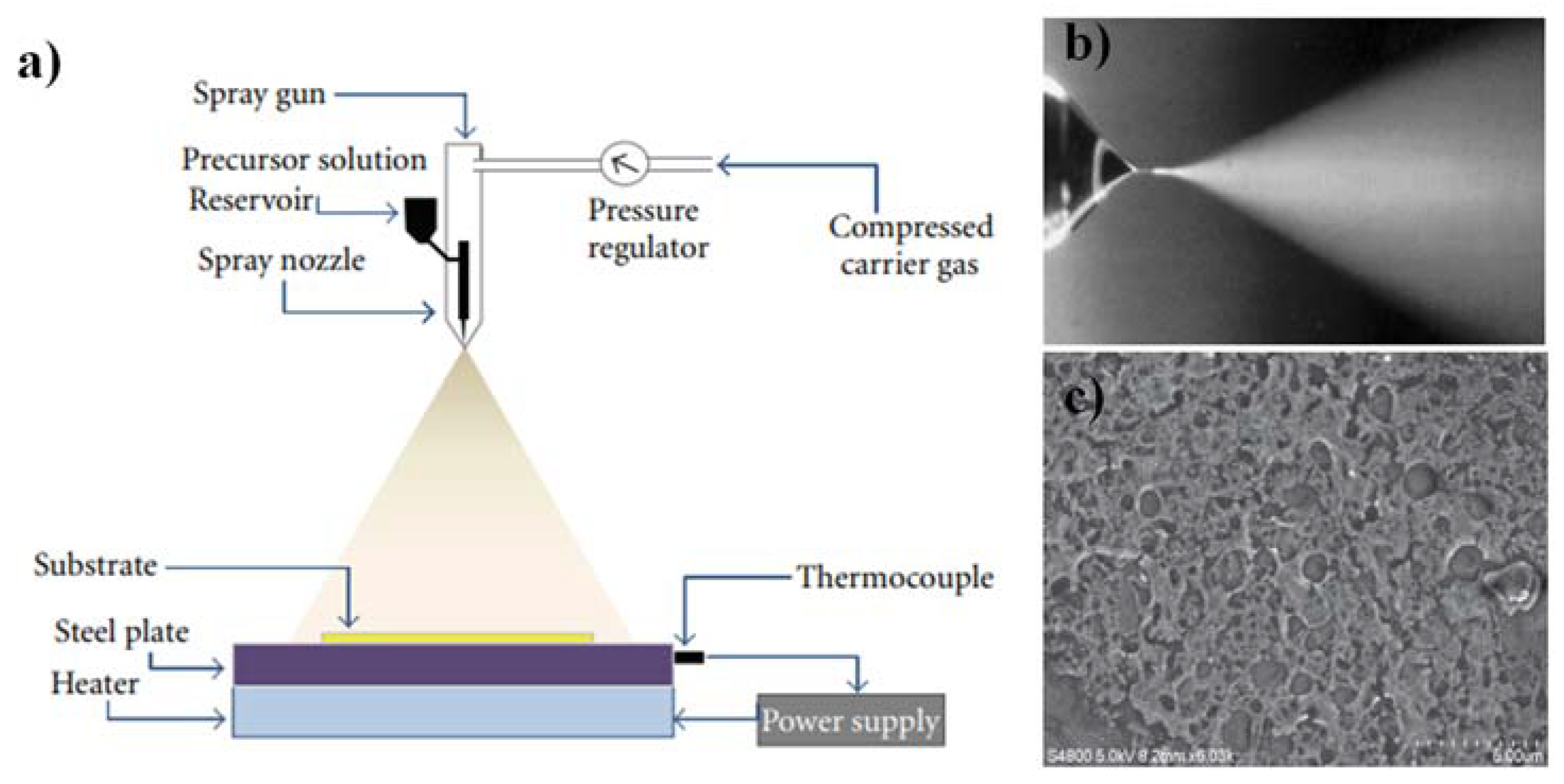
2. Refractive Index Modification Methods
The refractive index (RI) of a material is a number that describes how the light will propagate through it. Light travels at different speeds in the materials having different RI, which can be changed by modifying the density of the material.
2.1. Ion Exchange Process
2.2. Ion Implantation
2.3. Femtosecond-Laser Writing
This entry is adapted from the peer-reviewed paper 10.3390/ma15134591
References
- Jilani, A.; Abdel-Wahab, M.S.; Hammad, A.H. Advance deposition techniques for thin film and coating. In Modern Technologies for Creating the Thin Film Systems and Coatings; IntechOpen: London, UK, 2017; pp. 137–149.
- Hao, R.; Zhu, L.; Li, Z.; Fang, F.; Zhang, X. A Miniaturized and fast system for thin film thickness measurement. Appl. Sci. 2020, 10, 7284.
- Boolchandani, S.; Srivastava, S.; Vijay, Y.K. Preparation on InSe thin films by thermal evaporation method and their characterization: Structural, optical, and thermoelectrical properties. J. Nanotechnol. 2018, 2018, 9380573.
- Ali, N.; Teixeira, J.A.; Addali, A.; Saeed, M.; Al-Zubi, F.; Sedaghat, A.; Bahzad, H. Deposition of stainless steel thin films: An electron beam physical vapour deposition approach. Materials 2019, 12, 571.
- Ogugua, S.N.; Ntwaeaborwa, O.M.; Swart, H.C. Latest development on pulsed laser deposited thin films for advanced luminescence applications. Coatings 2020, 10, 1078.
- Opel, M.; Geprägs, S.; Althammer, M.; Brenninger, T.; Gross, R. Laser molecular beam epitaxy of ZnO thin films and heterostructures. J. Phys. D Appl. Phys. 2013, 47, 034002.
- Martin, P.M. Handbook of deposition technologies for films and coatings (Third edition). In Science, Applications and Technology; Elsevier Inc.: Amsterdam, The Netherlands, 2010; pp. 297–313.
- Randhawa, H.; Bunshah, R.; Brock, D.; Basol, B.; Stafsudd, O. Preparation of CuxS thin films by activated reactive evaporation technique. Sol. Energy Mater. 1982, 6, 445–453.
- Paul, R.; Hossain, M.; Muktadir, M.; Faisal, K. Fabrication of a cost effective thermal evaporation system for thin film deposition. In Proceedings of the 4th International Conference on Advances in Electrical Engineering (ICAEE), Dhaka, Bangladesh, 28–30 September 2017; pp. 703–706.
- Yu, H.K.; Lee, J.-L. Growth mechanism of metal-oxide nanowires synthesized by electron beam evaporation: A self-catalytic vapor-liquid-solid process. Sci. Rep. 2014, 4, 6589.
- Komalakrishna, H.; Augustin, A.; Udaya, B.K. Electron beam deposition of copper thin film on Aluminium substrate and its characterization. Am. J. Mater. Sci. 2015, 5, 19–24.
- Sharma, P.; Tripathi, N.; Gupta, N. Nanocrystalline silicon thin film prepared by e-beam evaporation for display application. J. Mater. Sci. Mater. Electron. 2016, 28, 3891–3896.
- Katzer, D.S.; Meyer, D.J.; Storm, D.F.; Nepal, N.; Wheeler, V.D. Silicon nitride thin films deposited using electron-beam evaporation in an RF plasma MBE system. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2014, 32, 02C117.
- Souk, J.H.; O’Hanlon, J.F.; Angillelo, J. Characterization of electron-beam deposited tungsten films on sapphire and silicon. J. Vac. Sci. Technol. A 1985, 3, 2289.
- Sontheimer, T.; Becker, C.; Ruske, F.; Klimm, C.; Bloeck, U.; Gall, S.; Kunz, O.; Young, T.; Egan, R.; Hupkes, J.; et al. Challenges and opportunities of electron beam evaporation in the preparation of poly-Si thin film solar cells. In Proceedings of the 2010 35th IEEE Photovoltaic Specialists Conference, Honolulu, HI, USA,, 20–25 June 2010; pp. 000614–000619.
- Sidqi, N.; Clark, C.; Buller, G.S.; Thalluri, G.K.V.V.; Mitrofanov, J.; Noblet, Y. Comparative study of dielectric coating materials for micro-cavity applications. Opt. Mater. Express 2019, 9, 3452–3468.
- Shamala, K.; Murthy, L.; Rao, K.N. Studies on optical and dielectric properties of Al2O3 thin films prepared by electron beam evaporation and spray pyrolysis method. Mater. Sci. Eng. B 2004, 106, 269–274.
- Ashfold, M.N.R.; Claeyssens, F.; Fuge, G.M.; Henley, S.J. Pulsed laser ablation and deposition of thin films. Chem. Soc. Rev. 2003, 33, 23–31.
- Rao, M.C. Pulsed laser deposition—Ablation mechanism and applications. Int. J. Mod. Phys. Conf. Ser. 2013, 22, 355–360.
- Snigirev, O.; Chukharkin, M.; Porokhov, N.; Rusanov, S.Y.; Kashin, V.V.; Tsvetkov, V.B.; Kalabukhov, A.; Winkler, D. Pulsed laser deposition of thin YBCO films on faceted YSZ single crystal fibers. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2014; Volume 507.
- Gulkowski, S.; Krawczak, E. RF/DC magnetron sputtering deposition of thin layers for solar cell fabrication. Coatings 2020, 10, 791.
- Zagho, M.M.; Dawoud, H.D.; Bensalah, N.; Altahtamouni, T.M. A brief overview of RF sputtering deposition of boron carbon nitride (BCN) thin films. Emergent Mater. 2018, 2, 79–93.
- Khan, N.A.; Akhavan, B.; Zhou, C.; Zhou, H.; Chang, L.; Wang, Y.; Liu, Y.; Fu, L.; Bilek, M.M.; Liu, Z. RF magnetron sputtered AlCoCrCu0.5FeNi high entropy alloy (HEA) thin films with tuned microstructure and chemical composition. J. Alloy. Compd. 2020, 836, 155348.
- Srivastava, P.K.; Vankar, V.D.; Chopra, K.L. R.F. magnetron sputtered tungsten carbide thin films. Bull. Mater. Sci. 1986, 8, 379–384.
- Kylián, O.; Shelemin, A.; Solař, P.; Pleskunov, P.; Nikitin, D.; Kuzminova, A.; Štefaníková, R.; Kúš, P.; Cieslar, M.; Hanuš, J.; et al. Magnetron sputtering of polymeric targets: From thin films to heterogeneous metal/plasma polymer nanoparticles. Materials 2019, 12, 2366.
- Subbarayudu, S.; Madhavi, V.; Uthanna, S. Growth of MoO3 films by RF magnetron sputtering: Studies on the structural, optical, and electrochromic properties. Int. Sch. Res. Not. 2013, 2013, 806374.
- Abdullah, S.; Nafarizal, N.; Sahdan, M.Z. Influence of Oxygen flow rate on the characteristics of the tungsten oxide using RF magnetron sputtering. Appl. Mech. Mater. 2015, 773–774, 657–661.
- Sproul, W.; Christie, D.; Carter, D. Control of reactive sputtering processes. Thin Solid Films 2005, 491, 1–17.
- Chen, S.; Tao, L.; Zeng, L.; Hong, R. RF magnetron sputtering aluminum oxide film for surface passivation on crystalline silicon wafers. Int. J. Photoenergy 2013, 2013, 792357.
- Mobtakeri, S.; Akaltun, Y.; Özer, A.; Kılıç, M.; Tüzemen, E.; Gür, E. Gallium oxide films deposition by RF magnetron sputtering; a detailed analysis on the effects of deposition pressure and sputtering power and annealing. Ceram. Int. 2020, 47, 1721–1727.
- Nur-E-Alam, M.; Lonsdale, W.; Vasiliev, M.; Alameh, K. Application-Specific oxide-based and metal–dielectric thin-film materials prepared by radio frequency magnetron sputtering. Materials 2019, 12, 3448.
- Greene, J.E. Review Article: Tracing the recorded history of thin-film sputter deposition: From the 1800s to 2017. J. Vac. Sci. Technol. A Vac. Surf. Films 2017, 35, 05C204.
- Lin, L.; Deng, B.; Sun, J.; Peng, H.; Liu, Z. Bridging the gap between reality and ideal in chemical vapor deposition growth of graphene. Chem. Rev. 2018, 118, 9281–9343.
- Woehrl, N.; Ochedowski, O.; Gottlieb, S.; Shibasaki, K.; Schulz, S. Plasma-enhanced chemical vapor deposition of graphene on copper substrates. AIP Adv. 2014, 4, 047128.
- Cohen, A.; Patsha, A.; Mohapatra, P.K.; Kazes, M.; Ranganathan, K.; Houben, L.; Oron, D.; Ismach, A. Growth-etch metal-organic chemical vapor deposition approach of WS2 atomic layers. ACS Nano 2021, 15, 526–538.
- Okada, H.; Baba, M.; Furukawa, M.; Yamane, K.; Sekiguchi, H.; Wakahara, A. Formation of SiO2 film by chemical vapor deposition enhanced by atomic species extracted from a surface-wave generated plasma. AIP Conf. Proc. 2017, 1807, 020006.
- Matsuura, Y.; Harrington, J.A. Hollow glass waveguides with three-layer dielectric coating fabricated by chemical vapor deposition. J. Opt. Soc. Am. A 1997, 14, 1255–1259.
- Matsuura, Y.; Harrington, J.A. Infrared hollow glass waveguides fabricated by chemical vapor deposition. Opt. Lett. 1995, 20, 2078–2080.
- Gismatulin, A.A.; Kamaev, G.N.; Kruchinin, V.N.; Gritsenko, V.A.; Orlov, O.M.; Chin, A. Charge transport mechanism in the forming-free memristor based on silicon nitride. Sci. Rep. 2021, 11, 2417.
- Bigiani, L.; Barreca, D.; Gasparotto, A.; Andreu, T.; Verbeeck, J.; Sada, C.; Modin, E.; Lebedev, O.I.; Morante, J.R.; Maccato, C. Selective anodes for seawater splitting via functionalization of manganese oxides by a plasma-assisted process. Appl. Catal. B Environ. 2020, 284, 119684.
- Blout, A.; Pulpytel, J.; Mori, S.; Arefi-Khonsari, F.; Méthivier, C.; Pailleret, A.; Jolivalt, C. Carbon nanowalls functionalization for efficient O2 reduction catalyzed by laccase using design of experiment. Appl. Surf. Sci. 2021, 547, 149112.
- Lusk, D.; Gore, M.; Boardman, W.; Casserly, T.; Boinapally, K.; Oppus, M.; Upadhyaya, D.; Tudhope, A.; Gupta, M.; Cao, Y.; et al. Thick DLC films deposited by PECVD on the internal surface of cylindrical substrates. Diam. Relat. Mater. 2008, 17, 1613–1621.
- Selvamanickam, V.; Xie, Y.; Reeves, J.; Chen, Y. MOCVD-Based YBCO-Coated Conductors. MRS Bull. 2004, 29, 579–582.
- Ruano, J.M.; Benoit, V.; Aitchison, J.S.; Cooper, J.M. Flame hydrolysis deposition of glass on silicon for the integration of optical and microfluidic devices. Anal. Chem. 2000, 72, 1093–1097.
- Tandon, P.; Boek, H. Experimental and theoretical studies of flame hydrolysis deposition process for making glasses for optical planar devices. J. Non-Cryst. Solids 2003, 317, 275–289.
- Kilian, A.; Kirchof, J.; Przyrembel, G.; Wischmann, W. Birefringence free planar optical waveguide made by flame hydrolysis deposition (FHD) through tailoring of the overcladding. J. Light. Technol. 2000, 18, 193–198.
- Holmes, C.; Gates, J.C.; Smith, P.R. Planarised optical fiber composite using flame hydrolysis deposition demonstrating an integrated FBG anemometer. Opt. Express 2014, 22, 32150–32157.
- Zhang, L.; Xie, W.; Wu, Y.; Xing, H.; Li, A.; Zheng, W. Optical and surface properties of SiO2 by flame hydrolysis deposition for silica waveguide. Opt. Mater. 2003, 22, 283–287.
- Saha, J.K.; Bukke, R.N.; Mude, N.N.; Jang, J. Significant improvement of spray pyrolyzed ZnO thin film by precursor optimization for high mobility thin film transistors. Sci. Rep. 2020, 10, 8999.
- Perednis, D.; Gauckler, L.J. Thin Film Deposition Using Spray Pyrolysis. J. Electroceramics 2005, 14, 103–111.
- Patil, G.E.; Kajale, D.D.; Gaikwad, V.B.; Jain, G.H. Spray Pyrolysis Deposition of Nanostructured Tin Oxide Thin Films. ISRN Nanotechnol. 2012, 2012, 275872.
- Cho, J.; Hwang, S.; Ko, D.-H.; Chung, S. Transparent ZnO Thin-Film Deposition by Spray Pyrolysis for High-Performance Metal-Oxide Field-Effect Transistors. Materials 2019, 12, 3423.
- Filipovic, L.; Selberherr, S.; Mutinati, G.C.; Brunet, E.; Steinhauer, S.; Köck, A.; Teva, J.; Kraft, J.; Siegert, J.; Schrank, F. Methods of simulating thin film deposition using spray pyrolysis techniques. Microelectron. Eng. 2014, 117, 57–66.
- Ramadhani, M.F.; Pasaribu, M.A.H.; Yuliarto, B. Nugraha Fabrication of ZnO nanorod using spray-pyrolysis and chemical bath deposition method. AIP Conference Proceedings 2014, 1586, 74–77.
- Gurav, A.; Kodas, T.; Pluym, T.; Xiong, Y. Aerosol Processing of Materials. Aerosol Sci. Technol. 1993, 19, 411–452.
- Leong, K. Morphological control of particles generated from the evaporation of solution droplets: Theoretical considerations. J. Aerosol Sci. 1987, 18, 511–524.
- Ju, S.H.; Hong, S.K.; Jang, H.C.; Kang, Y.C. Fine size cobalt oxide powders prepared by spray pyrolysis using two types of spray generators. J. Ceram. Soc. Jpn. 2007, 115, 507–510.
- Lengyel, M.; Atlas, G.; Elhassid, D.; Luo, P.; Zhang, X.; Belharouak, I.; Axelbaum, R. Effects of synthesis conditions on the physical and electrochemical properties of Li1.2Mn0.54Ni0.13Co0.13O2 prepared by spray pyrolysis. J. Power Sources 2014, 262, 286–296.
- Zhang, S.-C.; Messing, G.L.; Borden, M. Synthesis of solid, spherical zirconia particles by spray pyrolysis. J. Am. Ceram. Soc. 1990, 73, 61–67.
- Cho, S.-Y.; Kim, I.-T.; Kim, D.-Y.; Park, S.J.; Kim, B.-K.; Lee, J.-H. Effects of H2O2 on the morphology of ZrO2 powder prepared by ultrasonic spray pyrolysis. Mater. Lett. 1997, 32, 271–273.
- Kang, Y.; Roh, H.; Park, S. Preparation of Y2O3:Eu phosphor particles of filled morphology at high precursor concentrations by spray pyrolysis. Adv. Mater. 2000, 12, 451–453.
- Pantano, C.; Ganan-Calvo, A.; Barrero, A. Zeroth-order, electrohydrostatic solution for electrospraying in cone-jet mode. J. Aerosol. Sci. 1994, 25, 1065–1077.
- Ebelmen, M. Sur les combinaisons des acides borique et silicique avec les ethers. Annl. Chim. Phys. 1846, 16, 129–166.
- Thor, T.; Vaclavik, J. Sol-gel preparation of silica and titania thin films. In Proceedings of the Optics and Measurement International Conference, Liberec, Czech Republic, 11 November 2016; Volume 10151.
- Schroeder, H. Oxide layers deposited from organic solutions. In Physics of thin films: Advances in Research and Developments; Academic Press: London, UK; New York, NY, USA, 1969; pp. 87–141.
- Attia, S.M.; Wang, J.; Wu, G.; Shen, J.; Jianhua, M.A. Review on sol-gel derived coatings: Process, techniques and optical applications. J. Mater. Sci. Technol. 2002, 18, 211–217.
- Logan, M.N.; Prabakar, S.; Brinker, C.J. Sol-Gel-Derived silica films with tailored microstructures for applications requiring organic dyes. MRS Proc. 1994, 346, 74–77.
- Brinker, C.; Frye, G.; Hurd, A.; Ashley, C. Fundamentals of sol-gel dip coating. Thin Solid Films 1991, 201, 97–108.
- Fidalgo, A.; Ilharco, L.M. The defect structure of sol–gel-derived silica/polytetrahydrofuran hybrid films by FTIR. J. Non-Cryst. Solids 2001, 283, 144–154.
- Parin, R.; Rigon, M.; Bortolin, S.; Martucci, A.; Del Col, D. Optimization of hybrid sol-gel coating for dropwise condensation of pure steam. Materials 2020, 13, 878.
- Acosta, S.; Ayral, A.; Guizard, C.; Lecornec, C.; Passemard, G.; Moussavi, M. Sol-Gel derived silica layers for low-k dielectrics applications. MRS Proc. 2000, 612, D5.26.1–D5.26.6.
- Boudot, M.; Gaud, V.; Louarn, M.; Selmane, M.; Grosso, D. Sol–Gel based hydrophobic antireflective coatings on organic substrates: A detailed investigation of ammonia vapor treatment (AVT). Chem. Mater. 2014, 26, 1822–1833.
- Kim, T.; Song, K. Low-temperature preparation of superhydrophilic coatings using tetraethoxysilane and colloidal silica by sol-gel method. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129105.
- Hasaneen, M.; Shalaby, M.; Yousif, N.; Diab, A.; El Agammy, E. Structural and optical properties of transparent conducting oxide Cd1-xCrxO thin films prepared by the sol-gel dip-coating method. Mater. Sci. Eng. B 2022, 280, 115703.
- Niazmand, M.; Maghsoudipour, A.; Alizadeh, M.; Khahpour, Z.; Kariminejad, A. Effect of dip coating parameters on microstructure and thickness of 8YSZ electrolyte coated on NiO-YSZ by sol-gel process for SOFCs applications. Ceram. Int. 2022, 48, 16091–16098.
- Esfahani, M.B.; Eshaghi, A.; Bakhshi, S.R. Transparent hydrophobic, self-cleaning, anti-icing and anti-dust nano-structured silica based thin film on cover glass solar cell. J. Non-Cryst. Solids 2022, 583, 121479.
- Beldjebli, O.; Bensaha, R.; Panneerselvam, P. Effect of both sn doping and annealing temperature on the properties of dip-coated nanostructured TiO2 thin films. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1624–1636.
- Both, J.; Szabo, G.; Katona, G.; Muresan, L. Tannic acid reinforced sol-gel silica coatings for corrosion protecion of zinc substrates. Mater. Chem. Phys. 2022, 282, 125912.
- Rogozinski, R.; Karasinski, P. Optical waveguides produced in ion exchange process from the solutions of AgNO3-NaNO3 for planar chemical amplitude sensors. Opto-Electron. Rev. 2005, 13, 229–238.
- Izawa, T.; Nakagome, H. Optical waveguide formed by eletrically induced migration of ions in glass plates. Appl. Phys. Lett. 1972, 21, 584–586.
- Giallorenzi, T.; West, E.; Kirk, R.; Ginther, R.; Andrews, R. Optical waveguides formed by thermal migration of ions in glass. Appl. Opt. 1973, 12, 1240–1245.
- Findakly, T. Glass Waveguides by Ion Exchange: A Review. Opt. Eng. 1985, 24, 242244.
- Namba, S.; Aritome, H.; Nishimura, T.; Masuda, K.; Toyoda, K. Optical waveguides fabricated by ion implantation. J. Vac. Sci. Technol. 1973, 10, 936–940.
- Bi, Z.-F.; Wang, L.; Liu, X.-H.; Zhang, S.-M.; Dong, M.-M.; Zhao, Q.-Z.; Wu, X.-L.; Wang, K.-M. Optical waveguides in TiO2 formed by He ion implantation. Opt. Express 2012, 20, 6712–6719.
- Chandler, P.J.; Zhang, L.; Townsend, P.D. Double waveguide in LiNbO3 by ion implantation. Appl. Phys. Lett. 1989, 55, 1710–1712.
- Townsend, P.D.; Chandler, P.J.; Zhang, L. Optical Effects of Ion Implantation; Cambridge University Press: Cambridge, UK, 1994.
- Davis, K.M.; Miura, K.; Sugimoto, N.; Hirao, K. Writing waveguides in glass with a femtosecond laser. Opt. Lett. 1996, 21, 1729–1731.
- Gross, S.; Dubov, M.; Withford, M.J. On the use of the Type I and II scheme for classifying ultrafast laser direct-write photonics. Opt. Express 2015, 23, 7767–7770.
- Chen, G.Y.; Piantedosi, F.; Otten, D.; Kang, Y.Q.; Zhang, W.Q.; Zhou, X.; Monro, T.M.; Lancaster, D.G. Femtosecond-laser-written Microstructured Waveguides in BK7 Glass. Sci. Rep. 2018, 8, 10377.
- Ehrt, D.; Kittel, T.; Will, M.; Nolte, S.; Tünnermann, A. Femtosecond-laser-writing in various glasses. J. Non-Cryst. Solids 2004, 345–346, 332–337.
- Butt, M.A.; Nguyen, H.-D.; Ródenas, A.; Romero, C.; Moreno, P.; De Aldana, J.R.V.; Aguiló, M.; Solé, R.M.; Pujol, M.C.; Díaz, F. Low-repetition rate femtosecond laser writing of optical waveguides in KTP crystals: Analysis of anisotropic refractive index changes. Opt. Express 2015, 23, 15343–15355.
- Fedotov, S.S.; Butvina, L.N.; Okhrimchuk, A.G. Plastic deformation as nature of femtosecond laser writing in YAG crystal. Sci. Rep. 2020, 10, 19385.
- Pätzold, W.M.; Demircan, A.; Morgner, U. Low-loss curved waveguides in polymers written with a femtosecond laser. Opt. Express 2017, 25, 263–270.
- Nguyen, H.H.D.; Hollenbach, U.; Pfirrmann, S.; Ostrzinski, U.; Pfeiffer, K.; Hengsbach, S.; Mohr, J. Photo-structurable polymer for interlayer single-mode waveguide fabrication by femtosecond laser writing. Opt. Mater. 2017, 66, 110–116.
- Cerullo, G.; Osellame, R.; Taccheo, S.; Marangoni, M.; Polli, D.; Ramponi, R.; Laporta, P.; Silvestri, S.D. Femtosecond micromachining of symmetric wavegudies at 1.5 um by astigmatic beam focusing. Opt. Lett. 2002, 27, 1938–1940.
- Eaton, S.M.; Ng, M.; Osellame, R.; Herman, P.R. High refractive index contrast in fused silica waveguides by tightly focused, high-repetition rate femtosecond laser. J. Non.-Cryst. Solids 2011, 357, 2387–2391.
- Fletcher, L.B.; Witcher, J.J.; Troy, N.; Reis, S.T.; Brow, R.K.; Krol, D.M. Direct femtosecond laser waveguide writing inside zinc phosphate glass. Opt. Express 2011, 19, 7929–7936.
- Martínez, J.; Ródenas, A.; Stake, A.; Traveria, M.; Aguiló, M.; Solis, J.; Osellame, R.; Tanaka, T.; Berton, B.; Kimura, S.; et al. Harsh-Environment-Resistant OH-Vibrations-Sensitive Mid-Infrared Water-Ice Photonic Sensor. Adv. Mater. Technol. 2017, 2, 1700085.
- Lapointe, J.; Parent, F.; de Lima Filho, E.S.; Loranger, S.; Kashyap, R. Toward the integration of optical sensors in smartphone screens using femtosecond laser writing. Opt. Lett. 2015, 40, 5654–5657.
- Amorim, V.A.; Maia, J.M.; Viveiros, D.; Marques, P.V.D.S. Inscription of surface waveguides in glass by femtosecond laser writing for enhanced evanescent wave overlap. J. Opt. 2020, 22, 085801.
