Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Infectious Diseases
Monocytes and macrophages are a central component of the innate immune system and exert an important function in orchestrating inflammation.
- monocytes
- macrophages
1. Proinflammatory Immune Response
The human immune system is a complex interlinked system of cells, receptors and molecules that protect the human body from infections or tissue damage and orchestrate tissue healing and repair. The system has traditionally been divided into innate and adaptive. The adaptive immune system recognizes individual pathogens in a highly specific manner and for a long time it was assumed that it is solely responsible for the formation of immunological memory. It often offers the body lifetime immunity to re-infection with the same pathogen. The innate immune system on the other hand is the first line of defense against invading pathogens and relies on preserved receptors that detect the general characteristics of pathogens. These receptors are expressed on a variety of cells, facilitating rapid intervention in the event of an infection. It is believed that 99% of infections are responded to by the innate immune system [1]. The immune response can be divided into the following separate phases: homeostasis and surveillance; sensing and initiating the immune response (proinflammatory response); restoration of immune homeostasis and under certain physiological and pathological conditions, the generation of innate immune memory.
In addition to the tasks of immunological detection and the containment of infections, the innate immune system ensures the initiation of the adaptive immune response. The majority of cells of the innate immune system, mainly monocytes/macrophages, dendritic cells and granulocytes, derive from myeloid progenitor cells.
2. Inflammatory Monocytes and Macrophages
Monocytes and macrophages are a central component of the innate immune system and exert an important function in orchestrating inflammation [2]. They play a pivotal role not only in the generation of inflammatory mediators and in the regulation of the innate and adaptive immunity, but also contribute to the resolution of inflammation and the reestablishment of homeostasis (see Figure 1) [3,4]. Thus, the dysfunction of monocytes and macrophages is frequently involved in the pathophysiology of chronic infections and many severe sterile inflammatory and autoimmune diseases [5]. Monocytes account for 5–10% of all blood immune cells, and are bone-marrow-derived mononuclear cells with a life span of about 1–3 days [6,7]. In the steady state, they exert a homeostatic function and can differentiate into tissue macrophages. During an inflammatory process, monocytes are recruited to the site of inflammation and ultimately differentiate into inflammatory macrophages or dendritic cells [3].
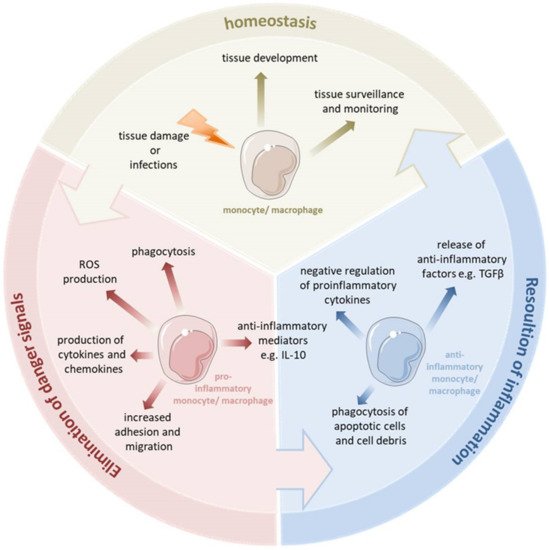
Figure 1. Monocytes and macrophages are a central component of the innate immune system. They are able to switch their phenotype from a homeostatic state to the proinflammatory state to eliminate pathogens and fight the inflammation. During uncomplicated inflammation, a switch from a proinflammatory to an anti-inflammatory phenotype occurs which enables the resolution of inflammation and the re-establishment of homeostasis. This enables the monocytes and macrophages to play diverse roles in the inflammatory response, both encouraging and discouraging this process. Depending on the kind of signal or pathophysiologic condition, monocytes and macrophages can undergo specific phenotypic polarization and thus acquire distinct functional phenotypes.
Macrophages reside in every tissue of the body and exhibit a great functional diversity. They play a critical role in tissue development, the surveillance and monitoring of tissue changes, as well as in maintaining tissue homeostasis [8]. Many tissue macrophages are prenatally established during embryonic development from progenitors derived from the yolk sac or fetal liver and in the steady state they are maintained independently from the bone marrow-derived monocytes [9]. In contrast, dermal, heart and intestinal macrophages are first seeded by embryonic liver-derived progenitors but quickly after birth are replaced by monocytes derived from hematopoietic stem cells [10]. Tissue resident macrophages show a proliferative potential and a self-renewal capacity. The bone marrow-derived cells can contribute to the macrophage pool in inflammatory infiltrates and can replace tissue resident macrophages of an embryonic origin, e.g., after severe inflammation [11]. Tissue macrophages can be affected by a variety of factors to change their phenotype and thus their function. Monocytes and macrophages are considered particularly plastic. They have several different phenotypic states and, depending on the tissue type and environmental cues, they can acquire distinct functional phenotypes. They are able to switch their phenotype from a basal state (homeostatic functions) to the proinflammatory state (eliminating pathogen and battling the inflammation). During uncomplicated inflammation, a kind of switch from a proinflammatory to an anti-inflammatory/pro-resolving phenotype occurs, thereby promoting the resolution of inflammation and the re-establishment of homeostasis. This enables the monocytes and macrophages to play diverse roles in the inflammatory response, both encouraging and discouraging this process. Depending on the different environmental signals or various pathophysiologic conditions, monocytes and macrophages can undergo different forms of phenotypic polarization and thus acquire distinct functional phenotypes.
The inflammatory response triggered by an infection or tissue damage involves the coordination of a multitude of cellular and molecular events. Pathogen recognition is considered as the most critical step for eliciting the adequate immune response during infection. Monocyte and macrophage activation and polarization are initiated through the recognition of pathogen- and tissue damage-associated conserved molecular motifs through pattern recognition receptors (PRRs) [12].
PRRs are germline-encoded host sensors that recognize the following two classes of molecules: pathogen-associated molecular patterns (PAMPs) which are evolutionarily conserved structures associated with pathogens such as viruses, bacteria, fungi and parasites and damage-associated molecular patterns (DAMPs), which are exposed in damaged host tissues. Currently, four different families of PRRs have been identified. These families include transmembrane receptors such as the Toll-like receptors (TLRs) and C-type lectin receptors (CLRs), as well as cytoplasmic proteins such as the Retinoic acid-inducible gene (RIG)-I-like receptors (RLRs) and NOD-like receptors (NLRs). With the exception of some NLRs, the sensing of PAMPs or DAMPs by PRRs upregulates the transcription of genes involved in inflammatory responses in monocytes and macrophages. The processes activated following the engagement of PRRs are rapid, induce conserved inflammatory patterns and include responses such as phagocytosis, cell locomotion, killing of pathogens or cells, and cytokine production. These innate immune mechanisms of monocytes and macrophages make them very effective in eliminating invading pathogens [13].
Among the PRRs, the TLR family has a unique capacity to sense the initial infection and induce an adequate inflammatory response [14]. TLRs are characterized by N-terminal leucine-rich repeats (LRRs) and a transmembrane region followed by a cytoplasmic Toll/IL-1R homology (TIR) domain. In humans, ten different TLRs have been identified. One of the most investigated TLRs present on the surface of monocytes and macrophages is TLR4, which is involved in the detection of Gram-negative bacteria and their associated endotoxins (e.g., Lipopolysaccharide, LPS), whereas lipoteichoic acid (LTA), peptidoglycan (PGN) and Pam3Cys of Gram-positive bacteria are recognized by TLR2 [15,16]. TLR signaling pathways consist, of at least a MyD88 (Myeloid differentiation primary response gene (88))-dependent pathway that is common to all TLRs and a MyD88-independent pathway that is peculiar to the TLR3 and TLR4 signaling pathways [17]. Endotoxin, or lipopolysaccharide (LPS), is an effective trigger of the inflammatory response during infection with Gram-negative bacteria and a very potent inducer of proinflammatory responses, especially in phagocytes. The activation of cells by LPS is initiated by an interaction with TLR4 and the formation of an LPS-binding complex with CD14 and MD-2 on the cell surface. Subsequently, the adaptor molecules MyD88 and TRIF (TIR-domain-containing adapter-inducing interferon-β) are recruited to the intracellular TLR4 domain resulting in the activation of either the “MyD88-dependent” or the “MyD88-independent” pathway in monocytes and macrophages.
In monocytes and macrophages, the “MyD88-dependent” pathway triggers the activation of downstream kinases, such as IL-1R-associated kinase-1 (IRAK-1), IRAK-4, phosphoinositide 3-kinase (PI3K), and mitogen-activated protein kinases (MAPK), and ultimately results in the liberation of the cytoplasmic nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells (NF-κB), its translocation to the nucleus and the subsequent transcription of inflammatory cytokines such as TNF-α, IL-6 and IL-1β. These cytokines promote the resulting immune response and induce the prolonged survival of monocytes [18]. The switching on of the “MyD88-independent” pathway in monocytes and macrophages leads to an activation of the transcription factor interferon regulatory factor 3 (IRF-3), and thereby induces interferon beta (IFN-β), finally resulting in the activation of several IFN-inducible genes [19,20,21]. Thus, macrophage activation leads to the induction of several potent mechanisms such as the production of ROS (reactive oxygen species), NO (nitrogen oxide) and the release of several cytokines to kill pathogens and combat the infection efficiently.
Over the last years, there has been growing evidence that TLRs play an important role not only in the recognition of pathogens by monocytes and macrophages but also in the sensing of DAMPs such as S100A8/S100A9, HMGB1 (high mobility group box protein-1), heat-shock proteins, uric acid and DNA. It has also been frequently reported that inflammatory processes induced by the activation of the TLR system via microbial products can be additionally modulated by endogenous ligands of TLR4.
3. S100-Alarmins: Potent Effector Proteins of Inflammatory Macrophages
The most abundantly expressed DAMPs by monocytes and neutrophils are S100A8 and S100A9, which are proteins that belong to the group of alarmins and form about 5% of the soluble cytosol content in monocytes [22].
S100A8 and S100A9 are known to form non-covalently associated heterodimeric complexes (also called calprotectin) which represent the physiologically relevant forms of these proteins. S100A8, S100A9 and the S100A8-S100A9 complex are highly released in various inflammatory diseases locally and systemically and extracellular S100A8 and S100A9 show proinflammatory activities on many cell types, e.g., endothelial cells, phagocytes, lymphocytes or osteoclasts [23,24,25,26]. Interestingly, S100A8/S100A9 have been described to specifically interact with the TLR4-CD14-MD2 complex, thus representing inflammatory components that amplify phagocyte activation during the sepsis upstream of TNF-α-dependent effects [27]. They are released during many inflammatory processes in humans including sepsis and endotoxemia and they have been demonstrated to promote sterile inflammation in the absence of any microbial trigger as well [24,28,29,30]. Thus, on the one hand, these alarmins play a key role in the initial host defence against many infections and represent an important factor in orchestrating coordinated immune reactions. On the other hand, an uncontrolled and excessive release of these alarmins leads to an overwhelming inflammatory response and contributes to the dysregulated processes seen in many inflammatory, allergic and autoimmune conditions [25,30]. In addition to these proinflammatory functions, the prolonged stimulation of cells with S100A8 and S100A9 can induce hypo-responsiveness in monocytes and macrophages similar to the well-known endotoxin tolerance and thereby subsequently trigger immune paralysis, which is the major risk factor for enhanced morbidity and mortality during sepsis and SIRS (systemic inflammatory response syndrome) [29].
This entry is adapted from the peer-reviewed paper 10.3390/cells11121979
This entry is offline, you can click here to edit this entry!
