Polyphenoloxidase (PPO) is an endogenous enzyme, which is naturally present in plant tissues, and it has also been referred to as polyphenol oxidase, catechol oxidase, tyrosinase, phenolase, catecholase, and o-diphenol oxidase. It is an oxidoreductase-copper-containing metalloprotein, which catalyses the degradation of phenolic fruit constituents to o-quinones in the presence of oxygen. The combination of ultrasound with temperature, and high pressure processing (HPP) with temperature to inactivate polyphenoloxidase enzyme in three fruits (pear, apple and strawberry) was investigated and compared with PPO inactivation exclusively by a thermal process.
- polyphenoloxidase
- high pressure processing
- fruit puree
- clean label
- sustainable
- sonication
- thermosonication
1. Enzymatic Browning by Polyphenoloxidase (PPO)
2. Thermal Pasteurization and Kinetics of PPO Enzyme Inactivation in Foods
3. Ultrasound and High Pressure Processing Pasteurization Technologies and Kinetics of PPO Enzyme Inactivation
3.1. Power Ultrasound
3.2. High Pressure Processing
4. Comparison of Three Pasteurization Technologies for the Inactivation of PPO in Three Fruits' Purees
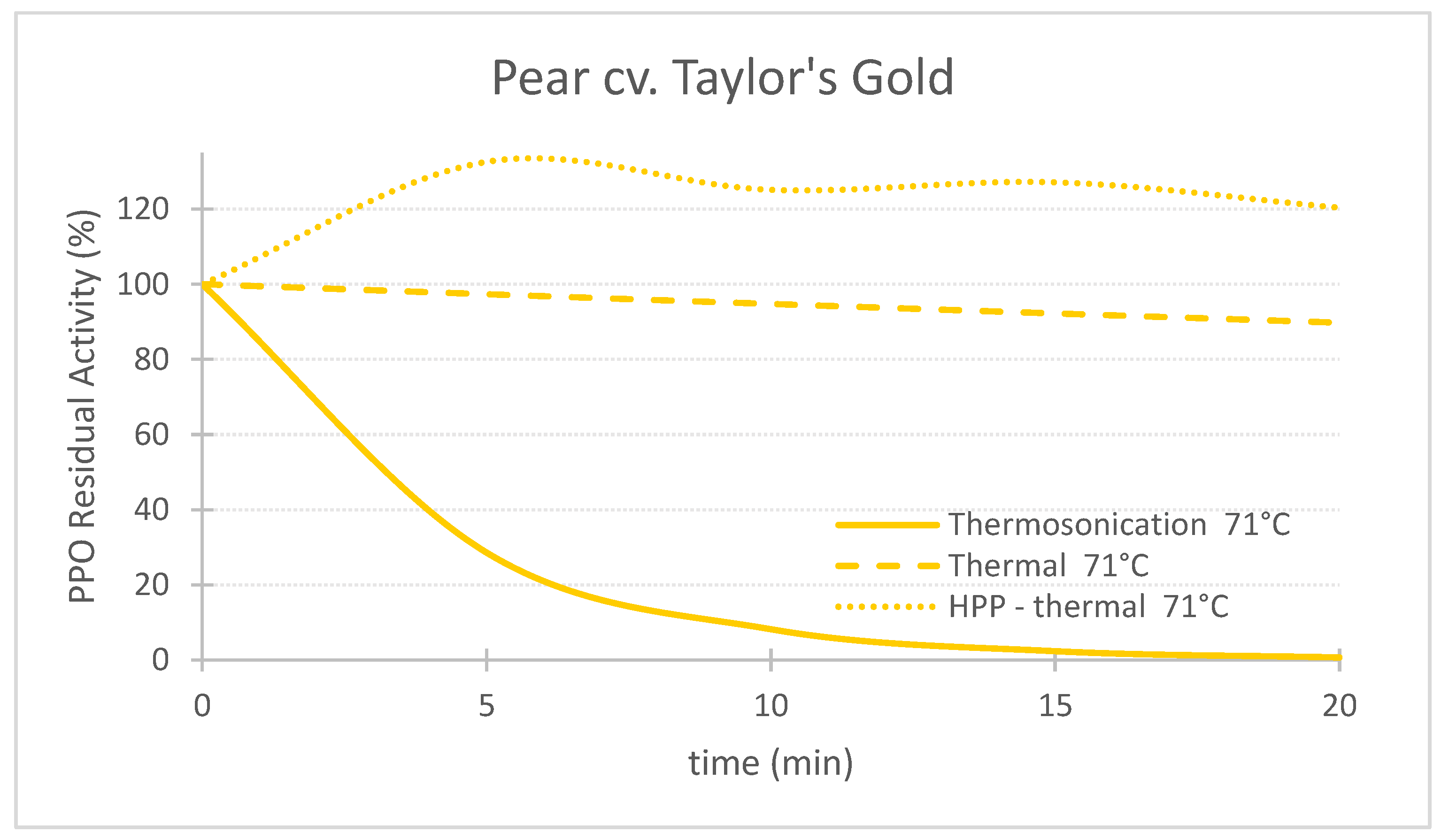 Figure 1. Comparing the effect of thermosonication (TS, 1.3 W/g—71 °C), heat assisted high pressure processing (HPTP, 600 MPa—71 °C) and thermal process alone (71 °C) on polyphenoloxidase enzyme residual activity during treatment time for ‘Taylor’s Gold’ pear puree.
Figure 1. Comparing the effect of thermosonication (TS, 1.3 W/g—71 °C), heat assisted high pressure processing (HPTP, 600 MPa—71 °C) and thermal process alone (71 °C) on polyphenoloxidase enzyme residual activity during treatment time for ‘Taylor’s Gold’ pear puree.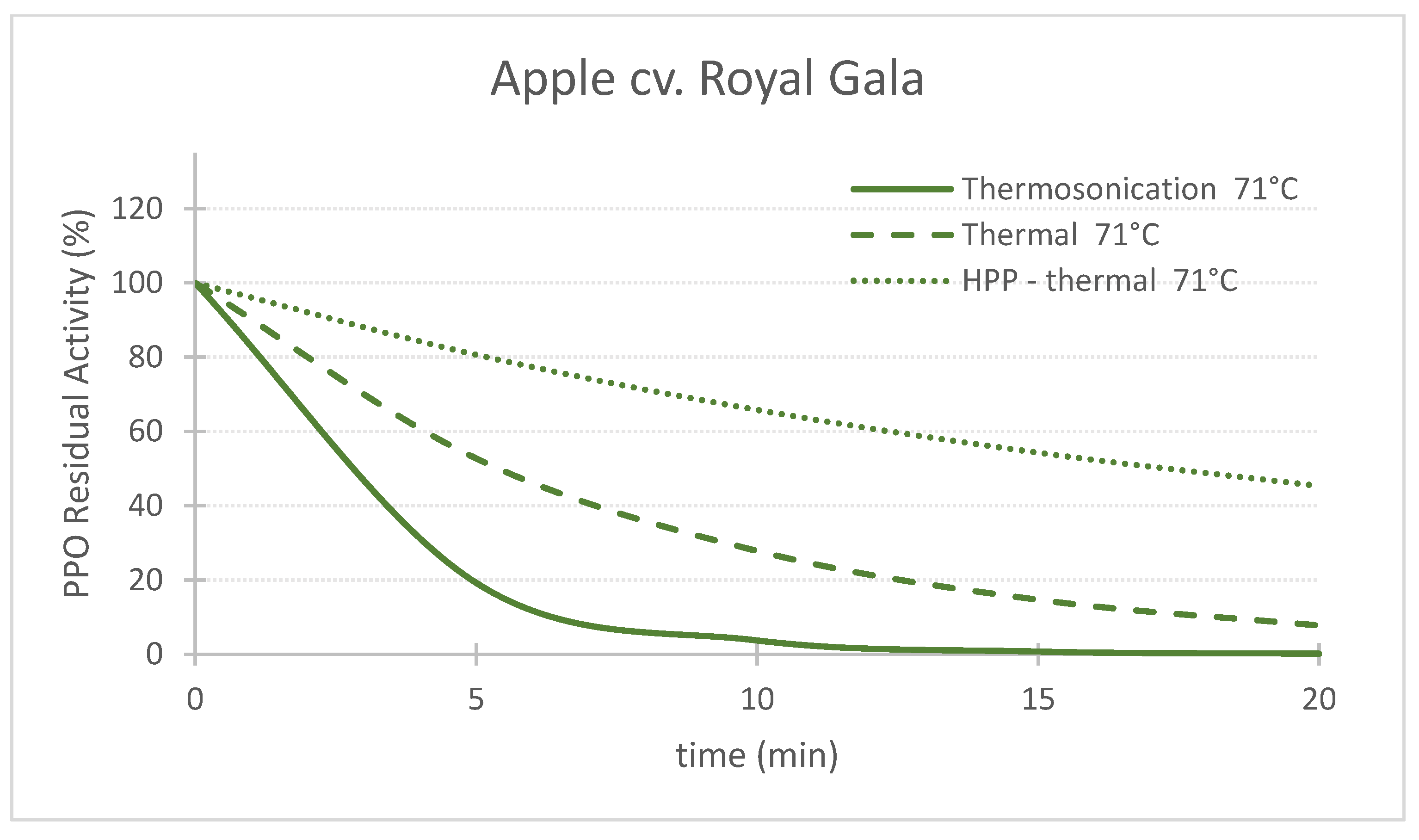 Figure 2. Comparing the effect of thermosonication (TS, 1.3 W/g—71 °C), heat-assisted high pressure processing (HPTP, 600 MPa—71 °C) and thermal process alone (71 °C) on polyphenoloxidase enzyme residual activity during treatment time for ‘Royal Gala’ apple puree.
Figure 2. Comparing the effect of thermosonication (TS, 1.3 W/g—71 °C), heat-assisted high pressure processing (HPTP, 600 MPa—71 °C) and thermal process alone (71 °C) on polyphenoloxidase enzyme residual activity during treatment time for ‘Royal Gala’ apple puree.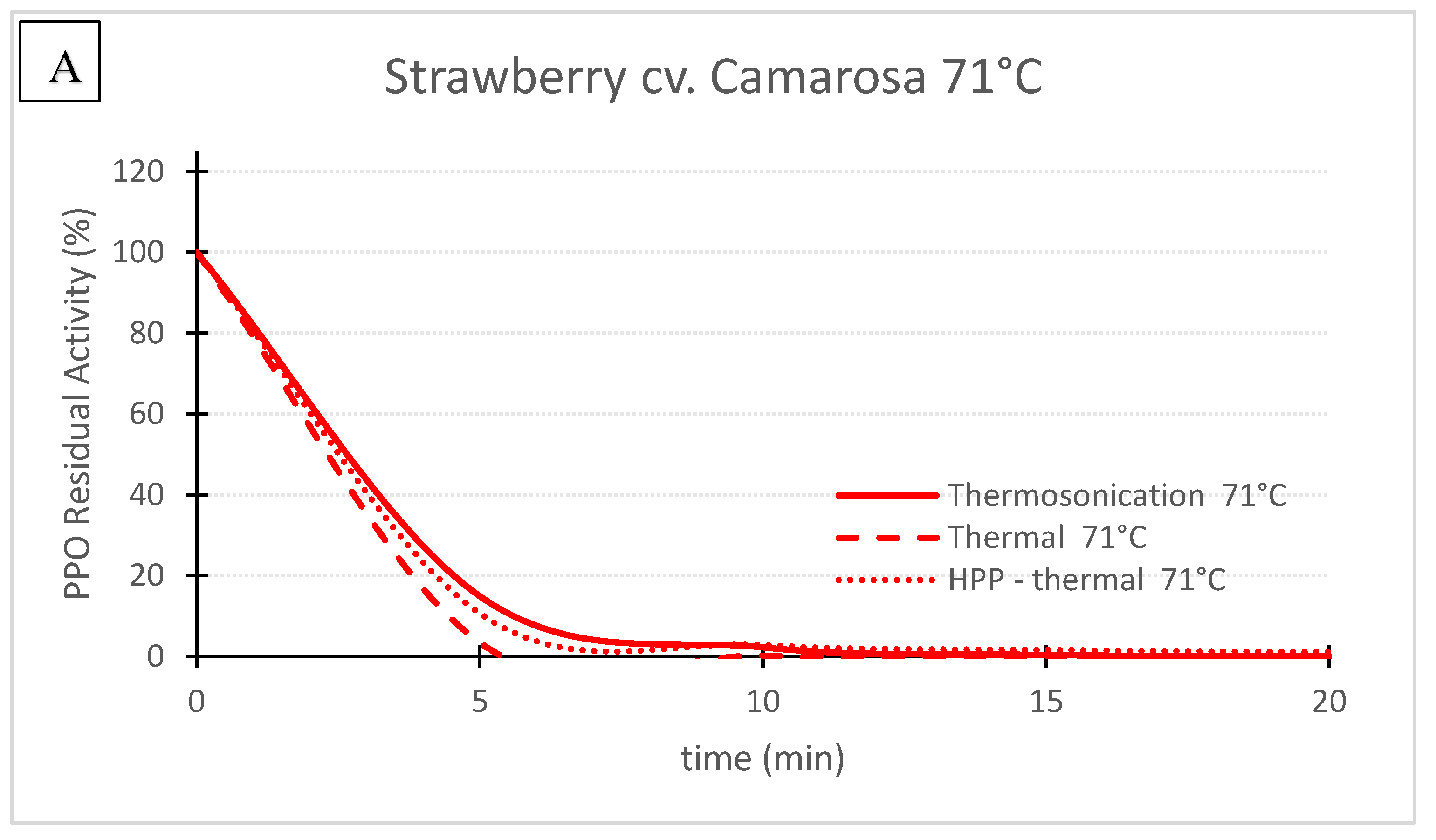
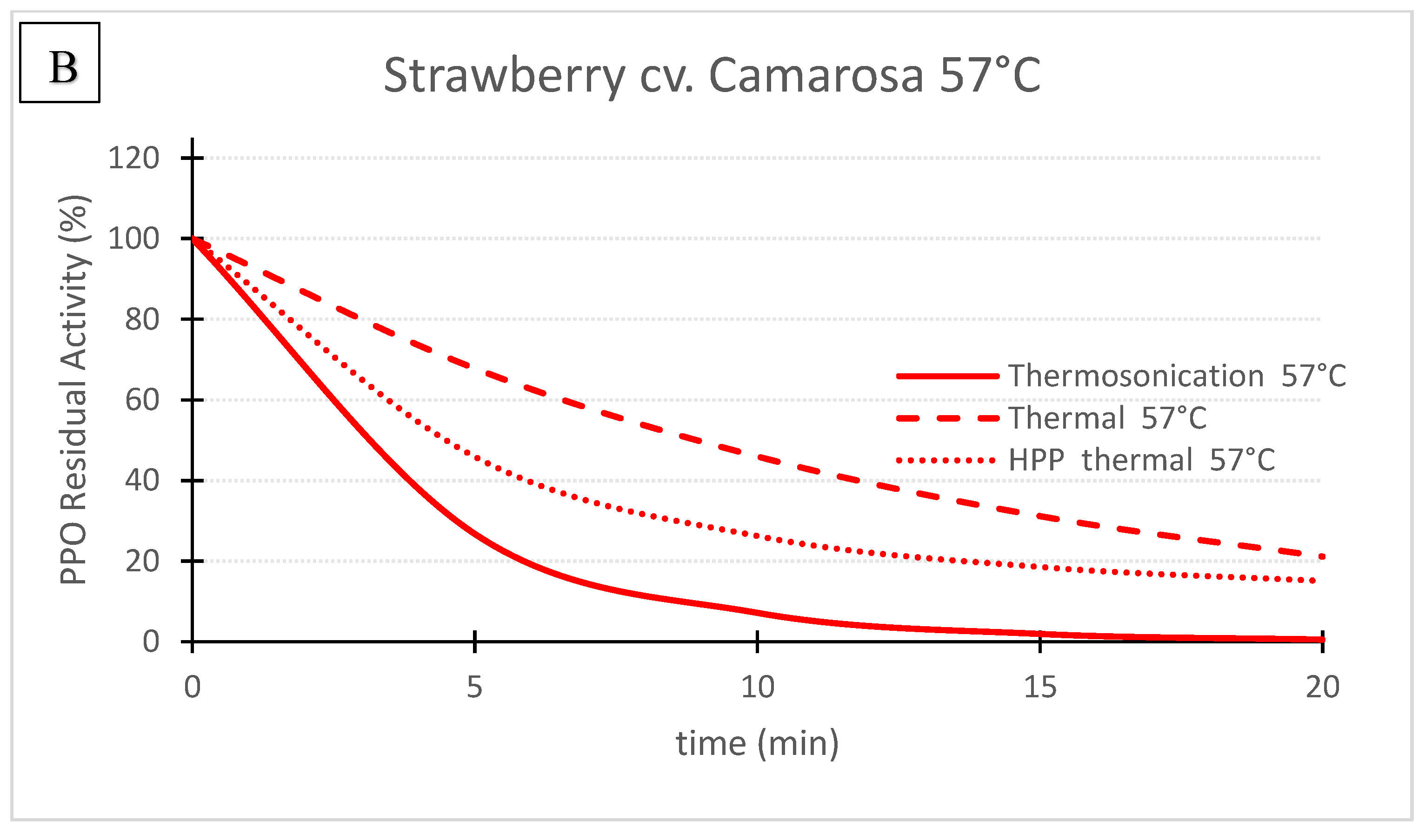 Figure 3. ‘Camarosa’ strawberry polyphenoloxidase residual activity after thermosonication (TS, 1.3 W/g), heat assisted high pressure processing (HPTP, 600 MPa) and thermal treatment alone at 71 °C (A) and 57 °C (B).
Figure 3. ‘Camarosa’ strawberry polyphenoloxidase residual activity after thermosonication (TS, 1.3 W/g), heat assisted high pressure processing (HPTP, 600 MPa) and thermal treatment alone at 71 °C (A) and 57 °C (B).| TS (1.3 W/g) |
Thermal | HPP-Thermal (600 MPa) |
|||
|---|---|---|---|---|---|
| Kinetic Model | First Order | First Order | First Order Biphasic | ||
| Fruit | Temperature (°C) | kT (min−1) |
kT (min−1) |
kL (min−1) |
kS (min−1) |
| Pear | 71 | 0.25 | 0.0054 | No inactivation | No inactivation |
| Apple | 71 | 0.33 | 0.13 | 0.061 | 0.018 |
| Strawberry | 71 | 0.38 | 0.68 | 0.51 | 0.081 |
| Strawberry | 57 | 0.26 | 0.078 | 0.21 | 0.018 |
5. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/foods11131942
References
- Chen, J.S.; Wei, C.; Rolle, R.S.; Otwell, W.S.; Balaban, M.O.; Marshall, M.R. Inhibitory effect of kojic acid on some plant and crustacean polyphenol oxidases. J. Agric. Food Chem. 1991, 39, 1396–1401.
- Mayer, A.M.; Harel, E. Polyphenoloxidases and their significance in fruits and vegetables. In Food Enzymology; Fox, P.F., Ed.; Elsevier Applied Science: London, UK, 1991; p. 373.
- Whitaker, J.R.; Lee, C.Y. Recent advances in chemistry of enzymatic browning. In Enzymatic Browning and Its Prevention; Lee, C.Y., Whitaker, J.R., Eds.; American Chemical Society: Washington, DC, USA, 1995; p. 3.
- Lee, C.Y. Browning reaction, enzymatic. In Wiley Encyclopedia of Food Science and Technology; Francis, F.J., Ed.; John Wiley & Sons: New York, NY, USA, 1999; p. 208.
- Silva, F.V.M.; Sulaiman, A. Polyphenoloxidase in Fruit and Vegetables: Inactivation by Thermal and Non-Thermal Processes. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019.
- Nogales-Delgado, S. Polyphenoloxidase (PPO): Effect, Current Determination and Inhibition Treatments in Fresh-Cut Produce. Appl. Sci. 2021, 11, 7813.
- Golangoldhirsh, A.; Whitaker, J.; Kahn, V. Relation between structure of polyphenol oxidase and prevention of browning. In Nutritional and Toxicological Aspects of Food Safety; Friedman, M., Ed.; Springer: New York, NY, USA, 1984; Volume 177, pp. 437–456.
- Vámos-Vigyázó, L. Prevention of enzymatic browning in fruits and vegetables—A review of principles and practice. In Enzymatic Browning and Its Prevention; Lee, C.Y., Whitaker, J.R., Eds.; American Chemical Society: Washington, DC, USA, 1995.
- Silva, F.V.M.; Gibbs, A. Principles of Thermal Processing: Pasteurization. In Engineering Aspects of Thermal Food Processing; Simpson, R., Ed.; Contemporary Food Engineering Series; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2009; pp. 13–48.
- Evelyn; Silva, F.V.M. Inactivation of Pathogenic Microorganisms in Foods by High Pressure Processing. In Food Safety and Protection; Rai, V.R., Bai, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 343–378.
- Evelyn; Silva, F.V.M. High Pressure Processing Effect on Microorganisms in Fruit and Vegetable Products. In High Pressure Processing of Fruit and Vegetable Products; Houska, M., Silva, F.V.M., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 3–38.
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards); Koutsoumanis, K.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. Scientific Opinion on the efficacy and safety of high-pressure processing of food. EFSA J. 2022, 20, e07128.
- Silva, F.V.M.; Gibbs, P. Target Selection in Designing Pasteurization Processes for Shelf-Stable High-Acid Fruit Products. Crit. Rev. Food Sci. Nutr. 2004, 44, 353–360.
- Silva, F.V.M.; Sulaiman, A. Advances in Thermosonication for the Inactivation of Endogenous Enzymes in Foods. In Ultrasound: Advances in Food Processing and Preservation; Bermudez-Aguirre, D., Ed.; Academic Press, Elsevier: Cambridge, MA, USA, 2017; pp. 101–130.
- Sulaiman, A.; Soo, M.J.; Farid, M.; Silva, F.V.M. Thermosonication for polyphenoloxidase inactivation in fruits: Modeling the ultrasound and thermal kinetics in pear, apple and strawberry purees at different temperatures. J. Food Eng. 2015, 165, 133–140.
- Feng, H.; Yang, W. Ultrasonic Processing. In Nonthermal Processing Technologies for Food; Zhang, H.Q., Barbosa-Canovas, G.V., Balasubramaniam, V.M., Dunne, C.P., Farkas, D.F., Yuan, J.T.C., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 135–154.
- Weiss, J.; Gulseren, I.; Kjartansson, G. Physicochemical effects of high-intensity ultrasonication on food proteins and carbohydrates. In Nonthermal Processing Technologies for Food; Zhang, H.Q., Barbosa-Canovas, G.V., Balasubramaniam, V.M., Dunne, C.P., Farkas, D.F., Yuan, J.T.C., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 109–134.
- Islam, M.N.; Zhang, M.; Adhikari, B. The inactivation of enzymes by ultrasound—A review of potential mechanisms. Food Rev. Int. 2014, 30, 1–21.
- Zhong, M.T.; Ming, X.W.; Su, P.W.; Ju, Q.K. Effects of ultrasound and additives on the function and structure of trypsin. Ultrason. Sonochem. 2004, 11, 399–404.
- Houška, M.; Silva, F.V.M. High Pressure Processing of Fruit and Vegetable Products; Contemporary Food Engineering Series; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2018; 178p.
- Terefe, N.; Buckow, R. High-Pressure Processing Effects on Endogenous Enzymes in Fruits and Vegetables. In High Pressure Processing of Fruit and Vegetable Products; Houska, M., Silva, F.V.M., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 39–62.
- Sulaiman, A.; Soo, M.J.; Yoon, M.M.L.; Farid, M.; Silva, F.V.M. Modeling the polyphenoloxidase inactivation kinetics in pear, apple and strawberry purees after High Pressure Processing. J. Food Eng. 2015, 147, 89–94.
- Hendrickx, M.; Ludikhuyze, L.; Van den Broeck, I.; Weemaes, C. Effects of high pressure on enzymes related to food quality. Trends Food Sci. Technol. 1998, 9, 197–203.
- Zawawi, N.A.F.; Hazmi, N.A.M.; How, M.S.; Kantono, K.; Silva, F.V.M.; Sulaiman, A. Thermal, High Pressure, and Ultrasound Inactivation of Various Fruit Cultivars’ Polyphenol Oxidase: Kinetic Inactivation Models and Estimation of Treatment Energy Requirement. Appl. Sci. 2022, 12, 1864.
- Mozhaev, V.V.; Lange, R.; Kudryashova, E.V.; Balny, C. Application of high hydrostatic pressure for increasing activity and stability of enzymes. Biotechnol. Bioeng. 1996, 52, 320–331.
- Asaka, M.; Hayashi, R. Activation of polyphenoloxidase in pear fruits by high-pressure treatment. Agric. Biol. Chem. 1991, 55, 2439–2440.
- Buckow, R.; Weiss, U.; Knorr, D. Inactivation kinetics of apple polyphenol oxidase in different pressure-temperature domains. Innov. Food Sci. Emerg. Technol. 2009, 10, 441–448.
- Bayindirli, A.; Alpas, H.; Bozoglu, F.; Hizal, M. Efficiency of high pressure treatment on inactivation of pathogenic microorganisms and enzymes in apple, orange, apricot and sour cherry juices. Food Control 2006, 17, 52–58.
- Tribst, A.A.L.; Júnior, B.R.C.L.; Oliveira, M.M.; Cristianini, M. High pressure processing of cocoyam, Peruvian carrot and sweet potato: Effect on oxidative enzymes and impact in the tuber color. Innov. Food Sci. Emerg. Technol. 2016, 34, 302–309.
- Matser, A.M.; Knott, E.R.; Teunissen, P.G.M.; Bartels, P.V. Effects of high isostatic pressure on mushrooms. J. Food Eng. 2000, 45, 11–16.
- Houška, M.; Silva, F.V.M.; Evelyn; Buckow, R.; Terefe, N.S.; Tonello, C. High Pressure Processing Applications in Plant Foods. Foods 2022, 11, 223.
- Milani, E.A.; Silva, F.V.M. Ultrasound assisted thermal pasteurization of beers with different alcohol levels: Inactivation of Saccharomyces cerevisiae ascospores. J. Food Eng. 2017, 198, 45–53.
