Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Since 1974, more than 800 disinfection byproducts (DBPs) have been identified from disinfected drinking water, swimming pool water, wastewaters, etc. Some DBPs are recognized as contaminants of high environmental concern because they may induce many detrimental health (e.g., cancer, cytotoxicity, and genotoxicity) and/or ecological (e.g., acute toxicity and development toxicity on alga, crustacean, and fish) effects. However, the information on whether DBPs may elicit potential endocrine-disrupting effects in human and wildlife is scarce.
- disinfection byproducts
- endocrine-disrupting effect
- adverse outcome pathways
- molecular initiating event
- receptor-mediated mechanism
1. Characterization of DBPs with Endocrine-Disrupting Data
As shown in Figure 1, these 131 disinfection byproducts (DBPs) could be divided into four classes (i.e., aromatic, aliphatic, alicyclic, and heterocyclic DBPs) on the basis of their chemical structure. Aromatic DBPs could be further classified into eight subgroups (i.e., halogenated phenyl esters, estrogen DBPs, halophenols, halogenated phenyl acids, halogenated phenyl aldehydes, halogenated phenyl nitriles, halonitrophenols, and nonhalogenated phenyl aldehydes). Aliphatic DBPs included seven subgroups (i.e., halogenated nitriles, halogenated acids, halogenated amides, halogenated alkanes, halogenated alcohols, nitrosamines and nitramines, and halogenated nitroalkanes). Alicyclic DBPs contained two subgroups (halogenated benzoquinones and others). Heterocyclic DBPs were represented by nonhalogenated furanone.
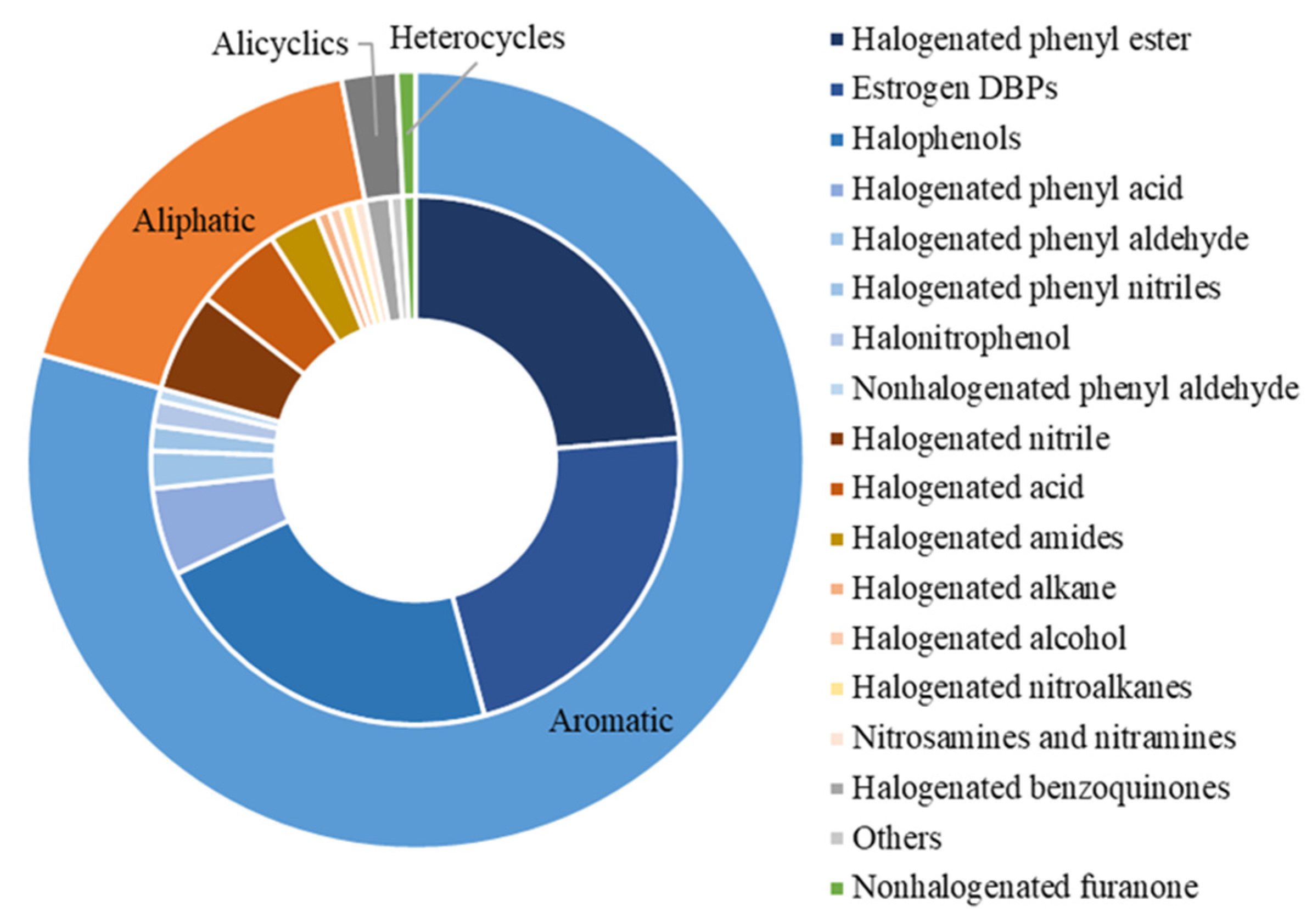
Figure 1. Summary of DBPs identified with endocrine-disrupting potential.
For each studied endocrine endpoint, the researchers also summarized the number of active compounds, inactive compounds, and compounds without available data. As shown in Figure 2, the number of active compounds for hERα was more than that of other endpoints. For hERα, human aryl hydrocarbon receptor (hAhR), and human androgen receptor (hAR), the number of active compounds was greater than that of inactive compounds. On the other hand, all the tested DBPs were active compounds for human transthyretin (hTTR), bullfrog transthyretin (bTTR), chicken transthyretin (cTTR), human serum albumin (HSA), peroxisome proliferator–activated receptor (hPPAR), human retinoic X receptor (hRXR), bullfrog thyroid receptor β (bTRβ), chicken thyroid receptor β (cTRβ), and human estrogen receptor β (hERβ).
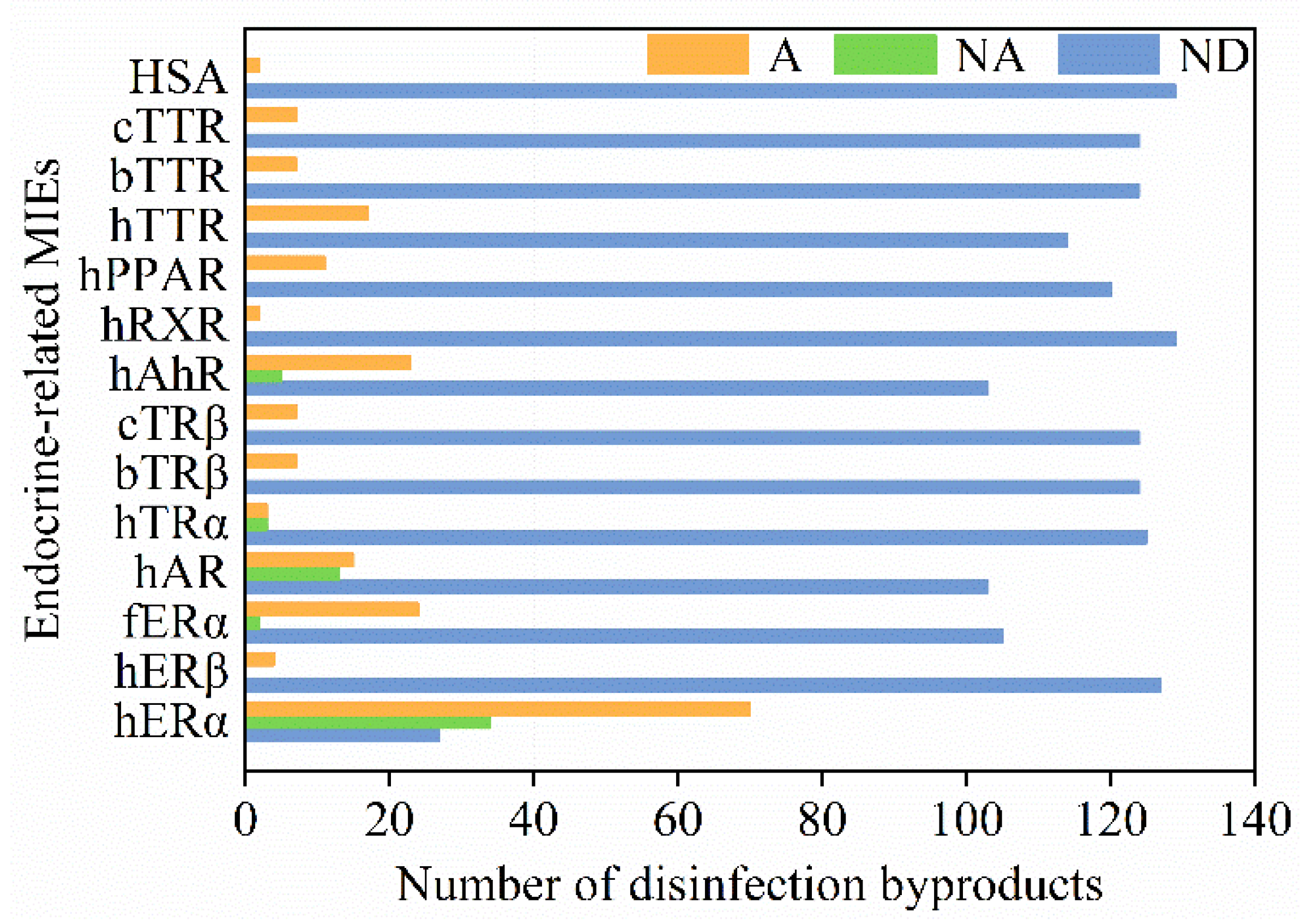
Figure 2. Overview of DBPs and their associated endocrine-disrupting effects. Orange, the number of active compounds (A); green, the number of inactive compounds (NA); blue, the number of compounds without available data (ND). Abbreviations: hERα—human estrogen receptor α; hERβ—human estrogen receptor β; fERα—medaka fish estrogen receptor α; hAR—human androgen receptor; hTRα—human thyroid receptor α; bTRβ—bullfrog thyroid receptor β; cTRβ—chicken thyroid receptor β; hAhR—human aryl hydrocarbon receptor; hRXR—human retinoic X receptor; hPPAR—peroxisome proliferator–activated receptor; hTTR—human transthyretin; bTTR—bullfrog transthyretin; cTTR—chicken transthyretin; HSA—human serum albumin.
2. Endocrine-Related MIEs of DBPs
DBPs can disturb normal endocrine homeostasis by regulating the hormone system for fundamental physiological and developmental control [1]. The perturbing mechanisms of DBPs include activating/inhibiting nuclear receptors and interfering with non-receptor-mediated pathways. It is reported that most of adverse outcomes of endocrine-disrupting chemicals (EDCs) are attributed to the fact that they interfere with nuclear receptor (NR)-mediated hormone signals [2]. The substance structure of some DBPs is similar to that of natural hormones; thus, they can directly bind with receptors, interfere with the hormone pathway, and show distinct disrupting activities. The mediated physiological and biochemical pathways of several receptors on which the Guidance for the Identification of Endocrine Disruptors (EFSA/ECHA, 2018) focuses [3], including androgen receptor (AR), estrogen receptor (ER), and thyroid receptor (TR), are of critical importance in significant biological studies of endocrine disruption effects. All the tested molecular-initiating events related to DBPs are illustrated in Figure 3.
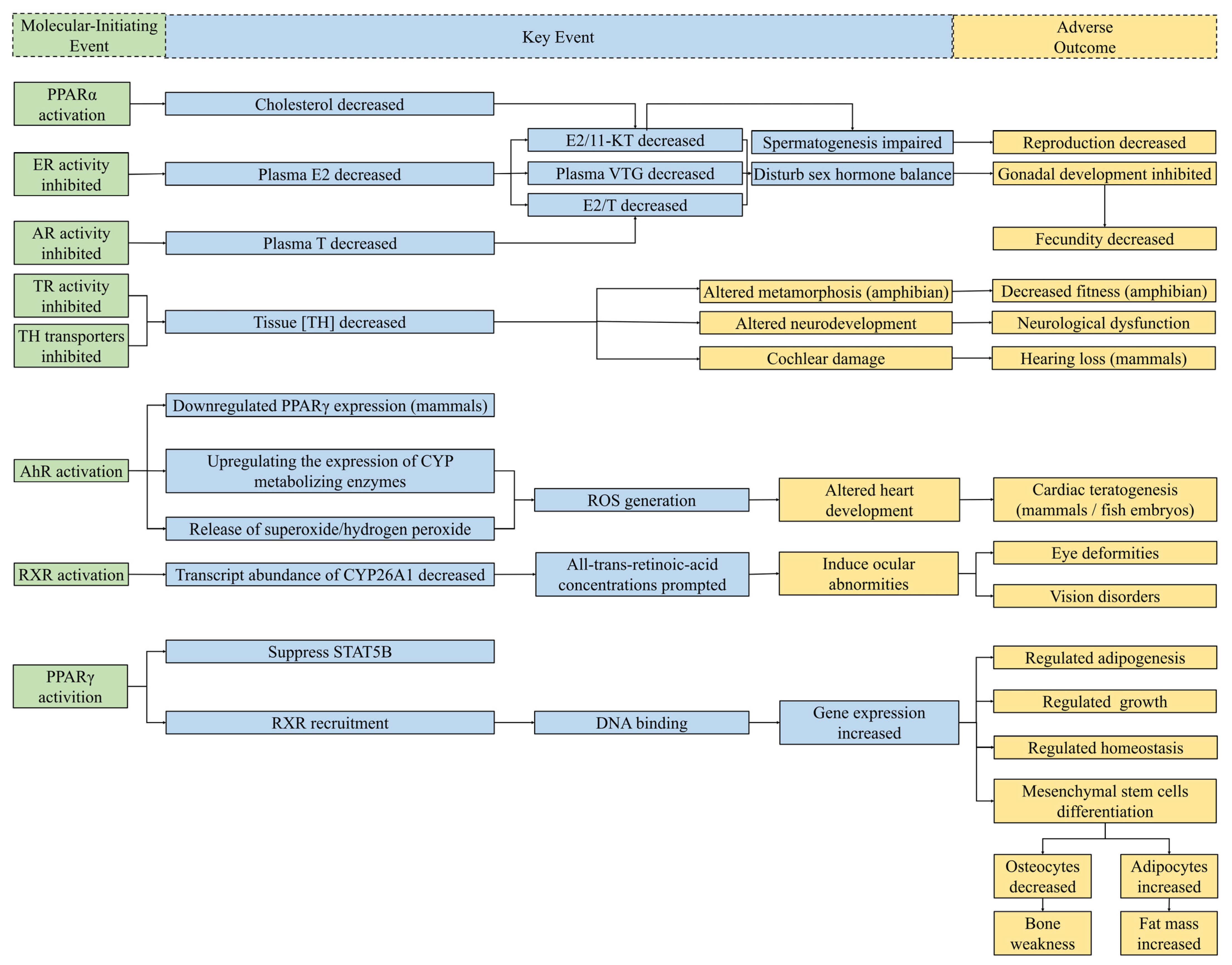
Figure 3. Schematic diagram of known MIEs for endocrine disruption of DBPs and several related potential KEs and AOs. The relationships between MIEs and potential KEs in this figure were collected from compounds other than DBPs [4][5][6][7][8][9]. Considering the significant relationships between each step, MIEs (left) bring about KEs (middle), and then lead to AOs (right). Green boxes, MIEs associated with endocrine perturbation of DBPs; blue boxes, KEs of endocrine disruptors collected from studies; yellow boxes, AOs for endocrine toxicity. Abbreviations: E2, estradiol; T, testosterone; 11-KT, 11-ketotestosterone; VTG, vitellogenin; TH, thyroid hormones; CYP, cytochrome P450; ROS, reactive oxygen species; STATSB, signal transducer and activator of transcription 5B.
2.1. Hormone Receptor-Mediated Mechanism of Endocrine Disruption
Estrogen receptors (ERs) have critical roles in the growth and development of organisms [10]. The recombinant yeast screening bioassay, the E-screen assay of MCF-7 and MVLN cell line, and the uterotrophic bioassay are usually adopted for identifying potential estrogenic disruptors [11][12][13]. The researchers' analysis results indicated that 70 DBPs have been proven to have estrogenic activity, i.e., they can interfere with ER. There is evidence in toxicological and epidemiological research in cell cultures that haloacetonitriles (HANs), e.g., dibromoacetonitrile (DBAN) and 2,3-dibromopropionitrile (DBPN), can invoke adverse effects on the endocrine system by binding to the human estrogen receptor and androgen receptor [14][15]. Additionally, Nakamura et al. [16] reported that halogenated derivatives of E1, E2, E3, and EE2 showed estrogenic activity, interfering with estrogen receptor α, using yeast two-hybrid assays between human and medaka fish (Oryzias latipes), and the ER-binding potency of halogenated DBPs of estrogens substituted at the 2- and 4-positions displayed a similar trend.
The androgen hormone regulates the androgen signaling pathway via binding with the androgen receptor (AR), and it plays an essential role in the physiological processes of human development and reproduction [17]. Iodoacetic acid (IAA) was observed to show AR binding in vitro [14]. Despite the discrepancies between this result and others, studies have still demonstrated that IAA is a potential disruptor of human AR (hAR) [11]. The differences in research results may be due to factors such as the selection of species of cells and diverse endpoints. Additionally, among haloacetamide DBPs, bromoacetamide (BAM) exhibited slight androgenic activity according to a yeast-based reporter bioassay [18]. Notably, iodoacetonitrile (IAN) generated from water disinfection processes was found to have a weak androgenic effect (11.4% induction) at the highest concentration [12].
Thyroid hormones (THs), a series of essential endocrine hormones, are synthesized and secreted by thyroid follicular cells. They exist in many tissues in the brain, heart, liver, etc., where they regulate metabolism and development [19]. THs, especially triiodothyronine (T3), mainly moderate gene transcription or protein expression via binding to thyroid hormone receptors (TRs) [20]. Halogenated derivatives of bisphenol A (BPA) have been shown to act as agonists/antagonists for TH receptors, affecting the levels of THs and invoking thyroid system disruption in organisms. 3,3’,5,5’-Tetrabromobisphenol A (TBBPA), 3,3’,5,5’-tetrachlorobisphenol A (TCBPA), and 3,3’,5-trichlorobisphenol A (3,3’,5-triClBPA) were proven to possess human TH agonist activity in a yeast two-hybrid assay incorporating hTRα [21]. In addition, Yamauchi et al. [22] investigated the influence of chlorinated compounds of BPA on T3 binding with the TR ligand-binding domains between chicken and bullfrog but demonstrated that they were unlikely to be TH system-disrupting compounds for these animals.
Some chemicals could bind to other receptors to indirectly participate in hormone regulation instead of acting directly on hormone receptors. For example, peroxisome proliferator-activated receptor gamma (PPARγ), expressed in the fatty tissue, is a critical transcription element in the development and metabolism of adipocytes [23]. The imbalance of PPAR might be associated with diseases such as diabetes, obesity, and dysgenesis [24]. A previous 293T cell-based luciferase reporter bioassay indicated that chlorinated BPS analogs enhanced PPAR activities as opposed to the parent compound, and their activities were correlated to the values of logKow [23]. TBBPA and TCBPA could also activate PPAR through direct interaction with humans or animals, and the activation potential highly relied on the halogenation degree [2][25]. The results from in vitro experiments revealed that halogenated products of BPF were also potential disruptors of PPAR, similar to those of BPA and BPS [26]. Taken together, the presence of DBPs of BPA, BPS, and BPF in disinfected water should be of concern because they could pose a potential risk to mitigation of inflammation.
Furthermore, human retinoic X receptor (RXRs) have also been shown to be endocrine-related targets for DBPs action. RXRs are key partners for the nuclear receptor signaling pathways of cell growth, differentiation, and metabolism [27]. Chlorination byproducts of BPA have been identified as RXRβ antagonists, the antagonist activities of which are much higher than that of BPA according to a yeast assay [28]. Considering that previous studies documented that BPA could exhibit several detrimental effects (e.g., endocrine-related harmful effects) on organisms [29][30][31][32][33], those results indicate that both BPA and its halogenated DBPs are potential endocrine disruptors. Experimental evidence for DBPs with respect to their AhR binding affinities is rather limited. In terms of structure, halogenated parabens are similar to halogenated aromatic hydrocarbons, which were determined to possess AhR potency. Experimental values obtained via a yeast bioassay and HepG2 cells showed that the AhR activity of monochlorinated parabens was more effective than that of their unsubstituted or chlorinated counterparts [34]. Analogously, this regular pattern is also applicable to monobrominated by-products. Promisingly, it was noted that 3-BrBP, 3-BrBnP, and 3-BriBP, compared with their unsubstituted and brominated corresponding counterparts, were proven to have the highest AhR activity with EC50 values of 3.9 nM, 9.0 nM, and 9.6 nM, respectively [35].
2.2. Non-Receptor-Mediated Mechanism of Endocrine Disruption
It has been recognized that activation or inhibition of nuclear receptors is not the only endocrine-disrupting pathway for DBPs to exert endocrine-perturbing effects [3]. Another toxicity pathway leading to an endocrine-related detrimental influence is the non-receptor-mediated mechanism [18]. Instead of acting directly on nuclear receptors, the pathway of non-receptor-mediated activity interference comprises inhibition of protein synthesis, destruction of β-galactosidase gene transcription, and inhibition of enzyme activity [36]. Endocrine disruptors can affect some links of the hypothalamus–pituitary–thyroid (HPT), hypothalamic–pituitary–gonadal (HPG), and hypothalamic–pituitary–adrenal (HPA) axes, and further disturb hormones biosynthesis, secretion, transport, metabolism, and feedback regulation [7][37]. There are three transporters in human blood that carry THs to target tissues: transthyretin (TTR), thyroxine-binding globulin (TBG), and albumin (ALB) [38].
The results from Yang et al. [39] revealed that 2,4,6-trihalo-phenols, 2,6-dihalo-4-nitrophenols, and 3,5-dihalo-4-hydroxybenzaldehydes, representing emerging polar phenolic DBPs, were identified as high-potency binders to compete with THs for binding to human TTR. Disrupting the transportation of TH might bring about DBPs being delivered to unexpected sites, which might further induce TH-related perturbing effects [37][40]. Previous evidence also showed that 2,6-dichloro-4-nonylphenol is a potent competitor of T3 interacting with chicken and bullfrog TTR, along with by-products of nonylphenol [22]. Furthermore, the comparison of TTR-binding activities among brominated derivatives of BPA indicated that the presence of a hydroxyl group at the para position and halogen substituents were conditions for TTR-binding effects [41]. These experimental results may confirm the conclusion that halogenated aromatic chemicals with phenol hydroxy groups can be considered as binders to TTR owing to their similar structure to the natural thyroxine (T4) [42]. ALB is also a potential endocrine-related target in the mechanism of TH transport disruption. According to competitive binding assays, 4-bromophenol and 2,4-dibromophenol were observed to interfere with human serum albumin (HSA) to form complexes [43]. Remarkably, 2,4-dibromophenol had a high binding affinity to HSA.
This entry is adapted from the peer-reviewed paper 10.3390/jox12030013
References
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342.
- Riu, A.; le Maire, A.; Grimaldi, M.; Audebert, M.; Hillenweck, A.; Bourguet, W.; Balaguer, P.; Zalko, D. Characterization of novel ligands of ERalpha, Erbeta, and PPARgamma: The case of halogenated bisphenol A and their conjugated metabolites. Toxicol. Sci. 2011, 122, 372–382.
- Stanojević, M.; Vračko Grobelšek, M.; Sollner Dolenc, M. Computational evaluation of endocrine activity of biocidal active substances. Chemosphere 2021, 267, 129284.
- Jin, H.; Ji, C.; Ren, F.; Aniagu, S.; Tong, J.; Jiang, Y.; Chen, T. AHR-mediated oxidative stress contributes to the cardiac developmental toxicity of trichloroethylene in zebrafish embryos. J. Hazard. Mater. 2020, 385, 121521.
- Li, Y.; Ma, H.; Chen, R.; Zhang, H.; Nakanishi, T.; Hu, J. Maternal Transfer of 2-Ethylhexyl Diphenyl Phosphate Leads to Developmental Toxicity Possibly by Blocking the Retinoic Acid Receptor and Retinoic X Receptor in Japanese Medaka (Oryzias latipes). Environ. Sci. Technol. 2021, 55, 5056–5064.
- Kirk, A.B.; Michelsen-Correa, S.; Rosen, C.; Martin, C.F.; Blumberg, B. PFAS and Potential Adverse Effects on Bone and Adipose Tissue Through Interactions with PPARgamma. Endocrinology 2021, 162, bqab194.
- Chen, P.; Yang, J.; Chen, G.; Yi, S.; Liu, M.; Zhu, L. Thyroid-Disrupting Effects of 6:2 and 8:2 Polyfluoroalkyl Phosphate Diester (diPAPs) at Environmentally Relevant Concentrations from Integrated In Silico and In Vivo Studies. Environ. Sci. Technol. Lett. 2020, 7, 330–336.
- Paul Friedman, K.; Watt, E.D.; Hornung, M.W.; Hedge, J.M.; Judson, R.S.; Crofton, K.M.; Houck, K.A.; Simmons, S.O. Tiered High-Throughput Screening Approach to Identify Thyroperoxidase Inhibitors Within the ToxCast Phase I and II Chemical Libraries. Toxicol. Sci. 2016, 151, 160–180.
- Park, C.B.; Kim, G.E.; On, J.; Pyo, H.; Park, J.W.; Cho, S.H. Sex-specific effects of bisphenol S with tissue-specific responsiveness in adult zebrafish: The antiandrogenic and antiestrogenic effects. Ecotoxicol. Environ. Saf. 2022, 229, 113102.
- He, J.; Peng, T.; Yang, X.; Liu, H. Development of QSAR models for predicting the binding affinity of endocrine disrupting chemicals to eight fish estrogen receptor. Ecotoxicol. Environ. Saf. 2018, 148, 211–219.
- Long, K.; Sha, Y.; Mo, Y.; Wei, S.; Wu, H.; Lu, D.; Xia, Y.; Yang, Q.; Zheng, W.; Wei, X. Androgenic and Teratogenic Effects of Iodoacetic Acid Drinking Water Disinfection Byproduct in Vitro and in Vivo. Environ. Sci. Technol. 2021, 55, 3827–3835.
- Park, C.G.; Jung, K.C.; Kim, D.H.; Kim, Y.J. Monohaloacetonitriles induce cytotoxicity and exhibit different mode of action in endocrine disruption. Sci. Total Environ. 2021, 761, 143316.
- Song, M.; Liang, D.; Liang, Y.; Chen, M.; Wang, F.; Wang, H.; Jiang, G. Assessing developmental toxicity and estrogenic activity of halogenated bisphenol A on zebrafish (Danio rerio). Chemosphere 2014, 112, 275–281.
- Holmes, B.E.; Smeester, L.; Fry, R.C.; Weinberg, H.S. Identification of endocrine active disinfection by-products (DBPs) that bind to the androgen receptor. Chemosphere 2017, 187, 114–122.
- Holmes, B.E.; Smeester, L.; Fry, R.C.; Weinberg, H.S. Disinfection Byproducts Bind Human Estrogen Receptor-alpha. Environ. Toxicol. Chem. 2019, 38, 956–964.
- Nakamura, H.; Shiozawa, T.; Terao, Y.; Shiraishi, F.; Fukazawa, H. By-products produced by the reaction of estrogens with hypochlorous acid and their estrogen activities. J. Health Sci. 2006, 52, 124–131.
- Zorn, K.M.; Foil, D.H.; Lane, T.R.; Hillwalker, W.; Feifarek, D.J.; Jones, F.; Klaren, W.D.; Brinkman, A.M.; Ekins, S. Comparison of Machine Learning Models for the Androgen Receptor. Environ. Sci. Technol. 2020, 54, 13690–13700.
- Kim, D.H.; Park, C.G.; Kim, Y.J. Characterizing the potential estrogenic and androgenic activities of two disinfection byproducts, mono-haloacetic acids and haloacetamides, using in vitro bioassays. Chemosphere 2020, 242, 125198.
- Xia, Y.; Mo, Y.; Yang, Q.; Yu, Y.; Jiang, M.; Wei, S.; Lu, D.; Wu, H.; Lu, G.; Zou, Y.; et al. Iodoacetic Acid Disrupting the Thyroid Endocrine System in Vitro and in Vivo. Environ. Sci. Technol. 2018, 52, 7545–7552.
- Paul-Friedman, K.; Martin, M.; Crofton, K.M.; Hsu, C.W.; Sakamuru, S.; Zhao, J.; Xia, M.; Huang, R.; Stavreva, D.A.; Soni, V.; et al. Limited Chemical Structural Diversity Found to Modulate Thyroid Hormone Receptor in the Tox21 Chemical Library. Environ. Health Perspect. 2019, 127, 097009.
- Terasaki, M.; Kosaka, K.; Kunikane, S.; Makino, M.; Shiraishi, F. Assessment of thyroid hormone activity of halogenated bisphenol A using a yeast two-hybrid assay. Chemosphere 2011, 84, 1527–1530.
- Yamauchi, K.; Ishihara, A.; Fukazawa, H.; Terao, Y. Competitive interactions of chlorinated phenol compounds with 3,3′,5-triiodothyronine binding to transthyretin: Detection of possible thyroid-disrupting chemicals in environmental waste water. Toxicol. Appl. Pharmacol. 2003, 187, 110–117.
- Zheng, S.; Shi, J.; Zhang, J.; Yang, Y.; Hu, J.; Shao, B. Identification of the disinfection byproducts of bisphenol S and the disrupting effect on peroxisome proliferator-activated receptor gamma (PPARgamma) induced by chlorination. Water Res. 2018, 132, 167–176.
- Swedenborg, E.; Rüegg, J.; Mäkelä, S.; Pongratz, I. Endocrine disruptive chemicals: Mechanisms of action and involvement in metabolic disorders. J. Mol. Endocrinol. 2009, 43, 1–10.
- Riu, A.; Grimaldi, M.; le Maire, A.; Bey, G.; Phillips, K.; Boulahtouf, A.; Perdu, E.; Zalko, D.; Bourguet, W.; Balaguer, P. Peroxisome proliferator-activated receptor gamma is a target for halogenated analogs of bisphenol A. Environ. Health Perspect. 2011, 119, 1227–1232.
- Zheng, S.; Shi, J.C.; Hu, J.Y.; Hu, W.X.; Zhang, J.; Shao, B. Chlorination of bisphenol F and the estrogenic and peroxisome proliferator-activated receptor gamma effects of its disinfection byproducts. Water Res. 2016, 107, 1–10.
- Tanaka, T.; De Luca, L.M. Therapeutic Potential of "Rexinoids" in Cancer Prevention and Treatment. Cancer Res. 2009, 69, 4945–4947.
- Li, N.; Jiang, W.; Ma, M.; Wang, D.; Wang, Z. Chlorination by-products of bisphenol A enhanced retinoid X receptor disrupting effects. J. Hazard. Mater. 2016, 320, 289–295.
- Henriksen, A.D.; Andrade, A.; Harris, E.P.; Rissman, E.F.; Wolstenholme, J.T. Bisphenol A Exposure in Utero Disrupts Hypothalamic Gene Expression Particularly Genes Suspected in Autism Spectrum Disorders and Neuron and Hormone Signaling. Int. J. Mol. Sci. 2020, 21, 3129.
- Ponzi, D.; Gioiosa, L.; Parmigiani, S.; Palanza, P. Effects of Prenatal Exposure to a Low-Dose of Bisphenol A on Sex Differences in Emotional Behavior and Central Alpha2-Adrenergic Receptor Binding. Int. J. Mol. Sci. 2020, 21, 3269.
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34.
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the Great Divide: A Review of Controversies in the Field of Endocrine Disruption. Endocr. Rev. 2009, 30, 75–95.
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155.
- Terasaki, M.; Yasuda, M.; Makino, M.; Shimoi, K. Aryl hydrocarbon receptor potency of chlorinated parabens in the aquatic environment. Environ. Sci. Water Res. Technol. 2015, 1, 375–382.
- Gouukon, Y.; Yasuda, M.T.; Yasukawa, H.; Terasaki, M. Occurrence and AhR activity of brominated parabens in the Kitakami River, North Japan. Chemosphere 2020, 249, 126152.
- Fic, A.; Žegura, B.; Gramec, D.; Mašič, L.P. Estrogenic and androgenic activities of TBBA and TBMEPH, metabolites of novel brominated flame retardants, and selected bisphenols, using the XenoScreen XL YES/YAS assay. Chemosphere 2014, 112, 362–369.
- Xi, Y.; Yang, X.; Zhang, H.; Liu, H.; Watson, P.; Yang, F. Binding interactions of halo-benzoic acids, halo-benzenesulfonic acids and halo-phenylboronic acids with human transthyretin. Chemosphere 2020, 242, 125135.
- Zhang, J.; Kamstra, J.H.; Ghorbanzadeh, M.; Weiss, J.M.; Hamers, T.; Andersson, P.L. In Silico Approach to Identify Potential Thyroid Hormone Disruptors among Currently Known Dust Contaminants and Their Metabolites. Environ. Sci. Technol. 2015, 49, 10099–10107.
- Yang, X.; Ou, W.; Xi, Y.; Chen, J.; Liu, H. Emerging Polar Phenolic Disinfection Byproducts Are High-Affinity Human Transthyretin Disruptors: An in Vitro and in Silico Study. Environ. Sci. Technol. 2019, 53, 7019–7028.
- Grimm, F.A.; Lehmler, H.J.; He, X.; Robertson, L.W.; Duffel, M.W. Sulfated metabolites of polychlorinated biphenyls are high-affinity ligands for the thyroid hormone transport protein transthyretin. Environ. Health Perspect. 2013, 121, 657–662.
- Meerts, I.; van Zanden, J.J.; Luijks, E.A.C.; van Leeuwen-Bol, I.; Marsh, G.; Jakobsson, E.; Bergman, A.; Brouwer, A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol. Sci. 2000, 56, 95–104.
- Weiss, J.M.; Andersson, P.L.; Zhang, J.; Simon, E.; Leonards, P.E.; Hamers, T.; Lamoree, M.H. Tracing thyroid hormone-disrupting compounds: Database compilation and structure-activity evaluation for an effect-directed analysis of sediment. Anal. Bioanal. Chem. 2015, 407, 5625–5634.
- Zhang, Z.; Yang, M.; Yi, J.; Zhu, Q.; Huang, C.; Chen, Y.; Li, J.; Yang, B.; Zhao, X. Comprehensive Insights into the Interactions of Two Emerging Bromophenolic DBPs with Human Serum Albumin by Multispectroscopy and Molecular Docking. ACS Omega 2019, 4, 563–572.
This entry is offline, you can click here to edit this entry!
