Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medicine, Research & Experimental
Biomarkers (BMs) are described as biological macromolecules or physiological parameters impartially measured to act as a marker or indicator of a normal or pathogenic cascade. Efficient biomarkers are crucial for the advancement of diagnoses, better molecular targeted therapy, along with therapeutic advantages in a broad spectrum of various diseases.
- biomarkers
- therapy
- cancer
- precision medicine
- translational medicine
- companion diagnostics
1. Applications of Biomarkers in Precision Medicine
1.1. Prevention and Early Intervention
A precedent in the above-mentioned area is to screen for BRCA1 and BRCA2 polymorphisms, which signify a genetic predisposition to breast and ovarian cancers [25]. Women with BRCA1 or BRCA2 have about 40–87% and 27–84% odds of developing breast cancer, respectively [26,27,28,29,30,31,32,33,34,35]. While for ovarian cancer, women with certain BRCA1 or BRCA2 gene mutations have a 16% to 60% and 11% to 27% likelihood of disease, respectively [28,33,36,37,38]. Indeed, the above tests may guide preventative actions, including prophylactic surgical intervention and chemoprevention.
1.2. Optimum Therapy Selection
Each person is unique in his/her genome and predisposition for diseases. The idea is that the “one drug fit for all” approach is leading to therapy failure or drug toxicity. Typically, a marketed drug works on an average for only 50% of the people who take it. According to a report, the percentages of patients for whom a particular drug is ineffective were 38% for antidepressants, 40% for asthma drugs, 43% for anti-diabetic drugs, 50% for anti-arthritis drugs, 70% for Alzheimer drugs, and 75% for anti-cancer drugs (Figure 1) [39]. Application of the concept of optimal therapy selection paves the way for stratified medicine (also known as, precision medicine or personalized medicine) [40]. The ramifications in terms of care cost and quality of care are considerable. The use of biomarkers permits the physician to choose an optimum therapy at the outset and circumvent the exasperating and costly practice of trial-and-error prescription. The most universal example given is HER2, which is used to identify the 25% to 30% of breast cancer patients who will benefit from receiving Herceptin® (trastuzumab). In metastatic colon cancer, about 40% of patients are doubtful to respond to two drugs, Erbitux® (cetuximab) and Vectibix® (panitumumab), due to mutations in their KRAS gene. Clinical practice guidelines recommend that those patients with only the KRAS gene normal form should be treated with these drugs in conjunction with chemotherapy.

Figure 1. Estimate of Ineffective Drug Responses. Estimates show that 75% of anti-cancer drugs, 70% of Alzheimer drugs, 50% of anti-arthritis drugs, 43% of anti-diabetic drugs, 40% of asthma drugs, and 38% of antidepressants are ineffective. Adapted from [39].
1.3. Drug Safety
Adverse drug reactions (ADRs) represent a serious consequence to patients. ADRs hospitalization is nearly 5.3% of admissions. Numerous ADRs are the results of genes coding for variations in the cytochrome P450 (CYP450) family of enzymes and other metabolizing enzymes. For instance, the FDA-approved Amplichip® CYP450 test helps clinicians make informed decisions regarding therapy options and drug dosages. Another FDA-approved example is the UGT1A1 assay™, which measures variations in the liver enzyme UDP-glucuronosyltransferase. The test predicts patients’ safety-related responses to Camptosar® (Irinotecan), which is a standard colon cancer treatment. The assay permits clinicians to alter the drug dosage for roughly 10% of the patients who metabolize the active form of the drug too slowly, where its accumulation in turn would lead to toxicity.
1.4. Patient Compliance
Non-compliance of patients during treatment results in, not only adverse health effects, but also increased costs. Patients are more inclined to comply with their therapy armed with knowledge and confidence. Personalized treatments, once proven to be more effective and/or present fewer side effects, will automatically encourage compliance. The above could be seen in treatments of other conditions such as diabetes or asthma, where non-compliance often makes it worse.
1.5. Improvement Rate of Success of Clinical Trials
The patient stratification strategy ensures drug validation success during clinical trials. Using biomarker testing in clinical trials, scientists may first choose individuals for study inclusion based on their anticipated benefit from the therapy and/or their susceptibility to negative side effects. Enriching the clinical trial pool will shorten, reduce, and/or lower the cost of clinical trials [40].
1.6. Healthcare Benefit Cost Reduction
Healthcare spending in the U.S. is rising. Over the last few years, the expense of the drug ineffectiveness for hypertensive and cholesterol medications alone has exceeded USD 1.2 billion–USD 3.8 billion. Precision medicine is critical for improving the healthcare system since it resolves issues with things such as untraceable drugs, unnecessary appointments to the hospital, and unsafe medical procedures. Research has been conducted, and it has been shown that personalized treatment creates clear economic advantages. For example, it is possible to save costs significantly by doing patient testing using UGT1A1 assay™ to identify patients who need lower doses of Irinotecan because of potential drug reactions. The study also found that if Vectibix® (panitumumab) or Erbitux® (cetuximab) were provided to metastatic colorectal cancer patients with the wild type KRAS gene, who are the only ones to get the benefits of the medicines, the country could save USD 604 million a year.
Another cost management example illustrates the power of Artificial Intelligence (AI). During the American Society of Hematology Annual Meeting (59th ASH), GNS Healthcare jointly with the Multiple Myeloma Research Foundation (MMRF) reported on their AI platform findings. The team found a biomarker that could qualify multiple myeloma patients’ stem cell transplantation eligibilities to guarantee benefit from the operation. AI analysis of 645 multiple myeloma patients identified the putative biomarker, CHEK1, which determines a patient’s response to stem cell treatment. Patients with low gene dosage received a 22-month Progression Free Survival (PFS) benefit from the operation while patients with high gene dosage did not receive any significant benefits. Discovering the link between a cancer patient’s genetic profile and treatment response ahead of undergoing a costly, invasive operation, such as stem cell transplantation, highlights the role of precision medicine in the making of difficult medical decisions in high-risk diseases.
2. Therapeutic Treatment and Biomarkers
The developments of personalized therapies and diagnostic tests are conducted simultaneously to provide essential data regarding the safe and effective use of a corresponding therapeutic treatment which is defined as companion diagnostics (CDx) by the US FDA [41,42]. The FDA has approved sixteen oncology medicines for 32 CDx tests from 1998 to 2016. The first approved CDx was Herceptin® which was introduced in early 1998 to treat breast cancer patients with HER-2/neu (Figure 2) [43,44,45]. The CDx tests are used for precise treatments that employ either small molecule inhibition of intracellular tyrosine kinase activity or monoclonal antibody inhibition of ligand-induced receptor activation.
Recently, two new CDx tests have been approved for cancer. The first drug is for patients with an aggressive and rare type of leukemia, i.e., AML with FLT3 mutation, and represents the new first treatment for this type of leukemia over two and half decades as the overall survival was significantly improved (23% reduction in the risk of death) [46,47,48]. The other CDx test is for advanced ovarian cancer patients, and it was the first drug that does not require BRCA mutation or other biomarker testing. Further, it is expected that around 15% of ovarian cancer patients would benefit from the BRCA analysis test.
A paradigm shift in the “one test, one drug model” is taking place that has defined CDx. More recently, Quest Diagnostics has collaborated with ThermoFisher Scientific to commercialize the company’s next-generation sequencing (NGS)-based CDx panel for non-small cell lung (NSCL) cancer, which was given FDA approval in June 2017. In total, the panel investigates 23-genes, and can determine whether an individual has one of three FDA-approved treatments-linked gene mutations, including those in EGFR, ROS1, and BRAF. Not only that, but it can examine whether or not additional variations exist in other genes. Oncomine Dx Target Test can be employed as a CDx for AstraZeneca’s EGFR inhibitor Iressa® (gefitinib), Pfizer’s ALK and ROS1 inhibitor Xalkori® (crizotinib), and a combination of trametinib (Novartis’ MEK inhibitor Mekinist®) and dabrafenib (RAF inhibitor Tafinlar®). In November 2017, FoundationOne CDx (F1CDx) received FDA approval for a 324-gene NGS-based test that detected genetic changes that are associated with several types of cancer including melanoma, colon cancer, breast cancer, ovarian cancer, and neuroendocrine tumors. F1CDx kit also offers information on the instability of microsatellites and the mutational burden of the tumor.
About 75% of the targeted and immune-oncology investigational therapies in development depend upon diagnostics to support their clinical end points and demonstrate response. The rationale of deploying a diagnostic in an oncology drug development is to mitigate risk, nevertheless, if not managed properly, a CDx can instead add significant risk to regulatory approval of novel oncology drugs. Thus, the CDxs have been shown to be vital tools in advance drug development due to: (1) fast and high chances of regulatory approval; (2) cost reduction, i.e., most suitable patients for a particular treatment (stratification of patient population).
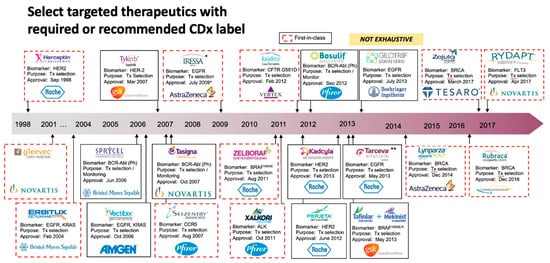

Figure 2. Overview of List of Approved Oncology CDx Tests on the Market. The first approved CDx was Herceptin® which was introduced in early 1998 to treat breast cancer patients with HER-2/neu. The CDx tests are used precisely for specific therapies that inhibit signal transduction pathways by either inhibiting the intracellular tyrosine kinase activities using a small molecule or preventing ligand-induced receptor activation with a monoclonal antibody.
3. Ethnic Disparities in Biomarkers
Developing genomic-based testing that quantifies the risk of cancers and helps understand the tumor profile, offers treatment guidance and management of patients’ risk/therapy and thereby, smarter care for patients from diverse ethnic groups. The genomic testing would deliver the promise and value of precision medicine targeting sub-populations with a high risk and the unique biology of every cancer. This is particularly important for breast cancer, which is the most frequently observed cancer among women, globally characterized by having a heterogeneous, highly complex, and multifactorial nature with several documented risk factors contributing to its initiation. Inflammatory breast cancer is a rare cancer type that merely affects 1–5% of patients in the USA [67,68]; nonetheless, is more common in North Africa, accounting for as high as 11% of cases in Egypt [69]. If one can distinguish and recognize the proteins and genes associated with the aggressive form of breast cancer, he/she will be able to delineate the mechanisms associated with the transition from localized and controlled breast cancer to the aggressive form. The present efforts to delineate the Arab genome [70,71,72,73] might aid in discovering new ethnic-specific biomarkers. Moreover, the outcomes of the analyses are expected to recognize Arab-specific variants that might play a critical role in the molecular pathology of various diseases, including cancers, and could contribute to identifying significant genotype–phenotype association. The above investigations may feasibly contribute to disease prognosis and management in the future, ultimately, providing a way forward for precision medicine to help to reduce the burden of disease. In the Arab world, particularly in Qatar, this type of knowledge makes a valuable contribution to the drive towards precision health. The State of Qatar has initiated two major projects. The Qatar Biobank (QBB) collects biosamples and health/lifestyle details from the Qatari populace, and The Qatar Genome Program (QGP) is mapping the genome of the resident population [70,71]. Collectively, data would recognize the association of genotype–phenotype pertinent to the Qatari population. Further, a comprehensive Qatari genotyping array, the Q-Chip, has been developed. Development of a second generation of Q-Chip is underway. The new version has more processed clinical content and is designed to satisfy the local health requirements, thus delivering on the commitment of precision medicine for the population which in turn enables the evolution of precision healthcare in Qatar to usher in a new era in patient-centric care [74].
This entry is adapted from the peer-reviewed paper 10.3390/pr10061107
This entry is offline, you can click here to edit this entry!
