Collagen is the major fibrillar protein in most living organisms. Among the different types of collagen, type I collagen is the most abundant one in the tissues of marine invertebrates. The present overview has focused on a brief history of collagen, basic structure and synthesis, nomenclature, types, and classifications, sources of collagen with the emphasis on sea cucumber collagen, and related functional properties.
- collagen, sea cucumber, characterization
1. Introduction
Considering the collagen as a biomaterial, it has many usages in several fields[1]. Application of collagen is diversified mainly due to its unique properties such as biocompatibility, low antigenicity, high biodegradability, and cell growth potential[2]. Apart from the food industry, collagens have been widely used in tissue engineering, pharmaceutical, and biomedical industries as well as various other fields, including cosmetics[3].
2. History of Collagen
As one of the most abundant fibrous proteins, collagen plays a vital role in connective tissues, thus animal skin and bone provide an extracellular framework for strength and flexibility[4]. Collagen is one of the major structural proteins in the extracellular matrix and the name is derived from the Greek word “kola,” which means “glue producing”. Findings of Schweitzer et al.[5] revealed the presence of intact collagen in the soft tissue of the fossilized bones of 68 million-year-old Tyrannoaurus rex, a genus of coelurosaurian theropod dinosaur. Sequences of studies have been conducted for decades to propose a structure for the collagen molecule. Among those studies, triple-helical “Madras Model” by Ramachandran and Kartha[6] contributed much to the currently accepted structure of collagen which was discovered by Cowan, North and Randall[7]. Further findings of Rich and Crick[8] also improved the identified structure of collagen[9]. Currently, more than 29 distinct types of collagen have been identified[10][11].
The molecular structure of collagen consists of three polypeptide α chains intertwined with each other to form a triple helix, approximately 300 nm in length with a molecular weight of 105 kDa[12] [8]. These molecules can either be homomeric (contain identical α chains) or heteromeric (genetically distinct α chains)[13]. Each strand is initially shaped into a left-handed symmetry prior to their conformation as a right-handed triple helix.
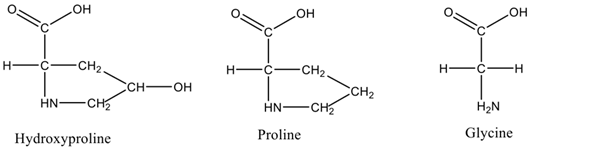
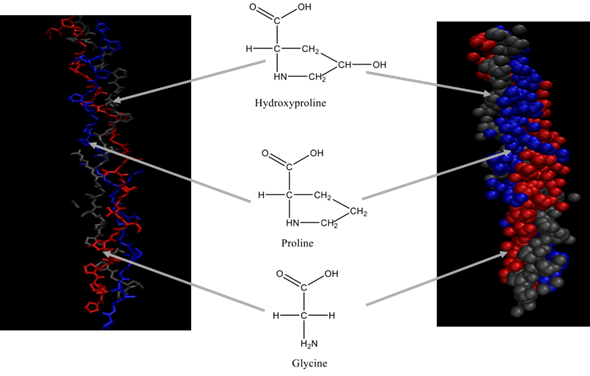
Each chain of the right-handed helical structure consists of a repeating sequence of glycine-X-Y, where often, X and Y are referred to proline or hydroxyproline, respectively (Figure 1)[11]. In this motif, all glycine residues are located inside the core, while other amino acids (X and Y) are located on the surface[13]. This rigid rod-like structure is further strengthened by interchain N-H (Gly) O=C(x) hydrogen bonds and electrostatic interactions[14]. The presence of triple helix (Figure 2) is the main feature in the collagen structure. However, triple helix can be varied according to the type of collagen present in the structure[15]. Moreover, this sequential uniformity can rarely be found in other proteins. Due to the uniformity of collagen, numerous studies have been conducted to determine their potential as a prospective biomaterial for a wide range of applications.
Figure 1. Amino acid residues present in triple helix.
Figure 2. Triple helix structure of collagen.
2.1. Basic Structure and Synthesis
The fundamental subunit of collagen is tropocollagen which is a three-stranded polypeptide unit. Collagen family is classified into various groups due to their complex structural diversity[16]. Different lengths of the helix, presence of non-helical components, interruptions in the helix, variations in the assembly of the basic polypeptide chains, and differences in the terminations of the helical domain directly lead to distinct types of collagens. Its general groups include fibrillar collagens, FACIT (Fibril Associated Collagens with Interrupted Triple Helices), FACIT-like collagen, beaded filament collagen, basement membrane collagen, short-chain collagen, transmembrane collagen, and unclassified collagen[11][17]. The length of the helix and portions of non-helical components are different depending on the type of collagen.
Numerous studies on collagen have revealed that collagen type I is the most ubiquitous form of collagen which belongs to the fibrillar group[11][13] [29,30]. Fibril forming collagen or fibrillar collagen is synthesized in the form of soluble precursor molecules (procollagen) by the process of fibrillogenesis. Each polypeptide chain is involved in the synthesis process and consists of N- and C- propeptides at each terminal position of the triple helix[18]. The fibrils produced have a visible banding, a direct result of the aggregating pattern of collagen. The stability of the fibrillar collagen depends on non-reducible covalent cross-links in the triple helix[19].
The name FACIT collagen implies that the association of fibrils is interrupted by non-helical domains. They are linked with the surface of collagen fibrils and the collagenous structure is disturbed by non-helical domains. Wang et al. [18] further described that the C-NC domain in FACITs is short compared to fibril forming collagens. Collagen types IX, XII, XIV, XVI, XIX, XX, XXI, and XXII belong to the FACIT group[15]. Wu, Woods, and Eyre[20] explained this scenario by depicting the structure of type IX collagen that lies anti-parallel to type II fibrils. Moreover, primary sequences of some FACIT collagens share similarities with fibrillar collagens[11].
The beaded filament collagen molecules assembled without undergoing the cleaving of terminal regions and the formation of the bead region in collagen filaments are facilitated by these uncleaved regions[11][15][20]. The most characteristic feature of this subgroup is having large N- and C- terminals. For example, type VI is having large N- and C- terminals even in their short triple- helical domains[11][13][21]. Furthermore, only type VI collagen belongs to the subgroup of beaded filament collagen[11].
The basement membrane and associated collagen are categorized under non-fibrillar collagen. They can be found mostly in tissue boundaries, which facilitate molecular filtration by forming a connected network, especially in basement membranes[16][18]. Apart from tissue boundaries, non-fibrillar collagen can be found in cavities of the epithelial lining, endothelium in the interior blood vessels, fat, muscle, and nerve cells. Based on the electron microscopic images, collagen IV belongs to the non-fibrillar collagen subgroup that appear as thin sheets and its molecules are relatively long compared to the fibrillar collagen[13]. Anchoring fibrils collagen VII are considered as essential for functional integrity[18]. Short-chain collagens are described as mesh forming collagen and are located in underlying endothelial cells. Some of the short-chain collagens are also present in mineralizing cartilage[10]. The short-chain collagen possesses a shorter triple-helical region (half of the length of fibrillar collagen). Type VIII and X are categorized under the subgroup of short-chain collagen. Among them, type VIII collagen involves the proliferation of cells as a growth enhancer[11].
The transmembrane collagens function as cell surface receptors as well as matrix components involved in adhesion[11][13][22]. Moreover, they possess a relatively long but interrupted triple-helical domain with a short N terminal domain[23]. Type XIII, XVII, XXIII, XXV, and other collagen-like proteins are categorized under transmembrane collagens[22][23].
2.2. Nomenclature, Types, and Classifications
After discovering type I, II, and III collagen, further research studies were evoked on the identification of possible molecular types of collagen. However, expanded studies indicated that type III collagen molecules also contained type I collagen and both types together could form mixed fibrils. This observation affected the terminology that existed then and became more complicated after the identification of type IV collagen[24]. Due to the variations of their histology, it was agreed to give a type number so, they are numbered with Roman numerals (I-XXIX) while polypeptide chains are named using α chains with Arabic numerals (α1, α2, α3, etc.). For instance, type I collagen with identical α1(I) chains and one chain α2(I) and the nomenclature for type I collagen is [α1(I)]2 α2(I)[23][24]. Table 1 represents the some of the common types of collagen with their nomenclature and distribution.
Table 1. Common types of collagen.
|
Collagen Type |
Chains |
Sub Family |
Distribution |
|
I |
α1(I) α2(I)
|
Fibrillar collagen |
Skin, tendon, bone, dermis, intestine, uterus |
|
II |
α1(II)
|
Fibrillar collagen |
Hyaline cartilage, vitreous, nucleus pulposus |
|
III |
α1(III)
|
Fibrillar collagen |
Dermis, intestine, large vessels, heart valve |
|
IV |
α1(IV) α2(IV) α3(IV) α4(IV) α5(IV) α6(IV)
|
Basement membrane and associated collagen |
Basement membranes |
|
V |
α1(V) α2(V) α3(V)
|
Fibrillar collagen |
Cornea, placental membranes, bone, large vessels |
|
VI |
α1(VI) α2(VI) α3(VI) |
Beaded filament forming collagen |
Descement’s membrane, skin, heart muscles |
|
VII |
α1(VII)
|
Basement membrane and associated collagen |
Skin, placenta, lung, cartilage, cornea |
|
VIII |
α1(VIII) α2(VIII) |
Short chain collagen |
Produced by endothelial cells, descemet’s membrane |
|
IX |
α1(IX) α2(IX) α3(IX) |
Fibril associated and related collagen |
Cartilage |
|
X |
α1(X)
|
Short chain collagen |
Hypertrophic and mineralizing cartilage |
|
XI |
α1(XI) α2(XI) α3(XI) |
Fibrillar collagen |
Cartilage, intervertebral disc, vitreous humor |
|
XII |
α1(XII)
|
Fibril associated and related collagen |
Chicken embryo tendon, bovine periodontal ligament |
|
XIII |
α1(XIII) |
Trans membrane collagens and collagen like proteins |
Cetal skin, bone, intestinal mucosa |
3. Sources of Collagen
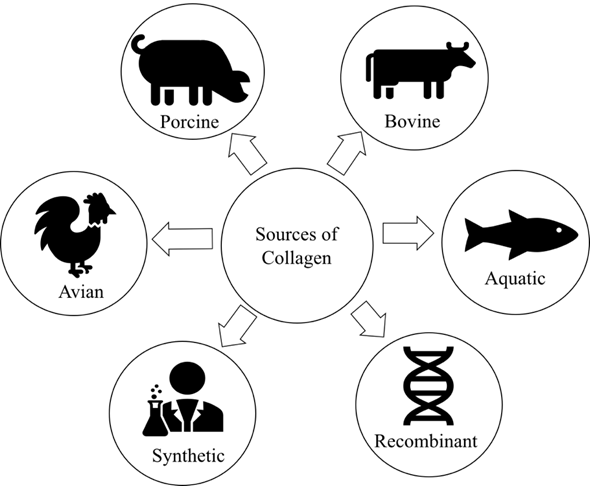
As the major structural proteins are in the skin and bones of most animals, collagen accounts for 30% of the total body protein[27]. The most common raw materials for collagen production are obtained from the slaughterhouse by-products, including hides, bones, tendons, and cartilages, or recombinant collagen. At the industrial-scale production, animals such as bovine and pigs are used as primary sources of collagen[4]. Figure 3 represents the most common sources of collagen. However, the outbreak of prion diseases such as bovine spongiform encephalopathy (BSE) resulted in some barriers for using bovine collagen whereas swine flu has limited the use of porcine collagen[28].
Figure 3. Popular sources of collagen.
In addition, due to various religious constraints, porcine or mammalian collagen for the development of kosher and halal products is limited[16][18][27]. Apart from the widely used species, several studies (Table 2) have extracted collagen from chicken[29], kangaroo tail[30], rat tail tendon[31], duck feet[32], equine tendon[17], alligators bone[33], birds’ feet[34][35][36], sheep tendon[37][38][39][40][41], and frog skin[42], while some studies have focused on using recombinant human collagen[3]. The high pathological risk for transmitted diseases and complicated extraction process have limited the applicability of using land animal collagen and created a growing concern towards finding alternative sources for collagen. The two primary sources of industrial collagen, including land animal by-products and marine organisms, are described in the following subsections.
Table 2. Alternative land animal sources for bovine and porcine collagen.
|
Source |
Extraction method |
Purpose of Extraction |
Reference |
|
Chicken feet
|
Acid extraction |
Optimization of extraction condition |
[29] |
|
Enzyme extraction |
Determination of pepsin digestion effect on the properties of extracted collagen |
[34] |
|
|
Acid extraction |
Preparation of edible films |
[43] |
|
|
Enzyme extraction (using papain and pepsin) |
Isolation and characterization of chicken feet originated collagen |
[44]
|
|
|
Acid extraction |
Use of chicken feet for protein films |
[45] |
|
|
Alkali, acid, and enzyme extraction |
Identification of best method of collagen extraction method and characterization of chicken feet collagen |
[36] |
|
|
Enzyme extraction
|
Optimization of extraction process and synthesis of chicken feet collagen based biopolymeric fibers |
[46] |
|
|
|
|
|
|
|
Rat tail tendon |
Acid extraction |
Preparation of type I collagen for tissue engineering applications |
[31] |
|
Alligator bone |
Acid and enzyme assisted extraction |
Determination of biochemical properties of alligator bone collagen |
[33] |
|
Silky fowl feet |
Combination of acid and enzyme extraction |
Identification of best combination for high quality collagen extraction method |
[35] |
|
Ovine tendon |
Acid extraction |
Determination of the biocompatibility of ovine tendon originated collagen with human dermal fibroblast |
[37] |
|
|
Acid extraction |
Determination of the biocompatibility of ovine tendon originated collagen with human dermal fibroblast Improve the mechanically strong ovine tendon originated collagen for tissue engineering purposes |
[38]
|
|
|
Acid extraction |
Characterization and fabrication of thin films from ovine tendon collagen for tissue engineering applications |
[39] |
|
|
Acid extraction |
Investigation of attachment, proliferation, and morphological properties of human dermal fibroblasts on ovine tendon collagen |
[40] |
|
Duck feet
|
Acid extraction |
Investigation of physicochemical properties of collagen derived from duck feet |
[32] |
|
Acid extraction |
Determination of feasibility of using duck feet collagen in improving physicochemical properties of surimi |
[47] |
|
|
Kangaroo tail |
Acid extraction |
Identification of alternative collagen sources for pre-clinical models for cell biology |
[30] |
|
Sheep bone |
Acid extraction |
Determination of effect of different collagen extraction protocols |
[41] |
|
Equine tendon |
Acid extraction |
Evaluation of the effects of different extraction methods on the collagen structure of equine tendons |
[17] |
3.1. Land Animal By-Products
In recent decades, inedible animal by-products are utilized to produce fertilizers, minerals, fatty acids, vitamins, protein hydrolysates, and collagen[48]. Bovine collagen is the primary source for the industrial collagen used in medicine, cosmetics, and other non-biomedical applications[49]. Sterilized purified collagen from cow skin is used as injectable bovine collagen[50]. Apart from BSE risk, around 3% of the population is allergic to bovine collagen, which hinders its usage[3].
Skins and bones of pigs are used to extract porcine collagen[3]. Pig rind is famous for processing food products such as sausage casings and edible films. Moreover, porcine collagen is used as a dermal substitute in the medical field as they are used widely as implants for reconstructive surgery[51]. Pig hides are used to extract porcine type I collagen and share similar properties to human collagen, hence it has a wide range of application in both medical and food industries[51][52][53].
Collagen extraction from poultry by-products such as skin, bones, and cartilage from chicken has also been reported. However, the usage was limited due to the occurrence of avian influenza[54]. The mammalian collagen is preferred in the industrial level applications over avian collagen. The limited applications of avian collagen correlate with the expensive and complicated extraction process.
3.2. Marine Organisms
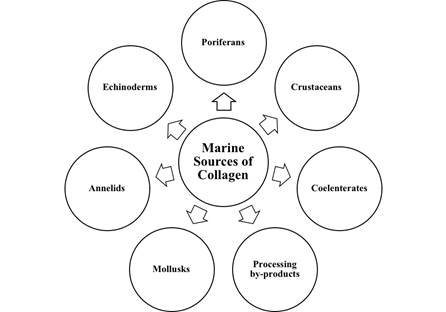
The marine-derived collagen is a promising alternative due to the occurrence of foot-and-mouth disease (FMD), BSE, and avian influenza like diseases, as well as religious and social constraints[55][56]. Several comprehensive reviews on marine-derived collagen and their application in various fields have appeared[3][2][57]. Recently, collagen from various marine organisms such as poriferans, coelenterates, annelids, mollusks, echinoderms, and crustaceans has been extensively investigated (Figure 4).
Figure 4. Marine sources of collagen.
The unique characteristics of marine collagen as a biomaterial with significant biocompatibility and biodegradability has been favored in many industrial applications over other alternate sources[55][58]. Mainly, marine by-products have been exploited to recover collagen and other collagen-derived biomaterials through a combination of different bioprocessing methods[59]. These include Japanese sea bass skin[60], clown feather back skin[61], bladder of yellow fin tuna[62], fin, scales, skins, bones, and swim bladders of big head carp[63], skin and bone from Japanese seerfish, cartilage from sturgeon and sponges, sea urchin[64], octopus[65] squid[66], cuttlefish[67], sea anemone[68], and sea cucumbers for extraction of marine collagen[69]. Particularly, collagen type I was extracted from the skin of silver carp[70], Japanese sea-bass[60], mackerel[71], bullhead shark[72], and sole fish[73] as well as from the bones of skipjack tuna[74], and scales of Nile tilapia[75].
Significant differences in the amino acid composition of collagen from various fish species are responsible for their unique characteristics[55]. Most of the fish collagen contains a lower proportion of hydroxyproline compared to mammalian and avian collagen. Consequently, their lower compatibility to crosslinking and stability compared to other types of collagen has been reported[76][55][77]. However, the content of hydroxyproline also depends on the habitat of fish species[78]. Moreover, the thermal stability of the collagen extracted from warm water species is found to be higher than cold water species[78].
The marine sources of collagen have received increasing attention due to their availability, easy processing techniques, safety (free of zoonosis), environmentally friendly extraction procedures, low molecular weight, less religious and ethical barriers, minor regulatory and quality control problems, a negligible amount of biological contaminants and toxins, low inflammatory response and excellent metabolic compatibility[3]. However, most studies have been conducted to identify the potential uses of collagen derived from marine vertebrates but reports on marine invertebrates are scarce[79][2]. Thus, current research interest is directed towards the use of marine invertebrates as potential sources of collagen, particularly for biomedical applications. Recent investigations have been concentrated on jellyfish[80] sponges[81], mussels[79][82], and sea cucumber[76][83][69][84][85][86][87][88][89][90][91][92][93][94] as potential candidates for producing marine-derived collagen.
3.3. Sea Cucumber as A Source of Collagen
Among the various bioactive compounds derived from sea cucumber, collagen plays a vital role. Primary intensive research on sea cucumber collagen has been initiated in the early 1970s by Eyre and Glimcher[95] and Matsumura, Shinmei, and Nagai[96]. Eyre et al.[97] studied the comparative biochemistry of the collagen crosslinks using sea cucumber Thyone briarius, a sponge Haliclona oculata, and sea urchin Strongylocentrotus droebachensis and reported the evidence for glycosylated crosslinks in collagen derived from the body wall of sea cucumber Thyone briarius. Matsumura et al.[96] then focused on the purification of collagen from sea cucumber Stichopus japonicus by disaggregating the connective tissue of body wall followed by the morphological study of the isolated collagen fibrils. Furthermore, the most extensive research studies were focused on the molecular structure and functional morphology of Cucumaria frondosa which led to a series of discoveries on the covalent composition and growth of collagen fibrils in the same species[98][99][100]. The dermal glycoprotein stiparin was identified as the main factor responsible for the aggregation of collagen fibrils from the dermis of sea cucumber Cucumaria frondosa[101] and Trotter et al.[102] characterized a sulphated glycoprotein, which inhibited fibril-aggregating activity.
Thurmond and Trotter[103] further investigated the morphology and biomechanics of the microfibrillar network of collagen derived from sea cucumber Cucumaria frondosa dermis and reported similar morphological characteristics with fibrillin microfibrils of vertebrates. Most of the early investigations of the sea cucumber collagen fibrils contributed to recent developments of the research related to collagen and other bioactivities from sea cucumber. Table 3 provides a cursory account of recent studies related to sea cucumber collagen.
Table 3. Recent studies on sea cucumber collagen.
|
Sea cucumber Species |
Focus of Study |
Major Findings |
Reference |
|
Stichopus japonicus
|
Chemical composition and subunit structure of collagen |
Collagen was comprised of 2 distinct subunits (α1 and α2 and rich in glutamic acid compared to other fibrillar collagen |
[76] |
|
|
Characterization and subunit composition of collagen |
Pepsin solubilized collagen resembled type I collagen and its amino acid composition was different from vertebrate collagen. |
[83] |
|
|
Changes of collagen during cooking |
Crude collagen fibers were more susceptible to heat treatment compared to pepsin-solubilized collagen |
[104] |
|
|
Identification of physicochemical properties and radical scavenging capacities of pepsin-solubilized collagen |
Extracted collagen maintained intact triple-stranded helices and high moisture retention and absorption capacities as well as exhibiting better radical scavenging ability compared to vitamins C and E. |
[84] |
|
|
Wound-healing effects on human keratinocyte (HaCaT) cell line of pepsin-solubilized collagen |
Pepsin-solubilized collagen has the potential to use as an alternative mammalian collagen in the nutraceutical and pharmaceutical industries |
[85] |
|
|
Effect of autolysis of intact collagen fibers related to the distributions of cathepsin L |
Lysosomal cathepsin L degrades the collagen fibers and speed and degree of autolysis is negatively correlated with the density of collagen. |
[105] |
|
|
Structural characteristics of sea cucumber collagen fibers in the presence of endogenous cysteine proteinases |
Collagen fibrils disaggregated into collagen fibrils by cysteine proteinases and the structural disorder of the native collagen fibers increased due to cysteine protease. |
[91] |
|
|
Structural and biochemical changes of collagen related to autolysis |
Collagen fibers and microfibrils gradually degraded with the autolysis and structural damage was less in monomeric collagen compared to other structural elements |
[92] |
|
|
Structural and thermal properties of sea cucumber collagen |
Distance between adjacent molecular chains in collagen molecules was decreased and CO2, NH3, H2O, CH4, NO2 and HCN gases released during the heat treatment |
[106] |
|
|
Enzymatic hydrolysis of collagen to determine the structural changes of collagen fibrils |
Collagen fibers were partially degraded into collagen fibrils by enzymatic (trypsin) treatments |
[107] |
|
|
Investigate the effect of collagenase type I on the structural features of collagen fibers |
Collagenase was responsible for partial depolymerization of collagen fibers into fibrils, uncoiled the fibrils, degrade monomeric collagen |
[108] |
| Parastichopus californicus
|
Purification and characterization of pepsin-solubilized collagen from skin and connective tissue |
Collagen extracted from skin and connective tissue contains type I collagen with three α1 chain. Amino acid composition is different from the mammalian type I collagen |
[109] |
|
Bohadschia spp |
Analysis of isolated pepsin-solubilized collagen |
Type I collagen was identified with three α1 chain |
[110] |
|
Stichopus vastus
|
Isolation and characterization of pepsin-solubilized collagen |
Purified collagen belongs to type I collagen contains three α1 chain with triple helical structure |
[88] |
|
|
Molecular mass distribution, amino acid composition and radical-scavenging activity of collagen hydrolysates prepared from isolated collagen |
β and α1 chains of the collagen were hydrolyzed by trypsin and molecular mass distribution ranged from 5 to 25 kDa. Hydrolysates contains high glycine, alanine, glutamate, proline and hydroxyproline residues and showed significant radical scavenging activity |
[111] |
|
|
Physicochemical and biochemical properties of pepsin solubilized collagen |
Glycine was the predominant amino acid present in purified collagen that possessed high moisture absorption and retention capacity |
[111] |
|
|
Identification of Angiotensin I converting enzyme (ACE) inhibitory and radical scavenging activities from collagen hydrolysates |
Novel bioactive peptides generated by optimized trypsin hydrolysis have the potential to use as ACE inhibitors and radical scavenging agents. |
[89] |
|
Holothuria parva
|
Purification and characterization of pepsin-solubilized collagen |
Isolated collagen constituted three α1 chain and was rich in glycine, proline, alanine and hydroxyproline |
[87] |
|
Stichopus monotuberculatus
|
Isolation and characterization of pepsin-solubilized collagen |
Isolated collagen was classified as type I collagen consisted of three α1 chain |
[90] |
|
Holothuria scabra
|
Determination of nano-collagen quality and extraction of acid solubilized collagen |
Extracted acid solubilized collagen had significant effect on physicochemical characteristics of nano-collagen particles |
[112] |
| Australostichopus mollis
|
Biochemical composition of isolated collagen |
Type I collagen was present with α1 and α2 chains, α chain dimers, β chains, and g components. Most abundant amino acids were glycine, alanine, threonine, serine, and proline. |
[113] |
|
Holothuria leucospilota
|
In vitro activity of anti-tyrosinase and anti-elastase activity of isolated collagen |
Isolated collagen exhibited weak anti-tyrosine activity and moderate anti-elastase activity |
[114] |
|
Acaudina leucoprocta
|
Extraction methods to remove heavy metals from the isolated collagen |
Pepsi- solubilized collagen showed two isoforms and amount of heavy metals present in the collagen were below the contaminant limit |
[115] |
|
Acaudina molpadioides
|
Preparation and characterization of antioxidative peptides from collagen hydrolysates |
Collagen peptides which showed highest antioxidant activity were rich in hydrophobic acid residues. |
[116] |
|
Stichopus vastus and Holothuria atra
|
Comparison of partial characteristics of two different sea cucumbers |
No significant difference in amino acid composition, yield, or whiteness |
[117] |
| Apostichopus japonicus
|
Type of constituent collagen using proteomics and bioinformatic strategies |
Heterogenicity of the sea cucumber collagen fibrils was revealed for the first time that provides novel insight into the composition of sea cucumber collagen |
[93] |
|
Analysis of the effect of epigallocatechin gallate (EGCG) on preserving molecular structure of collagen fibers during heating
|
EGCG protects the structure of crude collagen fibers in a dosage dependent manner and effects hydrogen bonds on the collagen which promotes protein aggregation |
[94] |
|
| Holothuria cinerascens |
Potential application of collagen in moisturizing cosmetics |
Collagen showed better moisture retention and moisture absorption capacity. Abundant hydrophilic groups in collagen increases their ability for cosmetic formulations |
[69] |
Sea cucumber research interests have been mainly focused on cultivation and bioactive molecules. Most of the research conducted on bioactive ingredients from sea cucumber has centered around proteoglycan and collagen[69]. The main edible portion of sea cucumber is the body wall composed of mutable connective tissue (MCT) with scattered cells[103]. The structural components of MCT consist of collagen, proteoglycan, and glycoprotein[107]. These assembled components form collagen fibrils, collagen fibers, and microfibrils. Among them, the majority of total body wall protein are comprised of insoluble collagen fibrils. Collagen fibers are surrounded and separated from the microfibrillar network in MCT and this network maintains the organization while providing a long-range restoring force[109].
The most abundant type of collagen found in sea cucumber is type I collagen and collagen fibrils of echinoderms are symmetrically spindle-shaped and short in length[102][109]. Moreover, at the molecular level, they are considered as bipolar collagen fibrils with surface associated proteoglycans[102]. Covalent crosslinks providing stabilization to collagen are internally present and similar to the mammalian collagen. Besides, absence of permanent crosslinks in the structure improves the isolation of collagen fibrils in their intact form[87][102]. It also helps to slide pass one another during lengthening and shortening of the tissue[103].The solubilized collagen from the body wall of sea cucumber (Stichopus japonicus) has distinct subunit structure of (α1)2 α2 and are rich in glutamic acid. Thermal denaturation of this type of collagen may impart unique textural properties[76]. A recent study on the molecular composition of collagen fibrils isolated from sea cucumber Aposticopus japonicus revealed that collagen fibrils are heterotypic containing two clade A, one clade B fibrillar collagens, and two FACIT collagens[93]. Fibrillar collagen α chains may be classified in to three clades according to their evolutionary steps. Clade A consists of α1(I), α2(I), α1(II), α1(III), and α2(V) chains; clade B contains α1(V), α3(V), α1(XI), and α2(XI) chains while clade C includes α1(XXIV) and α1(XXVII) chains[118].
Tian et al.[93] also reported the heterogenicity exhibited in the pepsin-solubilized collagen isolated from Aposticopus japonicus for the first time. Their novel findings on subunit compositions and constituents of sea cucumber collagen were, however, contradictory to the previous studies[76][83][87][88][90][91][98][104][109]. Most of the previous studies focused on pepsin-solubilized collagen (PSC), and structure analysis was conducted using sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). However, Tian et al.[93] used proteomic techniques and bioinformatic methods to analyze the constituents present in sea cucumber collagen. According to the phylogenetic analysis of identified collagen sequences revealed that reported sea cucumber collagen sequences did not belong to the branches of typical collagens. The authors concluded that the heterogenic and complex nature of the sea cucumber collagen is complicated and needs extensive investigations. Thus, previously reported studies on SDS-PAGE analysis are not considered adequate to conclude the fundamental molecular structure of collagen[93].
4. Functional Properties of Collagen
Interest in functional properties of collagen extracted from different sources, including animal, marine organism, and industrial by-products, has been increasing during the past few decades. According to Gomez-Guillen et al. [4], functional properties of collagen and gelatin can be divided into two main categories as properties associated with gelling behavior, and surface behavior. Properties associated with gelling behavior include (a) gel formation, (b) texturing, (c) thickening, and (d) water-binding capacity while properties related to their surface behavior include (a) emulsification, (b) foaming and stabilization, (c) adhesion and cohesion, (d) colloid function, and (e) film formation[4][119][120].
4.1. Gelling and Hydrophilic Properties
The process of collagen gelation is the aggregation of collagen molecules that can be achieved by heating either in acid or alkali[12] and induced by alterations of processing parameters such as ionic strength, pH, and temperature. During thermal solubilization of collagen, a considerable amount of intra- and intermolecular cross-links are cleaved. The aqueous solution of gelatin and collagen possesses the ability to swell by covalently linking with matrices[4]. Liu et al.[109] and Abedin et al.[88] evaluated and compared the gel-forming ability of sea cucumbers Parastichopus californicus and Stichopus vastus derived collagen with calfskin collagen. The findings of these studies revealed that ionic strength and pH were the predominant factors determining the gel-forming ability of collagen isolated from sea cucumbers. Moreover, calfskin collagen exhibited higher gel-forming ability compared to sea cucumber-derived collagen. The difference might be due to the low hydroxyproline content in sea cucumber collagen which has a direct influence on creating the three-dimensional branched network during gel-formation[109][121].
In addition, hydrolysis may occur in some amide bonds in the primary chain of collagen molecules during the gelation process[12]. The gelation process of collagen, as well as gelatin, are referred as thermo reversible processes[4]. Gel strength and gel melting point are significant physical properties of gelatin gels [12]. The melt-in-the-mouth property of gelatin is considered as one of the significant characteristics of gelatin, which is extensively utilized by both food and pharmaceutical industries[12].
Hydrophilic nature and swelling ability of solubilized collagen are used to minimize the dripping loss of frozen fish and meat products[122]. Moreover, for enhancing the sensory characteristics, collagenous materials are used widely in the food industry due to their gelling properties[123]. Apart from that, collagen and gelatin are utilized as wetting agents in food, pharmaceutical, and medical applications[4][12].
Dong et al.[104] studied the changes of collagen in sea cucumber Stichopus japonicas during cooking and reported that thermal treatments on the sea cucumber affect the appearance and the sensory properties of the final product. This is due to the alteration of water absorption ability of collagen. Zhu et al.[84] investigated the moisture absorption and retention capacities of PSC from sea cucumber and suggested that PSC might be an excellent functional ingredient for cosmetics as they exhibited a behavior comparable to that of glycerol. Li et al.[69] investigated the collagen from sea cucumber Holothuria cinerascens and evaluated its potential application in moisturizing cosmetic products. They reported that the polar groups, including carboxyl (-COOH) and hydroxyl (-OH) groups on the surface of the collagen molecule, promote the moisture retention of products.
4.2. Emulsifying Properties
Charged groups of collagens contain hydrophilic or hydrophobic amino acids that are responsible for its surface properties. In an aqueous system, hydrophobic and hydrophilic groups are involved in reducing surface tension by moving to the surface area of the emulsion[124][125]. Hydrophobic areas on the peptide chain have a major impact on the emulsifying and foaming properties of gelatin[124].
In addition, surface-active property and gel firmness are other crucial factors affecting emulsion properties. The emulsion capacity is increased with protein concentration[4]. In addition, molecular weight also influences the stabilization of the emulsion, as high-molecular-weight gelatin forms a more stable emulsion compared to low-molecular-weight one[4][126]. Moreover, factors like temperature, pH, concentration, and homogenization of the collagen may also affect the emulsifying and foaming properties of collagen[127]. Higher content of hydrophobic amino acid favors increased foam capacity of gelatin[126][127].
Furthermore, the stability of foams depends on various parameters including the rate of attaining equilibrium surface tension, bulk and surface viscosities, steric stabilization, and electrical repulsion between the two sides of the foam lamella[126].
4.3. Film Forming Properties
Biodegradable films made from edible protein-based biopolymers are gaining popularity in the food industry due to consumers’ awareness and their low impact on the environment[128][129]. However, the hygroscopic nature of gelatin limits its use as a protective barrier[4] and usually following the extraction process, collagen molecules tend to lose their mechanical properties compared to the native form[129]. Several investigations have been carried out to improve the mechanical and water resistance properties of these films with the addition of other biopolymers such as chitosan, hydrophobic and hydrophilic plasticizers, lipids, and protein isolates, among others[4][129].
Avena–Bustillos et al.[130] studied the water vapor permeability of mammalian and fish gelatin films and found lower water permeability in fish gelatin films compared to those from mammalian sources. Moreover, water vapor permeability of cold-water and warm-water fish gelatin are also different as warm-water fish always exhibits a higher water permeability compared to that of cold-water fish gelatin. However, excellent film-forming property of fish gelatin expands its usage in encapsulated drugs and frozen foods. The hydrophobicity of the protein is also an essential factor for its film formation. Notably, low hydrophobicity of marine collagen may be due to a lesser availability of proline and hydroxyproline for hydrogen bonding with water[4].
Furthermore, the film-forming ability of collagen and collagen-based derivatives like gelatin depends on their molecular weight distribution and amino acid composition that can directly affect the mechanical and barrier properties of films[131]. Recent research has been focusing on enriching these films with the addition of antioxidants and antimicrobial substances to enhance their application as a renewable biomaterial[132] .
This entry is adapted from the peer-reviewed paper 10.3390/md18090471
References
- Cinzia Ferrario; Francesco Rusconi; Albana Pulaj; Raffaella Macchi; Paolo Landini; Moira Paroni; Graziano Colombo; Tiziana Martinello; Luca Melotti; Chiara Gomiero; et al. From Food Waste to Innovative Biomaterial: Sea Urchin-Derived Collagen for Applications in Skin Regenerative Medicine. Marine Drugs 2020, 18, 414, 10.3390/md18080414.
- Fazli Subhan; Muhammad Ikram; Adeeb Shehzad; Abdul Ghafoor; Marine Collagen: An Emerging Player in Biomedical applications. Journal of Food Science and Technology 2014, 52, 4703-4707, 10.1007/s13197-014-1652-8.
- K. Silvipriya; K. Kumar; A. Bhat; B. Kumar; Anish John; Panayappan Lakshmanan; Collagen: Animal Sources and Biomedical Application. Journal of Applied Pharmaceutical Science 2015, 5, 123-127, 10.7324/japs.2015.50322.
- M.C. Gómez-Guillén; B. Giménez; M.E. López-Caballero; M.P. Montero; Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocolloids 2011, 25, 1813-1827, 10.1016/j.foodhyd.2011.02.007.
- Mary Higby Schweitzer; Zhiyong Suo; Recep Avci; John M. Asara; Mark A. Allen; Fernando Teran Arce; John R. Horner; Analyses of Soft Tissue from Tyrannosaurus rex Suggest the Presence of Protein. Science 2007, 316, 277-280, 10.1126/science.1138709.
- G N Ramachandran; G Kartha; Structure of Collagen. Nature 1954, 174, 269-270, 10.1038/174269c0.
- Cowan, P.M.; North, A.C.T.; Randall, J.T.; X-ray diffraction studies of collagen fibres. Soc. Exp. Biol. 1955, 9, 115-126, .
- Alexander Rich; F. H. C. Crick; The Structure of Collagen. Nature 1955, 176, 915-916, 10.1038/176915a0.
- Prashant K. Bhagwat; Padma B. Dandge; Collagen and collagenolytic proteases: A review. Biocatalysis and Agricultural Biotechnology 2018, 15, 43-55, 10.1016/j.bcab.2018.05.005.
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, 1–39.
- Sherman, V.R.; Yang, W.; Meyers, M.A. The materials science of collagen. J. Mech. Behav. Biomed. Mater. 2015, 52, 22–50.
- A.Abd Karim; Rajeev Bhat; Fish gelatin: properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocolloids 2009, 23, 563-576, 10.1016/j.foodhyd.2008.07.002.
- Samira Feyzi; Mehdi Varidi; Fatemeh Zare; Mohammad Javad Varidi; Fenugreek (Trigonella foenum graecum) seed protein isolate: extraction optimization, amino acid composition, thermo and functional properties. Journal of the Science of Food and Agriculture 2015, 95, 3165-3176, 10.1002/jsfa.7056.
- Anton V. Persikov; John A. M. Ramshaw; Barbara Brodsky; Prediction of Collagen Stability from Amino Acid Sequence. Journal of Biological Chemistry 2005, 280, 19343-19349, 10.1074/jbc.m501657200.
- Sylvie Ricard-Blum; The Collagen Family. Cold Spring Harbor Perspectives in Biology 2010, 3, a004978-a004978, 10.1101/cshperspect.a004978.
- Lin Wang; Qiufang Liang; Tingting Chen; Zhenbin Wang; Junmin Xu; Haile Ma; Characterization of collagen from the skin of Amur sturgeon (Acipenser schrenckii). Food Hydrocolloids 2014, 38, 104-109, 10.1016/j.foodhyd.2013.12.002.
- Alberta Terzi; Nunzia Gallo; Simona Bettini; Teresa Sibillano; Davide Altamura; Lorena Campa; Maria Lucia Natali; Luca Salvatore; Marta Madaghiele; Liberato De Caro; et al. Investigations of Processing–Induced Structural Changes in Horse Type-I Collagen at Sub and Supramolecular Levels. Frontiers in Bioengineering and Biotechnology 2019, 7, 1-15, 10.3389/fbioe.2019.00203.
- Lin Wang; Qiufang Liang; Zhenbin Wang; Junmin Xu; Yang Liu; Haile Ma; Preparation and characterisation of type I and V collagens from the skin of Amur sturgeon (Acipenser schrenckii). Food Chemistry 2014, 148, 410-414, 10.1016/j.foodchem.2013.10.074.
- Karl E. Kadler; Clair Baldock; Jordi Bella; Raymond P. Boot-Handford; Collagens at a glance. Journal of Cell Science 2007, 120, 1955-1958, 10.1242/jcs.03453.
- J J Wu; P E Woods; D R Eyre; Identification of cross-linking sites in bovine cartilage type IX collagen reveals an antiparallel type II-type IX molecular relationship and type IX to type IX bonding.. Journal of Biological Chemistry 1992, 267, 23007-23014, .
- Kazunori Mizuno; Sergei P. Boudko; Jürgen Engel; Hans Peter Bächinger; Kinetic Hysteresis in Collagen Folding. Biophysical Journal 2010, 98, 3004-3014, 10.1016/j.bpj.2010.03.019.
- Ertem, F.; Watson, A.R.; Rivers, C.R.; Babichenko, D.; Tang, G.; Schwartz, M.; Proksell, S.; Johnston, E.; Hashash, J.G.; Barrie, A.; et al. Mo1823 – endoscopic patterns and location of post-operative recurrence in crohn’s disease patients with side to side anastomosis following ileocecal resection. Gastroenterology 2019, 156, S-850–S-851, .
- Claus-Werner Franzke; Peter Bruckner; Leena Bruckner-Tuderman; Collagenous Transmembrane Proteins: Recent Insights into Biology and Pathology. Journal of Biological Chemistry 2004, 280, 4005-4008, 10.1074/jbc.r400034200.
- M Van Der Rest; R Garrone; Collagen family of proteins.. The FASEB Journal 1991, 5, 2814–2823, .
- Ramshaw, J.A.M.; Werkmeister, J.A.; Glattauer, V. Collagen-based biomaterials. Biotechnol. Genet. Eng. Rev. 1996, 13, 335–382.
- Friess, W. Collagen—Biomaterial for drug delivery. Eur. J. Pharm. Biopharm. 1998, 45, 113–136.
- Gaurav Kumar Pal; P.V. Suresh; Sustainable valorisation of seafood by-products: Recovery of collagen and development of collagen-based novel functional food ingredients. Innovative Food Science & Emerging Technologies 2016, 37, 201-215, 10.1016/j.ifset.2016.03.015.
- Phanat Kittiphattanabawon; Soottawat Benjakul; Wonnop Visessanguan; Fereidoon Shahidi; Isolation and characterization of collagen from the cartilages of brownbanded bamboo shark (Chiloscyllium punctatum) and blacktip shark (Carcharhinus limbatus). LWT - Food Science and Technology 2010, 43, 792-800, 10.1016/j.lwt.2010.01.006.
- D. C. Liu; Y. K. Lin; M. T. Chen; Optimum Condition of Extracting Collagen from Chicken Feet and its Characteristics. Asian-Australasian Journal of Animal Sciences 2001, 14, 1638-1644, 10.5713/ajas.2001.1638.
- James R. W. Conway; Claire Vennin; Aurélie S. Cazet; David Herrmann; Kendelle J. Murphy; Sean C. Warren; Lena Wullkopf; Alice Boulghourjian; Anaiis Zaratzian; Andrew M. Da Silva; et al. Three-dimensional organotypic matrices from alternative collagen sources as pre-clinical models for cell biology. Scientific Reports 2017, 7, 1-15, 10.1038/s41598-017-17177-5.
- Alexander J. Lausch; Lester C. Chong; Hasan Uludag; Eli D. Sone; Multiphasic Collagen Scaffolds for Engineered Tissue Interfaces. Advanced Functional Materials 2018, 28, 1-9, 10.1002/adfm.201804730.
- Nurul Huda; E.K. Seow; M.N. Normawati; N.M. Nik Aisyah; Preliminary Study on Physicochemical Properties of Duck Feet Collagen. International Journal of Poultry Science 2013, 12, 615-621, 10.3923/ijps.2013.615.621.
- Ashley Wood; Masahiro Ogawa; Ralph J. Portier; Mark Schexnayder; Mark Shirley; Jack N. Losso; Biochemical properties of alligator (Alligator mississippiensis) bone collagen. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 2008, 151, 246-249, 10.1016/j.cbpb.2008.05.015.
- Lin, Y.K.; Liu, D.C. Comparison of physical-chemical properties of type I collagen from different species. Food Chem. 2006, 99, 244–251.
- Cheng, F.Y.; Hsu, F.W.; Chang, H.S.; Lin, L.C.; Sakata, R. Effect of different acids on the extraction of pepsin-solubilised collagen containing melanin from silky fowl feet. Food Chem. 2009, 113, 563–567.
- Zhou, C.; Li, Y.; Yu, X.; Yang, H.; Ma, H.; Yagoub, A.E.G.A.; Cheng, Y.; Hu, J.; Otu, P.N.Y. Extraction and characterization of chicken feet soluble collagen. LWT-Food Sci. Technol. 2016, 74, 145–153.
- Busra, F.M.; Chowdhury, S.R.; Saim, A.B.; Idrus, R.B. Genotoxicity and cytotoxicity of ovine collagen on human dermal fibroblasts. Saudi Med. J. 2011, 32, 1311–1312.
- Amri, M.; Firdaus, M.; Fauzi, M.; Chowdhury, S.; Fadilah, N.; Hamirul, W.W.; Reusmaazran, M.; Aminuddin, B.; Ruszymah, B. Cytotoxic evaluation of biomechanically improved crosslinked ovine collagen on human dermal fibroblasts. Bio-Med. Mater. Eng. 2014, 24, 1715–1724.
- Fauzi, M.B.; Lokanathan, Y.; Aminuddin, B.S.; Ruszymah, B.H.I.; Chowdhury, S.R. Ovine tendon collagen: Extraction, characterisation and fabrication of thin films for tissue engineering applications. Mater. Sci. Eng. C 2016, 68, 163–171.
- Fauzi, M.B.; Lokanathan, Y.; Nadzir, M.M.; Aminuddin, S.; Ruszymah, B.H.I.; Chowdhury, S.R. Attachment, proliferation, and morphological properties of human dermal fibroblasts on ovine tendon collagen scaffolds: A comparative study. Malays. J. Med. Sci. 2017, 24, 33–43.
- Cersoy, S.; Zazzo, A.; Lebon, M.; Rofes, J.; Zirah, S. Collagen extraction and stable isotope analysis of small vertebrate bones: A comparative approach. Radiocarbon 2017, 59, 679–694.
- Junjie Zhang; Rui Duan; Characterisation of acid-soluble and pepsin-solubilised collagen from frog ( Rana nigromaculata ) skin. International Journal of Biological Macromolecules 2017, 101, 638-642, 10.1016/j.ijbiomac.2017.03.143.
- Poliana Fernandes De Almeida; Marleide Guimarães Oliveira De Araújo; J. C. Curvelo Santana; Collagen extraction from chicken feet for jelly production. Acta Scientiarum. Technology 2012, 34, 345–351, 10.4025/actascitechnol.v34i3.10602.
- Hashim, P.; Ridzwan, M.S.M.; Bakar, J.; Isolation and characterization of collagen from chicken Feet. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2014, 8, 147–151, 10.5281/zenodo.1091484.
- Ji-Hyun Lee; Jihyeon Lee; Kyung Bin Song; Development of a chicken feet protein film containing essential oils. Food Hydrocolloids 2015, 46, 208-215, 10.1016/j.foodhyd.2014.12.020.
- Damodar Dhakal; Pisut Koomsap; Anita Lamichhane; Muhammad Bilal Sadiq; Anil Kumar Anal; Optimization of collagen extraction from chicken feet by papain hydrolysis and synthesis of chicken feet collagen based biopolymeric fibres. Food Bioscience 2018, 23, 23-30, 10.1016/j.fbio.2018.03.003.
- Huda, N.; Seow, E.K.; Normawati, M.N.; Nik Aisyah, N.M.; Fazilah, A.; Easa, A.M.; Effect of duck feet collagen addition on physicochemical properties of surimi. Int. Food Res. J. 2013, 20, 537–544, .
- Francisco G.E. Nogueira; Nayara T. Do Prado; Luiz C.A. Oliveira; Ana R.R. Bastos; João H. Lopes; Janice G. De Carvalho; Incorporation of mineral phosphorus and potassium on leather waste (collagen): A new NcollagenPK-fertilizer with slow liberation. Journal of Hazardous Materials 2010, 176, 374-380, 10.1016/j.jhazmat.2009.11.040.
- Nasser Y. Mostafa; Extraction and Characterization of Collagen from Buffalo Skin for Biomedical Applications. Oriental Journal of Chemistry 2016, 32, 1601-1609, 10.13005/ojc/320336.
- Zahrani, A.R. Extraction and Isolation of Collagen Type I from Fish Skin. Master’s Thesis, The University of Otago, Dunedin, New Zealand, November 2011.
- Ginger A. Abraham; James Murray; Kristen Billiar; Susan J. Sullivan; Evaluation of the porcine intestinal collagen layer as a biomaterial. Journal of Biomedical Materials Research 2000, 51, 442-452, 10.1002/1097-4636(20000905)51:3<442::aid-jbm19>3.3.co;2-w.
- Chen, X.; Wu, J.-H.; Li, L.; Wang, S.-Y. The cryoprotective effects of antifreeze peptides from pigskin collagen on texture properties and water mobility of frozen dough subjected to freeze–thaw cycles. Eur. Food Res. Technol. 2017, 243, 1149–1156.
- Maione-Silva, L.; de Castro, E.G.; Nascimento, T.L.; Cintra, E.R.; Moreira, L.C.; Cintra, B.A.S.; Valadares, M.C.; Lima, E.M. Ascorbic acid encapsulated into negatively charged liposomes exhibits increased skin permeation, retention and enhances collagen synthesis by fibroblasts. Sci. Rep. 2019, 9, 1–14.
- Kumudini A Munasinghe; Jurgen G. Schwarz; Matthew Whittiker; Utilization of Chicken By-Products to Form Collagen Films. Journal of Food Processing 2015, 2015, 1-6, 10.1155/2015/247013.
- Shahidi, F.; Varatharajan, V.; Peng, H.; Senadheera, R. Utilization of marine by-products for the recovery of value-added products. J. Food Bioact. 2019, 6, 10–61.
- Dave, D.; Liu, Y.; Clark, L.; Dave, N.; Trenholm, S.; Westcott, J. Availability of marine collagen from Newfoundland fisheries and aquaculture waste resources. Bioresour. Technol. Reports 2019, 7, 100271
- Hashim, P.; Mohd Ridzwan, M.S.; Bakar, J.; Mat Hashim, D.; Collagen in food and beverage industries. Int. Food Res. J. 2015, 22, 1-8, .
- Bo Li; Beneficial Effects of Collagen Hydrolysate: A Review on Recent Developments. Biomedical Journal of Scientific & Technical Research 2017, 1, 1-5, 10.26717/BJSTR.2017.01.000217.
- Deepika Dave; Winny Routray; Current scenario of Canadian fishery and corresponding underutilized species and fishery byproducts: A potential source of omega-3 fatty acids. Journal of Cleaner Production 2018, 180, 617-641, 10.1016/j.jclepro.2018.01.091.
- Hyun Kyung Kim; Young Ho Kim; Yun Ji Kim; Hyun Jin Park; Nam Hyouck Lee; Effects of ultrasonic treatment on collagen extraction from skins of the sea bass Lateolabrax japonicus. Fisheries Science 2012, 78, 485-490, 10.1007/s12562-012-0472-x.
- Phanat Kittiphattanabawon; Soottawat Benjakul; Sittichoke Sinthusamran; Hideki Kishimura; Characteristics of collagen from the skin of clown featherback (Chitala ornata). International Journal of Food Science & Technology 2015, 50, 1972-1978, 10.1111/ijfs.12864.
- Onouma Kaewdang; Soottawat Benjakul; Thammarat Kaewmanee; Hideki Kishimura; Characteristics of collagens from the swim bladders of yellowfin tuna (Thunnus albacares). Food Chemistry 2014, 155, 264-270, 10.1016/j.foodchem.2014.01.076.
- Dasong Liu; Li Liang; Joe M. Regenstein; Peng Zhou; Extraction and characterisation of pepsin-solubilised collagen from fins, scales, skins, bones and swim bladders of bighead carp (Hypophthalmichthys nobilis). Food Chemistry 2012, 133, 1441-1448, 10.1016/j.foodchem.2012.02.032.
- Naga Vijaya Lakshmi Manchinasetty; Sho Oshima; Masanori Kikuchi; Preparation of flexible bone tissue scaffold utilizing sea urchin test and collagen. Journal of Materials Science: Materials in Medicine 2017, 28, 184, 10.1007/s10856-017-5993-5.
- Takeshi Nagai; Keiko Nagamori; Eiji Yamashita; Nobutaka Suzuki; Collagen of octopus Callistoctopus arakawai arm. International Journal of Food Science & Technology 2002, 37, 285-289, 10.1046/j.1365-2621.2002.00568.x.
- Phanat Kittiphattanabawon; Sitthipong Nalinanon; Soottawat Benjakul; Hideki Kishimura; Characteristics of Pepsin-Solubilised Collagen from the Skin of Splendid Squid (Loligo formosana). Journal of Chemistry 2015, 2015, 1-8, 10.1155/2015/482354.
- Mourad Jridi; Sana Bardaa; Dorsaf Moalla; Tarak Rebaii; Nabil Souissi; Zouheir Sahnoun; Moncef Nasri; Microstructure, rheological and wound healing properties of collagen-based gel from cuttlefish skin. International Journal of Biological Macromolecules 2015, 77, 369-374, 10.1016/j.ijbiomac.2015.03.020.
- Jean-Yves Exposito; Claire Larroux; Caroline Cluzel; Ulrich Valcourt; Claire Lethias; Bernard M. Degnan; Demosponge and Sea Anemone Fibrillar Collagen Diversity Reveals the Early Emergence of A/C Clades and the Maintenance of the Modular Structure of Type V/XI Collagens from Sponge to Human. Journal of Biological Chemistry 2008, 283, 28226-28235, 10.1074/jbc.m804573200.
- Po-Hsien Li; Wen-Chien Lu; Yung-Jia Chan; Wen-Ching Ko; Chao-Chuen Jung; Dung Thi Le Huynh; Yu-Xiang Ji; Extraction and characterization of collagen from sea cucumber (Holothuria cinerascens) and its potential application in moisturizing cosmetics. Aquaculture 2020, 515, 734590, 10.1016/j.aquaculture.2019.734590.
- Mehdi Abdollahi; Masoud Rezaei; Ali Jafarpour; Ingrid Undeland; Sequential extraction of gel-forming proteins, collagen and collagen hydrolysate from gutted silver carp (Hypophthalmichthys molitrix), a biorefinery approach. Food Chemistry 2018, 242, 568-578, 10.1016/j.foodchem.2017.09.045.
- Kanako Hashimoto; Shozo Kobayashi; Michiaki Yamashita; Comparison of connective tissue structure and muscle toughness of spotted mackerel Scomber australasicus and Pacific mackerel S. japonicus during chilled and frozen storage. Fisheries Science 2016, 83, 133-139, 10.1007/s12562-016-1042-4.
- Berillis, P.; Environment, A.; Envi-, A.; Ionia, N.; Marine Collagen: Extraction and applications. SM Gr. Open Access eBooks 2015, null, 1–13, .
- Gokula Krishnan Sivasundari Arumugam; Diksha Sharma; Raj Mohan Balakrishnan; P. E. JagadeeshBabu; Extraction, optimization and characterization of collagen from sole fish skin. Sustainable Chemistry and Pharmacy 2018, 9, 19-26, 10.1016/j.scp.2018.04.003.
- Di Yu; Chang-Feng Chi; Bin Wang; Guo-Fang Ding; Zhong-Rui Li; Characterization of acid-and pepsin-soluble collagens from spines and skulls of skipjack tuna (Katsuwonus pelamis). Chinese Journal of Natural Medicines 2014, 12, 712-720, 10.1016/s1875-5364(14)60110-2.
- Phanat Kittiphattanabawon; Chodsana Sriket; Hideki Kishimura; Soottawat Benjakul; Characteristics of acid and pepsin solubilized collagens from Nile tilapia (Oreochromis niloticus) scale. Emirates Journal of Food and Agriculture 2019, 31, 95-101, 10.9755/ejfa.2019.v31.i2.1911.
- Masataka Saito; N. Kunisaki; N. Urano; S. Kimura; Collagen as the Major Edible Component of Sea Cucumber (Stichopus japonicus). Journal of Food Science 2002, 67, 1319-1322, 10.1111/j.1365-2621.2002.tb10281.x.
- D Hickman; T J Sims; C A Miles; Allen J Bailey; M De Mari; M Koopmans; Isinglass/collagen: denaturation and functionality.. Journal of Biotechnology 2000, 79, 245-257, 10.1016/s0168-1656(00)00241-8.
- Joseph Wasswa; Jian Tang; Xiao-Hong Gu; Xiao-Qing Yuan; Influence of the extent of enzymatic hydrolysis on the functional properties of protein hydrolysate from grass carp (Ctenopharyngodon idella) skin. Food Chemistry 2007, 104, 1698-1704, 10.1016/j.foodchem.2007.03.044.
- F. Rodríguez; L. Morán; G. González; E. Troncoso; R. N. Zúñiga; Collagen extraction from mussel byssus: a new marine collagen source with physicochemical properties of industrial interest. Journal of Food Science and Technology 2017, 54, 1228-1238, 10.1007/s13197-017-2566-z.
- Birgit Hoyer; A. Bernhardt; Anja Lode; Sascha Heinemann; Judith Sewing; Matthias Klinger; Holger Notbohm; Michael Gelinsky; Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomaterialia 2014, 10, 883-892, 10.1016/j.actbio.2013.10.022.
- Dieter Swatschek; Wolfgang Schatton; Josef Kellermann; Werner E.G Müller; J. Kreuter; Marine sponge collagen: isolation, characterization and effects on the skin parameters surface-pH, moisture and sebum. European Journal of Pharmaceutics and Biopharmaceutics 2002, 53, 107-113, 10.1016/s0939-6411(01)00192-8.
- Michael H. Suhre; Melanie Gertz; Clemens Steegborn; Thomas Scheibel; Structural and functional features of a collagen-binding matrix protein from the mussel byssus. Nature Communications 2014, 5, 3392, 10.1038/ncomms4392.
- Feng Cui; Chang-Hu Xue; Zhao-Jie Li; Yong-Qin Zhang; Ping Dong; Xue-Yan Fu; Xin Gao; Characterization and subunit composition of collagen from the body wall of sea cucumber Stichopus japonicus. Food Chemistry 2007, 100, 1120-1125, 10.1016/j.foodchem.2005.11.019.
- Zhu, B.; Dong, X.; Zhou, D.; Gao, Y.; Yang, J.; Li, D.; Zhao, X.; Ren, T.; Ye, W.; Tan, H.; et al. Physicochemical properties and radical scavenging capacities of pepsin-solubilized collagen from sea cucumber Stichopus japonicus. Food Hydrocoll. 2012, 28, 182–188.
- Park, S.Y.; Lim, H.K.; Lee, S.; Hwang, H.C.; Cho, S.K.; Cho, M. Pepsin-solubilised collagen (PSC) from Red Sea cucumber (Stichopus japonicus) regulates cell cycle and the fibronectin synthesis in HaCaT cell migration. Food Chem. 2012, 132, 487–492.
- Wu, H.T.; Li, D.M.; Zhu, B.W.; Sun, J.J.; Zheng, J.; Wang, F.L.; Konno, K.; Jiang, X. Proteolysis of noncollagenous proteins in sea cucumber, stichopus japonicus, body wall: Characterisation and the effects of cysteine protease inhibitors. Food Chem. 2013, 141, 1287–1294.
- Adibzadeh, N.; Aminzadeh, S.; Jamili, S.; Karkhane, A.A.; Farrokhi, N. Purification and characterization of pepsin-solubilized collagen from skin of sea cucumber holothuria parva. Appl. Biochem. Biotechnol. 2014, 173, 143–154.
- Abedin, M.Z.; Karim, A.A.; Ahmed, F.; Latiff, A.A.; Gan, C.Y.; Che Ghazali, F.; Islam Sarker, M.Z. Isolation and characterization of pepsin-solubilized collagen from the integument of sea cucumber (stichopus vastus). J. Sci. Food Agric. 2013, 93, 1083–1088.
- Abedin, M.Z.; Karim, A.A.; Gan, C.Y.; Ghazali, F.C.; Barzideh, Z.; Zzaman, W.; Zaidul, I.S.M. Identification of angiotensin I converting enzyme inhibitory and radical scavenging bioactive peptides from sea cucumber (stichopus vastus) collagen hydrolysates through optimization. Int. Food Res. J. 2015, 22, 1074–1082.
- Zhong, M.; Chen, T.; Hu, C.; Ren, C. Isolation and characterization of collagen from the body wall of sea cucumber stichopus monotuberculatus. J. Food Sci. 2015, 80, C671–C679.
- Liu, Y.X.; Zhou, D.Y.; Ma, D.D.; Liu, Z.Q.; Liu, Y.F.; Song, L.; Dong, X.P.; Li, D.M.; Zhu, B.W.; Konno, K.; et al. Effects of endogenous cysteine proteinases on structures of collagen fibres from dermis of sea cucumber (Stichopus japonicus). Food Chem. 2017, 232, 10–18.
- Liu, Y.X.; Zhou, D.Y.; Liu, Z.Q.; Lu, T.; Song, L.; Li, D.M.; Dong, X.P.; Qi, H.; Zhu, B.W.; Shahidi, F. Structural and biochemical changes in dermis of sea cucumber (stichopus japonicus) during autolysis in response to cutting the body wall. Food Chem. 2018, 240, 1254–1261.
- Tian, M.; Xue, C.; Chang, Y.; Shen, J.; Zhang, Y.; Li, Z.; Wang, Y. Collagen fibrils of sea cucumber (apostichopus japonicus) are heterotypic. Food Chem. 2020, 316, 126272.
- Dong, X.; Shen, P.; Yu, M.; Yu, C.; Zhu, B.; Qi, H. (−)-Epigallocatechin gallate protected molecular structure of collagen fibers in sea cucumber apostichopus japonicus body wall during thermal treatment. LWT 2020, 123, 1–7.
- D.R. Eyre; M.J. Glimcher; Comparative biochemistry of collagen crosslinks: Reducible bonds in invertebrate collagens. Biochimica et Biophysica Acta (BBA) - Protein Structure 1971, 243, 525-529, 10.1016/0005-2795(71)90027-4.
- T Matsumura; M Shinmei; Y Nagai; Disaggregation of connective tissue: preparation of fibrous components from sea cucumber body wall and calf skin.. Journal of Biochemistry 1973, 73, 155-162, .
- D. R. Eyre; M. J. Glimcher; Evidence for Glycosylated Crosslinks in Body-Wall Collagen of the Sea Cucumber, Thyone briareus. Experimental Biology and Medicine 1973, 144, 400-403, 10.3181/00379727-144-37600.
- Trotter, J.A.; Lyons-Levy, G.; Thurmond, F.A.; Koob, T.J. Covalent composition of collagen fibrils from the dermis of the sea cucumber, cucumaria frondosa, a tissue with mutable mechanical properties. Comp. Biochem. Physiol. Part A Physiol. 1995, 112, 463–478.
- Trotter, J.A.; Chapman, J.A.; Kadler, K.E.; Holmes, D.F. Growth of sea cucumber collagen fibrils occurs at the tips and centers in a coordinated manner. J. Mol. Biol. 1998, 284, 1417–1424.
- Trotter, J.A.; Kadler, K.E.; Holmes, D.F. Echinoderm collagen fibrils grow by surface-nucleation-and-propagation from both centers and ends. J. Mol. Biol. 2000, 300, 531–540.
- John A Trotter; Gillian Lyons-Levy; David Luna; Thomas J. Koob; Douglas R. Keene; Mark A.L. Atkinson; Stiparin: A glycoprotein from sea cucumber dermis that aggregates collagen fibrils. Matrix Biology 1996, 15, 99-110, 10.1016/s0945-053x(96)90151-1.
- J. A. Trotter; Gillian Lyons-Levy; Kazumi Chino; Thomas J. Koob; Douglas R. Keene; Mark A.L. Atkinson; Collagen fibril aggregation-inhibitor from sea cucumber dermis. Matrix Biology 1999, 18, 569-578, 10.1016/s0945-053x(99)00050-5.
- Thurmond; Trotter; Morphology and biomechanics of the microfibrillar network of sea cucumber dermis. Journal of Experimental Biology 1996, 199, 1817–1828, .
- Xiu-Ping Dong; Beiwei Zhu; Liming Sun; Jie Zheng; Dan Jiang; Dayong Zhou; Haitao Wu; Yoshiyuki Murata; Changes of collagen in sea cucumber (Stichopus japonicas) during cooking. Food Science and Biotechnology 2011, 20, 1137-1141, 10.1007/s10068-011-0155-x.
- Yu-Xin Liu; Da-Yong Zhou; Dong-Dong Ma; Yan-Fei Liu; Dong-Mei Li; Xiuping Dong; Mingqian Tan; Ming Du; Bei-Wei Zhu; Changes in collagenous tissue microstructures and distributions of cathepsin L in body wall of autolytic sea cucumber (Stichopus japonicus). Food Chemistry 2016, 212, 341-348, 10.1016/j.foodchem.2016.05.173.
- Leilei Si; Yan Fan; Yuekun Wang; Leilei Sun; Bafang Li; Changhu Xue; Hu Hou; Thermal degradation behavior of collagen from sea cucumber ( Stichopus japonicus ) using TG-FTIR analysis. Thermochimica Acta 2018, 659, 166-171, 10.1016/j.tca.2017.12.004.
- Zi-Qiang Liu; Feng-Yan Tuo; Liang Song; Yu-Xin Liu; Xiu-Ping Dong; Dong-Mei Li; Da-Yong Zhou; Fereidoon Shahidi; Action of trypsin on structural changes of collagen fibres from sea cucumber (Stichopus japonicus). Food Chemistry 2018, 256, 113-118, 10.1016/j.foodchem.2018.02.117.
- Yu-Xin Liu; Zi-Qiang Liu; Liang Song; Qian-Ru Ma; Da-Yong Zhou; Bei-Wei Zhu; Fereidoon Shahidi; Effects of collagenase type I on the structural features of collagen fibres from sea cucumber (Stichopus japonicus) body wall.. Food Chemistry 2019, 301, 125302, 10.1016/j.foodchem.2019.125302.
- Zunying Liu; Alexandra C. M. Oliveira; Yi-Cheng Su; Purification and Characterization of Pepsin-Solubilized Collagen from Skin and Connective Tissue of Giant Red Sea Cucumber (Parastichopus californicus). Journal of Agricultural and Food Chemistry 2010, 58, 1270-1274, 10.1021/jf9032415.
- Siddiqui, Y.D.; Arief, E.M.; Yusoff, A.; Suzina, A.H.; Abdullah, S.Y.; Isolation of pepsin solubilized collagen (PSC) from crude collagen extracted from body wall of sea cucumber (bohadschia spp.). Int. J. Pharm. Pharm. Sci. 2013, 5, 555–559, .
- Zainul Abedin; Alias A. Karim; Aishah A. Latiff; Chee-Yuen Gan; Farid Che Ghazali; Zoha Barzideh; Sahena Ferdosh; Jahurul Haque Akanda; Wahidu Zzaman; Rezaul Karim; et al. Biochemical and radical-scavenging properties of sea cucumber (Stichopus vastus) collagen hydrolysates. Natural Product Research 2014, 28, 1302-1305, 10.1080/14786419.2014.900617.
- Desmelati; Sumarto; Dewita; Dahlia; Syafrijal; P A Sari; Determination of Nano-Collagen Quality from Sea Cucumber Holothuria scabra. IOP Conference Series: Earth and Environmental Science 2020, 430, 012005, 10.1088/1755-1315/430/1/012005.
- Feng Liu; Leonardo Zamora; Andrew Jeffs; Siew-Young Quek; Biochemical composition of the Australasian sea cucumber, Australostichopus mollis, from a nutritional point of view. Nutrire 2017, 42, 613, 10.1186/s41110-017-0036-z.
- Abdillah Syamsudin; Wijiyanti Gita; Setiawan Metta; Umroh Noor Siti; Mala Nurilmala; Syamsudin Abdillah; Gita Wijiyanti; Metta Setiawan; Siti Umroh Noor; In vitro anti-tyrosinase and anti-elastase activity of collagen from sea cucumber (Holothuria leucospilota). African Journal of Biotechnology 2017, 16, 771-776, 10.5897/AJB2016.15655.
- Saijun Lin; Ya-Ping Xue; Enli San; Tan Chee Keong; Lifang Chen; Yu-Guo Zheng; Extraction and Characterization of Pepsin Soluble Collagen from the Body Wall of Sea Cucumber Acaudina leucoprocta. Journal of Aquatic Food Product Technology 2017, 26, 502-515, 10.1080/10498850.2016.1222560.
- Huo-Xi Jin; Hong-Ping Xu; Yan Li; Qian-Wei Zhang; Hui Xie; Preparation and Evaluation of Peptides with Potential Antioxidant Activity by Microwave Assisted Enzymatic Hydrolysis of Collagen from Sea Cucumber Acaudina Molpadioides Obtained from Zhejiang Province in China. Marine Drugs 2019, 17, 169, 10.3390/md17030169.
- Yuniati, R.; Sulardiono, B.; Exploration of the collagen of non commercial sea cucumber Holothuria atra and commercial sea cucumber stichopus vastus in the Karimunjawa Islands, Indonesia. Ocean Life 2019, 3, 18-23, 10.13057/oceanlife/o030103.
- Yan Zhang; Wentao Liu; Guoying Li; Bi Shi; Yuqing Miao; Xiaohua Wu; Isolation and partial characterization of pepsin-soluble collagen from the skin of grass carp (Ctenopharyngodon idella). Food Chemistry 2007, 103, 906-912, 10.1016/j.foodchem.2006.09.053.
- Daniela Coppola; Maria Oliviero; Giovanni Andrea Vitale; Chiara Lauritano; Isabella D’Ambra; S. Iannace; Donatella De Pascale; Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Marine Drugs 2020, 18, 214, 10.3390/md18040214.
- Dasong Liu; Mehdi Nikoo; Gökhan Boran; Peng Zhou; Joe M. Regenstein; Collagen and Gelatin. Annual Review of Food Science and Technology 2015, 6, 527-557, 10.1146/annurev-food-031414-111800.
- Maria Sadowska; Ilona Kołodziejska; Celina Niecikowska; Isolation of collagen from the skins of Baltic cod (Gadus morhua). Food Chemistry 2003, 81, 257-262, 10.1016/s0308-8146(02)00420-x.
- F. Shahidi; J. Synowiecki; Protein hydrolyzates from seal meat as phosphate alternatives in food processing applications. Food Chemistry 1997, 60, 29-32, 10.1016/s0308-8146(96)00296-8.
- Aydın Erge; Ömer Zorba; Optimization of gelatin extraction from chicken mechanically deboned meat residue using alkaline pre-treatment. LWT - Food Science and Technology 2018, 97, 205-212, 10.1016/j.lwt.2018.06.057.
- Zayas, J.F. Emulsifying Properties of proteins. Functionality of Proteins in Food; Springer: Berlin/Heidelberg, Germany, 1997; pp. 134–227.
- Yi‐Nan Du; Xiao‐kun Guo; Yi‐Tong Han; Jia‐Run Han; Jia‐Nan Yan; Wen‐Hui Shang; Hai-Tao Wu; Hai-Tao Wu; Physicochemical and functional properties of protein isolate from sea cucumber ( Stichopus japonicus ) guts. Journal of Food Processing and Preservation 2019, 43, 1-11, 10.1111/jfpp.13957.
- Baha Eddine Abdelmalek; Joaquín Gómez-Estaca; Assaâd Sila; Oscar Martinez-Alvarez; M. Carmen Gómez-Guillén; Semia Chaabouni-Ellouz; Mohamed Ali Ayadi; Ali Bougatef; Characteristics and functional properties of gelatin extracted from squid (Loligo vulgaris) skin. LWT - Food Science and Technology 2016, 65, 924-931, 10.1016/j.lwt.2015.09.024.
- Madhuri V. Bhuimbar; Prashant K. Bhagwat; Padma B. Dandge; Extraction and characterization of acid soluble collagen from fish waste: Development of collagen-chitosan blend as food packaging film. Journal of Environmental Chemical Engineering 2019, 7, 102983, 10.1016/j.jece.2019.102983.
- Nor, M.H.M.; Nazmi, N.N.M.; Sarbon, N.M. Effects of plasticizer concentrations on functional properties of chicken skin gelatin films. Int. Food Res. J. 2017, 24, 1910–1918.
- Slimane, E.B.; Sadok, S. Collagen from cartilaginous fish by-products for a potential application in bioactive film composite. Mar. Drugs 2018, 16, 211.
- R.J. Avena-Bustillos; C.W. Olsen; D.A. Olson; B. Chiou; E. Yee; P.J. Bechtel; T.H. McHugh; Water Vapor Permeability of Mammalian and Fish Gelatin Films. Journal of Food Science 2006, 71, E202-E207, 10.1111/j.1750-3841.2006.00016.x.
- J.H Muyonga; C.G.B Cole; Kwaku G. Duodu; Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chemistry 2004, 86, 325-332, 10.1016/j.foodchem.2003.09.038.
- Bárbara Teixeira; António Marques; Carla Pires; Cristina Ramos; I. Batista; Jorge Alexandre Saraiva; Maria Leonor Nunes; Characterization of fish protein films incorporated with essential oils of clove, garlic and origanum: Physical, antioxidant and antibacterial properties. LWT - Food Science and Technology 2014, 59, 533-539, 10.1016/j.lwt.2014.04.024.